Published online Feb 10, 2016. doi: 10.4253/wjge.v8.i3.143
Peer-review started: July 31, 2015
First decision: September 29, 2015
Revised: October 7, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: February 10, 2016
Processing time: 185 Days and 15.6 Hours
Stents are tubular devices made of plastic or metal. Endoscopic stenting is the most common treatment for obstruction of the common bile duct or of the main pancreatic duct, but also employed for the treatment of bilio-pancreatic leakages, for preventing post- endoscopic retrograde cholangiopancreatography pancreatitis and to drain the gallbladder and pancreatic fluid collections. Recent progresses in techniques of stent insertion and metal stent design are represented by new, fully-covered lumen apposing metal stents. These stents are specifically designed for transmural drainage, with a saddle-shape design and bilateral flanges, to provide lumen-to-lumen anchoring, reducing the risk of migration and leakage. This review is an update of the technique of stent insertion and metal stent deployment, of the most recent data available on stent types and characteristics and the new applications for biliopancreatic stents.
Core tip: Biliary and pancreatic stents have become one of the major advances made in therapeutic endoscopy and the endoscopic placement of these devices has a universally recognized role in the management of numerous pancreatico-biliary diseases. This review is an update of the technical considerations and available devices for biliary and pancreatic stenting.
- Citation: Mangiavillano B, Pagano N, Baron TH, Arena M, Iabichino G, Consolo P, Opocher E, Luigiano C. Biliary and pancreatic stenting: Devices and insertion techniques in therapeutic endoscopic retrograde cholangiopancreatography and endoscopic ultrasonography. World J Gastrointest Endosc 2016; 8(3): 143-156
- URL: https://www.wjgnet.com/1948-5190/full/v8/i3/143.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i3.143
In 1980 the first case of biliary stent placement for drainage of malignant obstructive jaundice was published[1]. A single-pigtail stent was fashioned using the cut end of an angiography catheter. The procedure was technically successful, but ultimately, the stent migrated upstream.
Cotton[2] reported the use of a stent made with a double-pigtail design to prevent upward migration and Huibregtse et al[3] described the creation of side flaps in the wall of a straight stent instead of pigtails to prevent migration.
Today a variety of plastic stents (PSs) with different designs, diameters, lengths and plastic materials have been investigated and available in the market. At the end of the 80s, some authors described the insertion of a self-expandable metal stent (SEMS) across biliary stenosis[4,5]. Early SEMS had relatively poor stent patency because of over and ingrowth of tissue. Because of their non-removability partially covered (PC) and then fully covered (FC) SEMSs were developed. Such stents are covered by a biocompatible polymer resistant to organic degradation. Despite various original articles and reviews about the types and techniques of stenting for different bilio-pancreatic disorders[6-9], the majority are focused only on one or more than one pathology or focused to pancreatic or biliary disease. The aim of our review is to emphasize the update of the technique of stent insertion and metal stent deployment, considering the most recent data available on stent types and characteristics and the new applications for bilio-pancreatic stents, both for endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasonography (EUS), considering also the gallbladder drainage and pancreatic fluid collections (PFC).
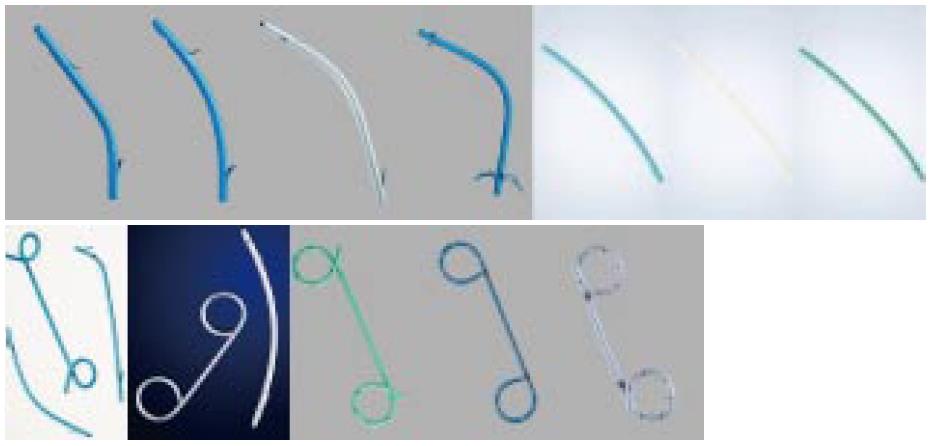
Ideally PSs should be technically easy to insert, should effectively relieve biliary obstruction, should not occlude, and should not cause injury to the bile duct or duodenal wall. Several different materials, sizes, and shapes have been used to optimize these aspects (Table 1 and Figure 1).
| Producer | Model | Diameter (Fr) | Length (cm) | Shape | Material |
| Boston Scientific | Advanix | 7, 8.5, 10 | 5-18 | Duodenal bend, centre bend, double pigtail | Polyethylene |
| ConMed | Hydroduct | 7, 10, 12 | 4-15 | Straight, angled, curved, double pigtail | Polyurethane with hydrophilic hydromer coating |
| Cook Endoscopy | Compass BDS | 7 | 5, 10, 15 | Double pigtail | Polyethylene |
| Cook Endoscopy | Cotton-Huibregtse | 7, 8.5, 10, 11.5 | 5-18 | Angled | Polyethylene |
| Cook Endoscopy | Cotton-Leung | 7, 8.5, 10, 11.5 | 5-18 | Curved | Polyethylene |
| Cook Endoscopy | Cotton-Leung Sof-Flex | 7, 10 | 5-15 | Curved | Polyethylene and polyurethane blend |
| Cook Endoscopy | Fusion Marathon Antireflux | 10 | 5-12 | Curved | Polyethylene with teflon sleeve |
| Cook Endoscopy | Soehendra-Tannenbaum | 8.5, 10, 11.5 | 5-15 | Curved | Teflon |
| Cook Endoscopy | Solus | 10 | 1-15 | Double pigtail | Polyethylene and polyurethane blend |
| Cook Endoscopy | Zimmon | 5, 6, 7, 10 | 4, 7, 10 | Double pigtail | Polyethylene |
| Endo-Flex | PE-Soft | 7, 8.5, 10, 11.5 | 3-15 | Bended, straight, curved, double pigtail, single pigtail | Polyethylene |
| Endo-Flex | PTFE-Strong | 7, 8.5, 10, 11.5 | 5-15 | Bended, straight, curved | Polytetrafluoroethylene |
| GI Supply | ViaDuct | 7, 10 | 5-15 | Winged straight | Polyurethane |
| Hobbs Medical | Biliary stent | 7, 10 | 4-15 | Curved, Double pigtail | Soft polymer blend |
| Indus Medical | CIBIDI | 7, 10 | 5-15 | Straight, curved, double pigtail | Polyurethane and teflon |
| Olympus | Double Layer | 10 | 4-15 | Duodenal bend, centre bend | Inner layer: Perfluoro; middle layer: Stainless steel; outer layer: Polyamide elastomer |
| Olympus | Biliary EVA | 7, 8.5, 10, 12 | 5-18 | Straight, proximal bend, centre bend, double pigtail | Ethylene vinyl acetate copolymers |
| Olympus | Biliary FEP | 7, 8.5, 10, 12 | 3-15 | Straight, proximal bend | Fluorinated ethylene propylene |
| Olympus | Biliary PE | 7, 8.5, 10, 12 | 3-15 | Straight, proximal bend, centre bend, double pigtail | Polyethylene |
| Pauldrach Medical | Gallengangs | 7, 8.4, 10 | 9 | Curved | Polyethylene |
Plastic biliary stents are composed of polyethylene, polyurethane, polytetrafluoroethylene (Teflon) and other plastic polymers. The diameters of PSs are measured in French (Fr), corresponding to 0.33 mm, and diameters range from 5 Fr to 12 Fr.
