Published online Feb 10, 2016. doi: 10.4253/wjge.v8.i3.122
Peer-review started: May 7, 2015
First decision: September 8, 2015
Revised: November 9, 2015
Accepted: December 1, 2015
Article in press: December 2, 2015
Published online: February 10, 2016
Processing time: 277 Days and 14.6 Hours
In recent years, water jet instruments have been used in the field of gastrointestinal endoscopy, mainly in two clinical situations: Investigation and treatment under endoscopic view. Injecting water jet into the gastrointestinal lumen is helpful for maintaining a clear endoscopic view, washing away blood or mucous in the lumen or on the surface of the tip of the endoscope. This contributes to reducing time and discomfort of examination. Water jet technology is an alternative method for dissecting soft tissue; this method does not harm the small vessels or cause mechanical or thermal damage. However, its use in clinical settings has been limited to the transmucosal injection of water into the submucosal layer that elevates the mucosa to prepare for endoscopic mucosal resection or endoscopic submucosal dissection, instead of tissue dissection, which may occur because of the continuous water jet. A preclinical study has been conducted using a pulsed water jet system as an alternative method for submucosal dissection by reducing intraoperative water consumption and maintenance of dissection capability. This review introduces recent studies pertaining to using a water jet in gastrointestinal endoscopy and discusses future prospects.
Core tip: This review provides an overview of recent clinical and preclinical studies of water jet instruments in gastrointestinal endoscopy. Water jets have been used to keep the endoscopic view clear which contributed to reduce time and discomfort of endoscopic examination, and the technology provides an alternative method for endoscopic tumor resection. However, continuous flow is used in the transmucosal injection of water into the submucosal layer for elevating the mucosa to prepare for endoscopic mucosal resection. A preclinical study has used a pulsed water jet system as an alternative method to achieve dissection of submucosal layer.
- Citation: Nakano T, Sato C, Sakurai T, Kamei T, Nakagawa A, Ohuchi N. Use of water jet instruments in gastrointestinal endoscopy. World J Gastrointest Endosc 2016; 8(3): 122-127
- URL: https://www.wjgnet.com/1948-5190/full/v8/i3/122.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i3.122
Incidences of colorectal cancer are increasing in the developed world; in comparison with other types of examinations such as the stool occult blood test, barium enema, and computed tomography colonography, colonoscopy enables enhanced diagnostic specificity and sensitivity[1]. The incidence of gastric cancer remains high in Asian countries, including Japan. The demand for upper gastrointestinal endoscopy has been increasing annually, especially in Asian countries[2]. It requires highly advanced techniques and a learning curve exists for digestive endoscopy[1,2]. When the endoscope first appeared, it was a struggle to maintain a clear endoscopic view. The introduction of the forceps hole into the endoscope has been useful for injecting water vigorously into the gastrointestinal lumen to keep the endoscopic view clear. Endoscopes with incorporated water jet systems have been developed and released for clinical practice and are in widespread use. Water jets have also been recently used for endoscopic treatment, i.e., in endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). This review provides an overview of recent clinical and preclinical studies of water jet instrument in gastrointestinal endoscopy.
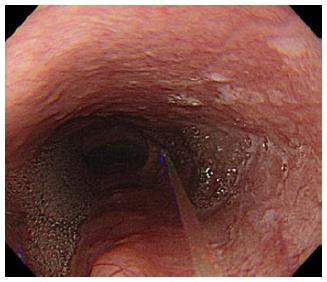
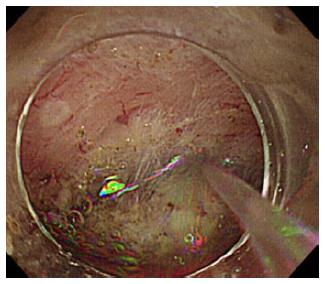
Water jet instruments were initially used to facilitate endoscopic observation. During gastrointestinal endoscopy, blood, food residue, and bubbles can impede the endoscopic view. Specifically in colonoscopy, colonic cleaning with polyethylene glycol method (PEG) helps with finding small lesions[3]. However, PEG can result in a lot of bubbles forming, hindering observation as much as the feces[4]. It is necessary to wash these out to discover the minute lesions or to treat under a clear endoscopic view. During gastroendoscopy, premedication with mucolytic agents, such as pronase, N-acetylcysteine, or dimethylpolysiloxane before upper gastrointestinal endoscopy improves the mucosal visibility of the stomach[5,6]. It is still necessary to wash away the bubbles caused by saliva or mucus (Figure 1). Recently, upper gastrointestinal endoscopy using nasal endoscope has rapidly become popular, as it is less painful and causes minimum vomiting reflux[7-10]. However, problems to be solved with this technique include lower camera resolution, insufficient light intensity, and the longer duration of the procedure as compared with that of an oral endoscopy. Attempts to use fluids such as oolong tea to clean the lens surface have been reported[11]. Manual water jet pumping prolongs inspection time[12]; Takahashi et al[13] reported that the introduction of a water jet operated by a foot switch in the nasal gastrointestinal endoscopy reduced the average inspection time from 561 ± 123 s to 503 ± 98 s (P = 0.0002). Using a water jet to maintain a clear endoscopic view is useful for reducing time and the discomfort of examination. A water jet from an automatic lavage pump is useful to keep endoscopic view clear[14]. This is currently supplied in products from several companies. Some models of upper gastrointestinal and colonic endoscope have separate water supply and forceps holes, which make it possible to inject water during endoscopic treatment such as hemostasis, EMR, or ESD (Figure 2). Hemostatic procedure is one of the important techniques during endoscopic treatment like EMR or ESD. So water jet systems are widely used to find the bleeding point and to make a view during hemostasis.
Water jet technology was used in liver[15] and cardiovascular[16] surgeries, as well as in neurosurgery in the late 1980s[17]. When used in liver surgery, this system reduces blood loss and parenchymal trauma better than both ultrasonic aspiration and blunt dissection[18,19]. Using the water jet instrument as a surgical device provides energy using the kinetic energy of the water flowing from a nozzle at the tip of the delivery device. This energy is transmitted to the tissue surface where it ejects particles of tissue, making an incision through the organ or tissue. Mass reduction can also be achieved using water jets[15,20]. Water jet has several features pertaining to dissection that are superior to conventional instruments, including selective tissue removal with vessel preservation based on the different tensile strengths of the tissues. Water jet devices using a continuous water flow[20] allow organ dissection while preserving vessels that are > 100-200 μm in diameter[21,22]. Another notable advantage is that it helps avoid thermal damage to the surrounding parenchyma, which would otherwise be inevitable using an electric scalpel, electromagnetic, ultrasonic, and laser instruments[23,24