PS with a diameter of 10 Fr require a 3.7 mm operative endoscope channel, and, when the diameter is larger (≥ 11.5 Fr) a 4.2 mm operative channel is needed.
PSs have lengths ranging from 1 to 18 cm, and custom-made models may be requested from some manufacturers. A given stent length represents the entire length of the stent, although for some it is the distance between the end flaps. The length of a PS is generally selected to allow the shortest length possible while simultaneously ensuring adequate drainage. The length of plastic stents chosen is that which allows the ends to extent one to two cm over the proximal edge of the biliary lesion and 1 cm inside the duodenum.
Different types of PSs are commercially available. Plastic pig-tail stents are coiled at their proximal and distal extremities, or only at the distal (double pig-tail or single pig-tail, respectively). Side hole are generally placed at the coiled end. PSs may be straight or curved, with a flap on the proximal and the distal end and a side hole or with 4 flaps at both ends, without side holes (Tannenbaum stent). The role of side holes is to maintain biliary or pancreatic flow if the ends of the stent became occluded by bile or food impaction.
However, it has been hypothesized that side-holes can contribute to the formation of sludge. Moreover, the Tannenbaum stent (with multiple flap at its extremities but without side-holes) was designed to prevent migration. The aim of the development in biliary stenting in the recent years has been to increase the patency of the stents, improving the materials used for coating, a double-layer design, and a star-shaped stent winged stent without a central lumen. Finally, PSs are visualized radiographically, and some stents contain radiopaque markers at the proximal and/or distal ends. Introducing kits can be included in the stent package or available individually.
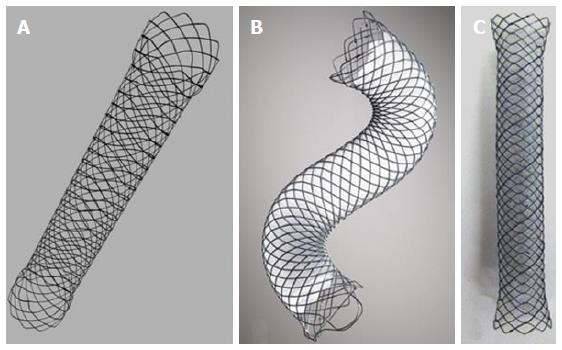
The first widely used SEMS were made of stainless steel, whereas today most SEMS are made of nitinol. SEMS are available as uncovered, partially (PC-SEMS) or fully covered (FC-SEMS) (Figure 2, Tables 2 and 3). Different materials contribute to the cover of the PFC-SEMS and of the FC-SEMS such as polytetrafluoroethylene, silicone and polyurethane, present on the exterior or interior of the SEMS.
| Producer | Model | Material | Diameter (mm) | Length (cm) | Shortening | Reconstrain | Characteristics |
| Boston Scientific | Wallflex® | Platinol | 8, 10 | 4, 6, 8, 10 | Yes | Yes | --------- |
| ConMed | Flexxus | Nitinol | 8, 10 | 4, 6, 8, 10 | Yes | No | Pistol delivery system |
| Cook Endoscopy | Zilver® | Nitinol | 6, 8, 10 | 4, 6, 8 | No | No | --------- |
| Cook Endoscopy | Evolution® | Nitinol | 8, 10 | 4, 6, 8, 10 | Yes | Yes | Pistol delivery system |
| Ella-CS | SX-ELLA® Nitinella Plus | Nitinol | 8, 10 | 4, 6, 8, 10 | Yes | Yes | --------- |
| Endochoice | Bonastent® | Nitinol | 8, 10 | 4, 5, 6, 8, 10, 12 | Yes | Yes | --------- |
| Endo-Flex | BIL-stent | Nitinol | 10 | 6, 8, 10 | Yes | No | --------- |
| Endo-Technik | NIT-BIL-1010® | Nitinol | 10 | 4, 6, 8, 10 | Yes | Yes | --------- |
| Leufen Medical | Aixstent® Gallengang | Nitinol | 8, 10 | 4, 6, 8 | Yes | No | --------- |
| Leufen Medical | Aixstent® Gallengang BDL - BDH | Nitinol | 8, 10 | 4, 6, 8, 10, 12 | Yes | No | The open cell design allows for Y stenting at the hilar region |
| Merit Endotek | Alimaxx-B® | Nitinol | 8, 10 | 4, 6, 8 | Yes | No | The open cell design allows for Y stenting at the hilar region |
| M.I. Tech | Hanarostent® | Nitinol | 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | Yes | Yes | --------- |
| M.I. Tech | Hanarostent® Hilar | Nitinol | 10 | 8 | Yes | No | The large cell design allows for Y stenting at the hilar region |
| Micro-Tech | BD stents Classic or Platinum-Line | Nitinol | 10 | 4, 6, 8, 10 | Yes | No | --------- |
| Olympus | NIRflex | Nitinol | 8, 10 | 4, 6, 8, 10 | Yes | No | --------- |
| S and G Biotech | EGIS® Biliary DC Stent | Nitinol | 8, 10, 12 | 4, 5, 6, 7, 8, 9, 10, 12 | Yes | No | Single or double bare |
| TaeWoong Medical | LCD® | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | Yes | No | The large cell design allows for Y stenting at the hilar region |
| TaeWoong Medical | Niti-S® D-type | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | Yes | No | --------- |
| TaeWoong Medical | Niti-S® S-type | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 9, 10, 12 | Yes | No | --------- |
| Producer | Model | Material | Diameter (mm) | Length (cm) | Shortening | Reconstrain | Shape | Covering |
| Allium Medical | Allium BIS® | Nitinol | 8, 10 | 6, 8, 10, 12 | No | No | Straight with anchoring segment | FC in polyurethane |
| Boston Scientific | Wallflex® | Platinol | 8, 10 | 4, 6, 8, 10 | Yes | Yes | Two flanges | PC and FC in permalume |
| Cook Endoscopy | Evolution® | Nitinol | 8, 10 | 4, 6, 8, 10 | Yes | Yes | Two flanges | PC and FC in silicone |
| Ella-CS | SX-ELLA® Nitinella Plus | Nitinol | 8, 10 | 4, 6, 8, 10 | Yes | Yes | Two flanges | PC and FC in silicone |
| Endochoice | Bonastent® | Nitinol | 8, 10 | 4, 5, 6, 8, 10, 12 | Yes | Yes | Two flanges | FC in silicone |
| Endo-Flex | BIL-stent | Nitinol | 10 | 6, 8 | Yes | No | Straight | FC in silicone |
| Endo-Technik | NIT-BIL-1010® | Nitinol | 10 | 4, 6, 8, 10 | Yes | No | Straight | PC in silicone |
| Gore Medical | Viabil® | Nitinol | 8, 10 | 4, 6, 8, 10 | No | No | Straightwith anchoring fins | FC in PTFE with/without drainage holes |
| Leufen Medical | Aixstent® Gallengang | Nitinol | 8, 10 | 4, 6, 8 | Yes | No | Two flanges | PC and FC in polyurethane |
| M.I. Tech | Hanarostent® BCT | Nitinol | 10 | 4, 6, 8, 10 | Yes | Yes | One flange with flaps and lasso | FC in silicone |
| M.I. Tech | Hanarostent® BCS | Nitinol | 10 | 4, 6, 8, 10, 12 | Yes | No | One flange and with flaps | FC in silicone |
| M.I. Tech | Hanarostent® BPE | Nitinol | 8, 10 | 8, 10 | Yes | No | One flange and with flaps | PC in silicone |
| Micro-Tech | BD stents | Nitinol | 10 | 4, 6, 8, 10 | Yes | No | Two flanges | PC and FC in silicone |
| S and G Biotech | EGIS® Biliary DC Stent | Nitinol | 8, 10, 12 | 4, 5, 6, 7, 8, 9, 10, 12 | Yes | No | Two flanges | PC in PTFE |
| TaeWoong Medical | Niti-S® S-type covered | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 10, 12 | Yes | No | Two flanges | FC in silicone |
| TaeWoong Medical | Niti-S® Kaffes | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8 | Yes | No | Tapered with long lasso | FC in silicone |
| TaeWoong Medical | Niti-S® Bumpy | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 10, 12 | Yes | No | Two flanges | FC in silicone and PTFE |
| TaeWoong Medical | Niti-S® Giobor | Nitinol | 8, 10 | 8, 10 | Yes | No | One flange | PC in silicone |
| TaeWoong Medical | Niti-S® ComVi | Nitinol | 6, 8, 10 | 4, 5, 6, 7, 8, 10, 12 | Yes | No | Straight | FC in PTFE |
Mechanical properties of SEMS are related to the stent design, type of wire, and covering materials. As a result of combinations of these variables, radial force and axial force were proposed as major mechanical properties that affect clinical outcomes. Radial force is well known as an expanding force, while axial force is a straightening or recovery force when SEMS are bent.
Radial force affects stent patency in that dilation of a biliary stricture and maintenance of luminal patency depend on the expanding force of the SEMS. Two factors in radial force exist in terms of time course. Immediate stent expansion at the time of stent deployment affects short-term outcomes, and chronic resistant force against tissue compression affects long-term outcomes. In general, the chronic resistant radial force is higher than the immediate stent expanding force because SEMS are made of a type of shape-memory alloy. This characteristic means that SEMS partially expand immediately after deployment and then gradually expand to their full extent, even though the radial force may be high. Axial force is considered to define conformability of SEMS in the bile duct and may have a greater relationship with clinical outcomes than radial force. After deployment in the bile duct, SEMS are fixed at the stricture by the tissue and axial force causes compression to the bile duct at both stent ends. As axial force increases, so does the compression of the bile duct or cystic duct or pancreatic duct orifice. Clinically, this situation may cause kinking of the bile duct with resultant cholecystitis or pancreatitis. In addition, less conformability of SEMS in the bile duct leads to stent migration. In general, axial force affects clinical outcomes such as stent migration and pancreatitis more than radial force.
SEMS have lengths ranging from 4 to 12 cm and diameters from 6 to 10 mm. The stents are mounted on a delivery system accepting a wire of 0.035 diameter, and the newest models can be also used with a single operator system. The diameters of the delivery systems range between 5.0 and 10.5 Fr. The smaller the catheter the easier it is to cross the stenosis without mechanical or pneumatic dilation. The same can be said for patients with Klatskin neoplasia.
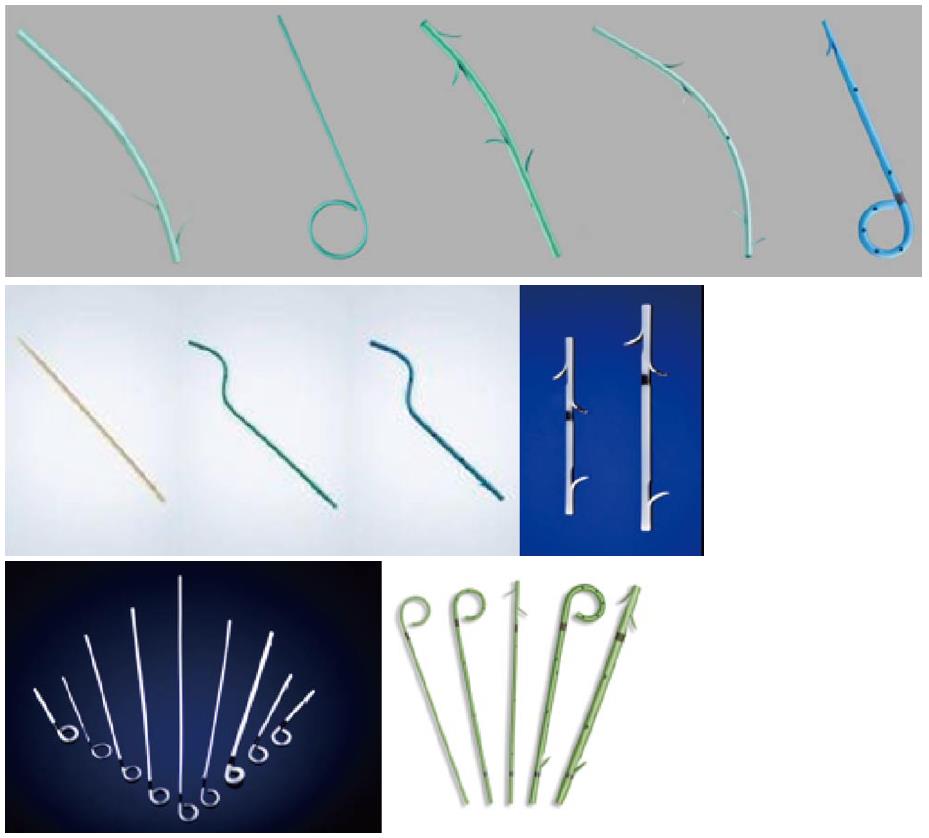
The majority of the delivery kits are resistant, avoiding kinking during insertion, allowing correct placement; the outer sheath of the kit is transparent for the visualization of the distal stent extremity during SEMS release. During stent placement, the outer sheath is gently pulled inside the operative endoscope channel to allow the release and expansion of the SEMS. Rarely, the stent is constrained by a thread that, when removed, allows SEMS expansion. Generally, SEMS can be recaptured, until 80% of their opening and all metal stents are visible fluoroscopically. The majority of SEMS have a marker at both extremities and, in some models, one at the middle. Some models of FC-SEMS have anti-migration flaps or flared ends to avoid distal or proximal migration (Figure 3).
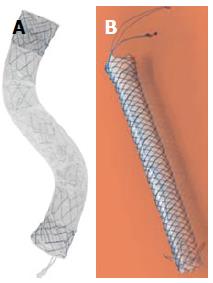
Recently, a new type of FC-SEMS is produced with the intent to diminish proximal and distal migration (Figure 4). The Hanaro, M.I. Tech, Seoul, South Korea has an “anchoring-flap” system made of four flaps in the proximal end, flared ends and one proximal and one distal lasso for retrieval. TaeWoong produces the Bumpy®-Niti-S stent, with a membrane of silicone (distal extremity) and polytetrafluoroethylene (body of the stent). This stent has both flared ends and a string for the removal, at the distal extremity. The characteristic of this FC-SEMS are the irregular meshes; it contributes to a different radial force in every point of the stent, conferring conformability and adaptability in the lumen of the duct, preventing migration.
Before stent placement a cholangiogram is performed to confirm successful biliary cannulation and to evaluate the location and length of the stricture or leak[10-12].
The correct choice of PS length is often based on operator’s experience. Alternatively after bile duct contrast medium injection, or using a centimeter guidewire. An alternative way to measure the length of the strictures for the choice of the stent is to gently withdrawing the catheter from the proximal to the distal end of the strictures, measuring with a ruler the centimeters of the device out of the operating channel.
Endoscopic sphincterotomy (ES) is not necessary for inserting a single PS, while is indispensable for multiple plastic stenting. If the stricture is tight, dilation with a balloon or a bougie before stenting may be useful. Balloon dilatation of strictures is usually helpful for placement of hilar PSs, particularly when bilateral stenting is attempted. Moreover, in these strictures, there is still a role for stents of smaller diameter and the tapered pigtail stent design. For example, if bilateral stenting is required in patients with hilar obstruction, it is often easier to place two 7 Fr stents initially to gradually dilate the bile duct and then replace them later with 10 Fr stents. Tapered pigtail stents are sometimes helpful to allow passage across very tight strictures.
The PS stent is loaded on a guide-catheter, over the guidewire, with the pusher-catheter. The guide-catheter and guidewire need to be made wet using a saline solution because they are hydrophilic. The entire stent insertion loaded kit is introduced inside the operative channel. When the PS is placed across the stricture, moving the endoscope in anti-clockwise rotation and with alternately moving the elevator up and down, the guide-catheter is pulled back, pushing the stent inside the CBD with the pusher-tube. When the guide-catheter is completely pulled back, the pusher-catheter can be removed from the channel.
During stent placement, maintaining the endoscope close to the Vater’s papilla facilitates tent insertion because it avoids looping of the delivery system in the duodenal lumen.
If the guide-catheter is inadvertently withdrawn from the inside of the PS, it may be possible to readvance it, continuing the stent placement. When stent insertion is challenging, the “long position” of the endoscope might be useful. This position allows to the operator to then use the straightening maneuver and, maintaining the elevator in up position, insert the stent into the duct. If the PS is damaged during insertion in the bile duct it can be removed over-the-wire, by passing a dilation balloon inside the PS or by using the Soehendra retriever, leaving the wire in place, and replacing the a new PS delivery system.
A final radiographic image should be obtained to verify if contrast medium drains through the stent. For implantation of a SEMS an ES is often performed, though is not mandatory. Then, under fluoroscopic examination (for biliary strictures), the length, presence or absence of a gallbladder and the relationship of the cystic duct with the CBD is determined for the correct choice of the type of the SEMS (length, diameter and covered vs uncovered).
Because of a potential risk of cholecystitis, some endoscopists prefer to use uncovered SEMS in the presence of a gallbladder, to avoid the cystic duct occlusion, or to place a FCSEMS, when indicated and a small diameter plastic stent inside the cystic duct. Before their insertion into the duct, the uncovered SEMS and the FC-SEMS are generally wet with saline solution in the guidewire channel and inside the outer sheath.
The release of the SEMS is performed under X-ray control, withdrawing the outer sheath of the device, pulling down the elevator, maintaining the stent in the correct position during the release, pulling back the device as it tends to move away from the operator and proximally into the duct. Most of the stent can be recaptured until 80% of the complete release. At the end of the procedure, after metal stent release a cholangiography is required to confirm the correct position of the SEMS and flow of contrast medium flow into the duodenal lumen. If the SEMS is released too proximally, it can be withdrawn distally grasping the distal extremity, or the distal thread, with a rat-tooth forceps. If these attempts fail, a second stent can be deployed, with the distal extremity inside the proximal one of the previous stent. Contrariwise, if the SEMS is released too distally into the duodenum, it can be completely removed by a rat-tooth forceps or the excess stent cut using argon plasma coagulation.
Different techniques are utilized for the drainage of the hepatic hilum. Preoperative magnetic resonance cholangiopancreatography or high-resolution CT should be performed in all patients with suspected proximal biliary stenosis to delineate the anatomy before the procedure. SEMS insertion can be performed using the “side-by-side” (SBS) or the “stent-in-stent” (SIS -“Y”) technique.
When SBS technique is performed, two or more guidewires are placed inside different biliary ducts to be drained. After the release of the first metal stent, the insertion of the delivery system of the second SEMS can be difficult because of the impaction of the distal ends of the first SEMS with the delivery of the second one. A way to overcome this difficulty is the insertion of a temporary plastic stent to maintain an accessory space between the first SEMS and the duct wall. In SBS technique the first lobe to drain is the left because the SEMS insertion in the right lobe is easier.
With the SIS technique, the second stent is deployed inside the meshes of the first stent. Balloon dilation of the first SEMS meshes might be helpful to facilitate positioning of the second SEMS device. Some SEMS are designed with large diameter meshes of the middle part to facilitate the deployment of the second one (Y-shaped stent).
In recent years, EUS has evolved from a diagnostic to a therapeutic procedure, and is now increasingly used to guide biliary drainage (BD) after failed ERCP. For therapeutic EUS, the use of a linear-array endoscope with a 3.8 mm operative channel is preferable to allow the passage of large diameter accessories. There are two possible puncture routes for EUS-BD; transgastric for the intra-hepatic bile duct drainage or transduodenal (bulb) for extrahepatic bile duct drainage. During therapeutic EUS Color Doppler is mandatory, to prevent damage to interposed vessels between the endoscope and the ducts. The puncture of the duct to drain can be performed with a fine needle aspiration (FNA) needle of 19- or 22-gauge (G). The 19 G needle is generally used because the capability of support a 0.035-inch guidewire, which provides more stiffness. The 22 G needle accommodates only a 0.018-inch guidewire, which carries a greater risk of dislodgement of the guidewire during the procedure. After duct access with the EUS needle, contrast medium injection from the needle is required to perform cholangiography for the confirmation of the correct position of the needle inside the biliary tree. After that, under fluoroscopic guidance, the guidewire can be placed into the duct, advancing it inside the needle[13-16].
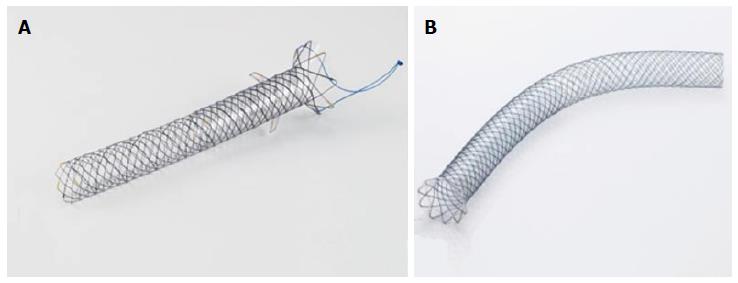
If the drainage is performed transmurally from the stomach, only intrahepatic ducts can be drained [hepaticogastrostomy (HPG)], and if performed from the duodenal bulb the extrahepatic bile duct are more accessible [choledochoduodenostomy (CLD)]. If the guidewire exits the papilla, the drainage can be integrated by ERCP, using the rendezvous technique. When the deployment of the stent is performed through the puncture route or deployed across the stricture or the ampulla in an antegrade fashion, different devices can be used for dilation of the site, such as bougie (6 or 7 Fr), pneumatic dilation balloon (4 or 6 mm) or a cystotome (8.5 Fr). Both plastic and metal stents are used for HPG or CLD although PC and FC SEMS are most often used to prevent stent migration and bile leakage. Uncovered SEMS should not be used for HPG or CLD. Recently two new SEMS have emerged specifically designed for EUS-BD (Figure 5).
The Giobor Niti-S, Taewoong, is a PC-SEMS with the inner part (intra-biliary) uncovered to prevent intrahepatic bile duct obstruction and migration, and covered in the trans and intragastric part to prevent bile leakage; it also has a single lasso for possible retrieval. The BPE, Hanaro MI Tech, is a PC-SEMS, the proximal portion, which is 15 to 55 mm in length, is uncovered for the prevention of duct obstruction, while the distal end, 35 mm in length, has a silicone cover for the prevention of bile leakage. The BPE stent and has anti-migration flaps at both extremities, for prevention of stent migration.
Cystic duct negotiation is the most challenging part of transpapillary gallbladder stenting. Methods to reach the cystic duct are cholangiography and fluoroscopy arm longitudinal and transversal axis rotation to allow for identification of the level of its insertion into the CBD[17,18].
For a left-side cystic duct take-off, a flexible-tip catheter or a rotatable sphincterotome may be used, while for a right-sided take-off, a standard sphincterotome may be used because it usually bows toward the cystic duct when it takes off on the right side. A 0.035” or 0.025” guidewire (stiff or hydrophilic) is used to enter into the cystic duct orifice. The angled tip guidewires are preferable to enter and pass through the spiral valves of Heister while minimizing the risk of perforation. In difficult cannulation of the cystic duct, an inflated Fogarty balloon up to the cystic duct insertion, with an angled-tip guidewire passed alongside may be useful for its negotiation. After cystic duct negotiation, the guidewire is advanced and coiled within the gallbladder lumen and an accurate study of the course and diameter of the duct must be performed for the correct choice of the stent. The catheter is then removed and the stent placed over the wire. Double pigtail 6 to 10 Fr PSs are preferable because of their superior anchorage into the gallbladder lumen compared with straight stents. The length of the stent is chosen based upon the distance between the major duodenal papilla and the gallbladder (usually 12-15 cm long stents are used) and the stent size according to the diameter of the cystic duct and common bile duct. When 10 Fr stents are placed, an ES should be performed to minimize the risk of post-ERCP pancreatitis caused by the fulcrum effect.
EUS guided gallbladder drainage (EUS-GD) is performed using a large channel (3.7 or 3.8 mm) echoendoscope with fluoroscopic guidance[19-22].
The best way to visualize the gallbladder is the pre-pyloric area, in the stomach, or from the duodenal bulb. The puncture is performed in the site in which gallbladder is in contact with the bowel. The more stable the echoendoscope position the easier the procedure. Color Doppler is mandatory, before gallbladder puncture, to avoid puncture of interposed blood vessels.
A 19 G FNA needle is usually used to obtain gallbladder access. After gallbladder puncture and removal of the stylet, cholecystography is performed by injecting contrast medium through the needle. After cholecystography, a guidewire is inserted and coiled inside the gallbladder. After the removal of the needle, the access-site can be enlarged using either a mechanical (6 or 7 Fr bougie or balloon catheters) or electrocautery (6 or 10 Fr cystotome or needle-knife) device. After dilation, the stent is advanced over the wire and into the gallbladder.
Recently, a single-step device allowing access, dilation and plastic stent placement has been developed for EUS-GD (Giovannini Needle Wire Oasis, Cook Ireland Ltd, Limerick, Ireland).
Plastic stents, standard or modified tubular covered SEMSs and lumen apposing metal stents (LAMSs) are used. Plastic stents were used for EUS-GD in early studies. However, the PSs can become occluded and may not allow complete sealing between the gallbladder and duodenal or gastric wall with a relative risk of bile leak in the abdomen.
To circumvent the limitations of plastic stents tubular FCSEMS were used for EUS-GD. Metal stents, with their high radial force and covering can reduce this risk. The larger diameters may facilitate draining of thick or necrotic debris, pus or sludge reducing the risk of stent clogging.
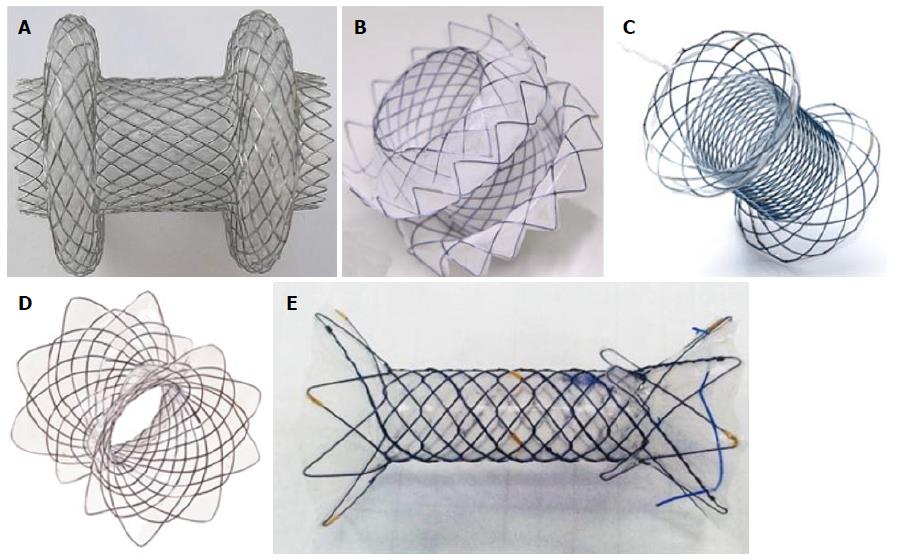
However when metal and plastic stents designed for ERCP are used migration remains an important risk. Recently LAMS have been developed to obtain better anchorage between the gallbladder or bile ducts and the bowel wall, reducing the risk of stent migration and bile leakage. These include the Axios stent (Boston Scientific, Natick, MA, United States) (Figure 6A) and Spaxus Niti-S stent (Taewoong Medical, Seoul, South Korea) (Figure 6B).
Pancreatic stents (Table 4 and Figure 7) are made of polyethylene; the shape and design resemble those of biliary stents, save for the presence of side holes along the length of the stent. The side holes allow draining of pancreatic juice from side branches.
| Producer | Model | Diameter (Fr) | Length (cm) | Shape | Material |
| Boston scientific | Advanix | 3, 4, 5, 7, 10 | 2-18 | Straight or single pigtail with or without internal flap | Polyethylene |
| Cook endoscopy | Geenan Sof-Flex | 5 | 3-12 | Curved with or without internal flap | Polyethylene and polyurethane blend |
| Cook endoscopy | Geenan | 3, 5, 7 | 3-15 | Curved | Polyethylene |
| Cook endoscopy | Johlin Wedge | 8.5, 10 | 8-22 | Wedge | Polyethylene and polyurethane blend |
| Cook endoscopy | Zimmon | 3, 5, 7 | 2-12 | Single pigtail with or without internal flap | Polyethylene |
| Endo-Flex | PTFE-Strong | 5, 7 | 3-9 | Curved | Polytetrafluoroethylene |
| GI supply | ViaDuct | 5, 7 | 3-12 | Winged straight or single pigtail with or without internal flap | Polyurethane |
| Hobbs medical | Freeman Flexi-Stents | 3, 4, 5, 7 | 2-18 | Straight or single pigtail with or without internal flap | Soft polymer |
| Olympus | Pancreatic PE | 7, 8.5, 10 | 3-15 | Straight, S-shaped | Polyethylene |
Pancreatic stents have lengths between 2 and 25 cm and diameters between 3 and 11.5 Fr. Different types of stents are now commercially available, with different shapes as straight, winged or with curved distal end or wedged proximal end. Some of these have a “J” or single pigtail shape to prevent migration into the pancreatic duct. There is also an S-shaped stent with many side holes and made in ethylene-vinyl-acetate (EVA). EVA has more flexibility compared to polyethylene.
Pancreatic stents with S-shape are made for a better adapting to the profile of the main pancreatic duct. A winged stent (Via-Duct stent, GI Supply) is made to allow pancreatic juice to flow through the wings of the stent.
Pancreatic PSs without a proximal flap are designed to allow spontaneous distal migration, when the stent are only to be used for a short time. Pancreatic PSs with a distal end pig-tail are designed for avoidance of proximal migration.
The majority of pancreatic PSs are deployed over-the-wire, only with the push-catheter, without the use of the guide-catheter, because of their small diameter. Pancreatic plastic stent with a diameter more than 8.5 Fr requires the use of a guide-catheter.
The only self-expandable stent designed for drainage of the main pancreatic duct (MPD) is the TaeWoong Bumpy® - Niti-S, that presents a non-regular cell mesh. It results in a different radial force in every part of the stent, avoiding compression of the side branches of the pancreatic duct.
However, other FC-SEMS are used off-label with good outcomes in selected situations, such as the WallFlex (Boston Scientific) and the Viabil (Gore Medical). The Viabil stent is fully covered and available with side holes designed to allow cystic duct drainage and which may allow drainage of some pancreatic duct side branches.
The pancreatic PSs placement technique is the same as used for the biliary tree. After MPD cannulation, the stent is inserted inside the duct over the wire; hydrophilic guidewire of 0.035” is used for placement of PSs from 5 to 10 Fr; 0.018” guidewires are used for 3 Fr PSs, generally reserved for cases of minor pancreatic duct stenting and temporary placement for prevention of post-ERCP pancreatitis. Pancreatic sphincterotomy is not always necessary for placement of PSs. In case of bilio-pancreatic sphincterotomy, pancreatic sphincterotomy is generally performed after biliary sphincterotomy[23].
The PSs diameter must not be greater than the maximum diameter of the pancreatic duct. Five and 7 Fr PSs are generally implanted in absence of duct dilation; 10 Fr PSs, or more than 10 Fr, are instead used when MPD stenosis with upstream duct dilation occurs. When very tight strictures are present, the placement of a PSs can be challenging. In this situation balloon dilation or a bougienage dilation are often helpful to, allow stent placement.
For implantation of a SEMS a pancreatic sphincterotomy is typically performed (often also with biliary sphincterotomy). The metal stent diameter and length are determined on the basis of a combination of location of lesion (stricture or leak), ductal configuration and in cases of stricture the diameter of dilated upstream duct proximal to the lesion.
For MPD strictures dilation is typically performed before SEMS placement and the stent is deployed through the ductal lesion. The distal portion of the SEMS is left in the duodenum for prevention of proximal migration and easy removal.
To perform EUS-guided drainage of the pancreatic duct (PDD) a large channel echoendoscope (3.7 or 3.8 mm) is required. The most common site for pancreatic duct (PD) access is the stomach (gastric body), usually the most straightforward and stable, but also transbulbar access is used (impossible in those with prior pancreatoduodenectomy)[24-28].
However, the route is selected on the basis of the pancreatic anatomical site to be treated. The aim of the drainage is to gain access the shortest way between the echoendoscope and the PD. The shorter the distance the easier the procedure, considering over-the-wire exchanges of devices. Pancreatic duct access may be performed with a 19-G FNA needle followed by either 0.035” or 0.025” guidewire placement via the needle or with a 22-G FNA needle that allows only the passage of an 0.018” guidewire.
After PD access, the wire is placed inside the duct, advancing it into the duodenal lumen, through the Vater’s papilla, or into the jejunal lumen in presence of a pancreatico-jejunal anastomosis. During guidewire placement and device exchanges, the use of fluoroscopy is helpful.
After guidewire placement, PD stenting can be performed in retrograde fashion, with EUS-guided PD rendezvous technique, with a side-viewing duodenoscope or with a frontal-viewing endoscope, in patients with postoperative anatomy, or in antegrade fashion, from the stomach or from the duodenal bulb, with EUS-guidance.
For antegrade stenting, dilation of the gastric wall or duodenal bulb wall and dilation of pancreatic parenchyma with a balloon is helpful before stent placement. In many cases a cystotome is used to gain access to the PD, after wire placement, creating an “electrocautery-tunnel”, to allow subsequent stent deployment. During EUS-guided PDD, a plastic stent is generally preferred to a metallic one, considering the risk of leakage if uncovered SEMS are used. Finally, when PSs are used, to avoid leakage and migration, the diameter of the stent should not be less than the diameter of the dilated tract.
Endoscopic drainage of PFCs are performed with different approaches as the trans-papillary (i.e., using endoscopic retrograde pancreatography), or transmural (cystoenterostomy), or both[29-35].
For transpapillary drainage, before implantation of the stent, a major or minor papilla pancreatic sphincterotomy is typically performed. Following this, a large-bore stent is placed. When the stent is placed, its proximal part can be placed inside the PFC or, in case of leakage, across the disruption of the PD. If a stricture of the PD is present downstream to the PFC, judicious dilation by bougie or dilation balloons needs to be performed before application of the stent.
PFC drainage can be performed or through the stomach (transgastric) or through the duodenum (transduodenal). More rarely drainage is performed through the esophageal wall (transesophageal). The drainage can be undertaken with or without EUS guidance.
When non-EUS-guided techniques are performed a large channel gastroscope or duodenoscope with a 4.2-mm working channel is used.
The side-viewing endoscope is most often used because it permits better visualization of the posterior wall of the gastric body, allowing placement of large diameter accessories (deployment of 10 Fr stents) with assistance of the elevator.
The initial PFC puncture for transmural drainage is generally performed at level of visible bulging on the gastric or duodenal wall. To obtain good endoscope stability, the short position, when possible, is recommended, and the angle between the needle and the gastric/duodenal wall needs to be closer to 90°. The closer to 90° results in shorter distance to traverse.
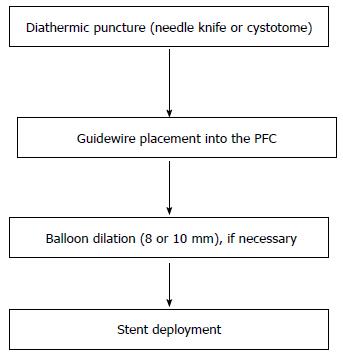
To access a PFC with the side-viewing endoscope, diathermic puncture technique or the Seldinger technique are used. The diathermic puncture technique involves the use of a needle-knife sphincterotome (double or triple-lumen), or a 10-Fr cystotome that is a catheter with a diathermic ring and a 5-Fr inner catheter housing a low-profile, 0.38” needle knife to facilitate close apposition of the PFC to the enteral lumen. A pure cutting current is recommended and the electrocautery should be discontinued immediately upon entry of the needle into the PFC cavity to avoid thermal injury to surrounding structures.
Following this, aspiration of fluid (which can be sent for analysis) and gentle injection of contrast under fluoroscopic guidance confirm position within the cavity. The needle is exchanged for a standard catheter. After that, the guidewire is placed inside the PFC, and coiled for 2-3 times.
Following deep access with a guidewire, the catheter is exchanged for an 8 or 10 mm pneumatic balloon, to dilate the tract. After dilation, the balloon is removed and a plastic or metallic stent is deployed over the guidewire. Alternatively, a cystotome can be used for single-step drainage, avoiding balloon dilation.
When the Seldinger technique is used, a 19-G aspirating needle is used for initial puncture of the PFC. After fluid aspiration contrast is injected inside the PFC for the confirmation of the correct position of the needle. Through the needle a guidewire is passed, coiling it inside the fluid collection. Leaving the wire in place, the needle is withdrawn and a cystotome or a dilation balloon is passed over the wire. Finally the cystotome (or the dilating balloon) is removed and a stent is placed over the guidewire. Moreover balloon dilation can be performed after the creation of the fistula with the cystotome.
There are two techniques for EUS-guided drainage (EUS-GD) of PFC: The 2-step approach and the 1-step approach. For 2-step approach larger (3.7 or 3.8 mm) and smaller (2.8 or 3.2 mm) mm working channel echoendoscopes can be used.
The PFC is located and studied by EUS, identifying the best site for drainage of the collection which is closest to the transducer. Color Doppler helps to avoid puncture of interposed vessels during drainage. This site can be marked with a biopsy forceps, with a metal clip or with India ink and the echoendoscope withdrawn and replaced with a side-viewing duodenoscope to perform the drainage. Otherwise, the PFC puncture is directly performed with a 19 G needle and, after puncture, a guidewire is placed inside the collection. After wire placement, the echoendoscope can be withdrawn, leaving the guidewire in place inside the PFC, and replaced with a side-viewing endoscope over the guidewire, and the drainage can be performed using this endoscope. These exchange of endoscope approaches are now used infrequently.
With the 1-step approach the echoendoscope is used for the entire procedure. An echoendoscope with a large operative channel is required. It allows the use large diameter accessories (deployment of 10 Fr stents) with the assistance of an elevator.
The PFC puncture is usually performed using a 19-G needle under endosonographic view. The collection contents can be aspirated for biochemical analysis, gram stain, culture and cytology. Through the lumen of the needle a 0.025” or 0.035” guidewire is advanced until it coils in the PFC which adds stabilisation of the position and access by forming anchoring extra loops in the cavity.
Fistula dilation is achieved by balloon dilatation over the guidewire, or using a cytostome and diathermy needle. Finally the stent is placed over the guidewire.
The 1-step approach PFC drainage avoids guidewire displacement during the exchange of the echoendoscope with the side-viewing endoscope. When more than one stent, or an additional naso-cystic drainage (NCD) are placed, two guidewires can be inserted inside the same catheter to avoid recannulation of the PFC. Recently, a 3-layer puncture kit, allowing synchronous placement of two guidewires has been described. This kit is composed of a 6 Fr catheter made of Teflon, inside an outer catheter of 8.5 Fr and a 22G FNA needle inside the 6 Fr catheter. Puncture of the collection is performed with a 22 G needle using electrocautery, under EUS-guidance. After puncture the 6 Fr inner catheter and the 8.5 French outer catheter are advanced inside the PFC. When the entire kit is inside the PFC, both needle and inner catheter are removed, and two guidewires can be inserted into the PFC through the outer catheter. Then, two stents, or one stent and one NCD, are placed.
After initial puncture and dilation some endoscopists described the use of the Soehendra dilator or a cystotome 10 Fr outer catheter for passage of two guidewires.
The “Navix-access-device” (Boston Scientific, Natick, MA, United States) consists of a 19-gauge trocar with a short extendable side blade. The retractable blade creates a cystoenterostomy without the use of cautery. It has an anchoring and dilating balloon (10 mm), as well as 2 guidewire ports to permit double wire advancement with the same puncture for sequential stent placement.
Traditionally, more than one plastic pigtail stent is used for PFC tansmural drainage. The fistula tract between the gastrointestinal wall and the PFC is maintained by placement of double pigtail plastic stents for preventing dislocation and migration. When 7 Fr stents are used the occlusion rates are higher. To further improve transmural drainage of PFCs, tubular FCSEMS (available for the treatment of biliary strictures) have recently been used as an alternative for the traditionally used plastic double-pigtail stents. Fully covered SEMS have larger diameters (10 mm) and placement of a single stent can provide a wide drainage opening. Furthermore, due to the larger diameter, there is a reduced risk of occlusion, especially for collections containing a significant amount of solid debris.
However, these stents are designed for drainage related to a luminal stricture and not to a transluminal route. When a bile duct stent is used for PFC drainage, protrusion of the ends of the stent both into the GI tract and inside the PFC can increase the risk of stent migration or bleeding, caused by a contact ulceration of the stent within the wall. They are not ideal in cases when the PFC is not firmly attached to the gastrointestinal wall because they do not apply any anchorage force and resultant leakage may occur.
To overcome limitations associated with the use of tubular biliary SEMS for transmural drainage, novel drainage stents have been developed.
These new lumen apposing metal stents (Table 5), are specifically designed for transmural drainage (Figure 6). These stent are fully-covered for preventing ingrowth of tissue and have large flanges at the distal ends, with a length from 10 to 40 mm. The flanges are designed to provide lumen-to-lumen anchoring and a low migration and leakage risk. The diameter of the stents, 10 and 15 mm, enable direct necrosectomy through the lumen of the stent. A flow-chart for the traditional transmural endoscopic drainage of PFC is summarized in Figure 8.
| Producer | Model | Internal diameter (mm) | Length (mm) | Flange diameter (mm) |
| Boston Scientific | Axios | 10, 15 | 10 | 21, 24 |
| Leufen Medical | Aix | 10, 14 | 20 | 14/16, 18/20 |
| M.I. Tech | Hanarostent BCF | 10, 12 | 30, 40 | 25 |
| TaeWoong Medical | Spaxus | 8, 10, 16 | 20 | 25 |
| TaeWoong Medical | Nagi | 10, 12, 14, 16 | 10, 20, 30 | 22, 24, 26, 28 |
Biliary and pancreatic stents are important advancements in therapeutic endoscopy and have revolutionized the approach to pancreaticobiliary disorders. The new designs of plastic and metal stents have allowed an increased use in a large, broad range of biliary and pancreatic benign and malignant conditions, replacing interventional radiologic approaches and surgery in most cases.
| 1. | Soehendra N, Reynders-Frederix V. Palliative bile duct drainage - a new endoscopic method of introducing a transpapillary drain. Endoscopy. 1980;12:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 248] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Cotton PB. Duodenoscopic placement of biliary prostheses to relieve malignant obstructive jaundice. Br J Surg. 1982;69:501-503. [PubMed] |
| 3. | Huibregtse K, Haverkamp HJ, Tytgat GN. Transpapillary positioning of a large 3.2 mm biliary endoprosthesis. Endoscopy. 1981;13:217-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Neuhaus H, Hagenmüller F, Classen M. Self-expanding biliary stents: preliminary clinical experience. Endoscopy. 1989;21:225-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 59] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Huibregtse K, Cheng J, Coene PP, Fockens P, Tytgat GN. Endoscopic placement of expandable metal stents for biliary strictures--a preliminary report on experience with 33 patients. Endoscopy. 1989;21:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 107] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Uppal DS, Wang AY. Advances in endoscopic retrograde cholangiopancreatography for the treatment of cholangiocarcinoma. World J Gastrointest Endosc. 2015;7:675-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 7. | Bang JY, Hawes R, Bartolucci A, Varadarajulu S. Efficacy of metal and plastic stents for transmural drainage of pancreatic fluid collections: a systematic review. Dig Endosc. 2015;27:486-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Kawakami H, Itoi T, Sakamoto N. Endoscopic ultrasound-guided transluminal drainage for peripancreatic fluid collections: where are we now? Gut Liver. 2014;8:341-355. [PubMed] |
| 9. | Baron TH. Best endoscopic stents for the biliary tree and pancreas. Curr Opin Gastroenterol. 2014;30:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Dumonceau JM, Heresbach D, Devière J, Costamagna G, Beilenhoff U, Riphaus A. Biliary stents: models and methods for endoscopic stenting. Endoscopy. 2011;43:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Lee JH. Self-expandable metal stents for malignant distal biliary strictures. Gastrointest Endosc Clin N Am. 2011;21:463-480, viii-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Srinivasan I, Kahaleh M. Metal stents for hilar lesions. Gastrointest Endosc Clin N Am. 2012;22:555-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Binmoeller KF, Nguyen-Tang T. Endoscopic ultrasound-guided anterograde cholangiopancreatography. J Hepatobiliary Pancreat Sci. 2011;18:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Itoi T, Isayama H, Sofuni A, Itokawa F, Kurihara T, Tsuchiya T, Tsuji S, Ishii K, Ikeuchi N, Tanaka R. Stent selection and tips on placement technique of EUS-guided biliary drainage: transduodenal and transgastric stenting. J Hepatobiliary Pancreat Sci. 2011;18:664-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Perez-Miranda M, Barclay RL, Kahaleh M. Endoscopic ultrasonography-guided endoscopic retrograde cholangiopancreatography: endosonographic cholangiopancreatography. Gastrointest Endosc Clin N Am. 2012;22:491-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Itoi T, Coelho-Prabhu N, Baron TH. Endoscopic gallbladder drainage for management of acute cholecystitis. Gastrointest Endosc. 2010;71:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 18. | Hasan MK, Itoi T, Varadarajulu S. Endoscopic management of acute cholecystitis. Gastrointest Endosc Clin N Am. 2013;23:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Súbtil JC, Betes M, Muñoz-Navas M. Gallbladder drainage guided by endoscopic ultrasound. World J Gastrointest Endosc. 2010;2:203-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 20. | Widmer J, Singhal S, Gaidhane M, Kahaleh M. Endoscopic ultrasound-guided endoluminal drainage of the gallbladder. Dig Endosc. 2014;26:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 21. | Choi JH, Lee SS. Endoscopic ultrasonography-guided gallbladder drainage for acute cholecystitis: from evidence to practice. Dig Endosc. 2015;27:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Peñas-Herrero I, de la Serna-Higuera C, Perez-Miranda M. Endoscopic ultrasound-guided gallbladder drainage for the management of acute cholecystitis (with video). J Hepatobiliary Pancreat Sci. 2015;22:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 23. | Testoni PA. Endoscopic pancreatic duct stent placement for inflammatory pancreatic diseases. World J Gastroenterol. 2007;13:5971-5978. [PubMed] |
| 24. | Irisawa A, Hikichi T, Shibukawa G, Takagi T, Wakatsuki T, Takahashi Y, Imamura H, Sato A, Sato M, Ikeda T. Pancreatobiliary drainage using the EUS-FNA technique: EUS-BD and EUS-PD. J Hepatobiliary Pancreat Surg. 2009;16:598-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Shami VM, Kahaleh M. Endoscopic ultrasound-guided cholangiopancreatography and rendezvous techniques. Dig Liver Dis. 2010;42:419-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Perez-Miranda M, de la Serna C, Diez-Redondo P, Vila JJ. Endosonography-guided cholangiopancreatography as a salvage drainage procedure for obstructed biliary and pancreatic ducts. World J Gastrointest Endosc. 2010;2:212-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Itoi T, Kasuya K, Sofuni A, Itokawa F, Kurihara T, Yasuda I, Nakai Y, Isayama H, Moriyasu F. Endoscopic ultrasonography-guided pancreatic duct access: techniques and literature review of pancreatography, transmural drainage and rendezvous techniques. Dig Endosc. 2013;25:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Fujii-Lau LL, Levy MJ. Endoscopic ultrasound-guided pancreatic duct drainage. J Hepatobiliary Pancreat Sci. 2015;22:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Babich JP, Friedel DM. Endoscopic approach to pancreatic pseudocysts: An American perspective. World J Gastrointest Endosc. 2010;2:77-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Samuelson AL, Shah RJ. Endoscopic management of pancreatic pseudocysts. Gastroenterol Clin North Am. 2012;41:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Seewald S, Ang TL, Teng KY, Groth S, Zhong Y, Richter H, Imazu H, Omar S, Polese L, Seitz U. Endoscopic ultrasound-guided drainage of abdominal abscesses and infected necrosis. Endoscopy. 2009;41:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Giovannini M. Endoscopic ultrasonography-guided pancreatic drainage. Gastrointest Endosc Clin N Am. 2012;22:221-230, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Fabbri C, Luigiano C, Maimone A, Polifemo AM, Tarantino I, Cennamo V. Endoscopic ultrasound-guided drainage of pancreatic fluid collections. World J Gastrointest Endosc. 2012;4:479-488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Singhal S, Rotman SR, Gaidhane M, Kahaleh M. Pancreatic fluid collection drainage by endoscopic ultrasound: an update. Clin Endosc. 2013;46:506-514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (3)] |
| 35. | Braden B, Dietrich CF. Endoscopic ultrasonography-guided endoscopic treatment of pancreatic pseudocysts and walled-off necrosis: new technical developments. World J Gastroenterol. 2014;20:16191-16196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kawaguchi T, Robles-Medranda C S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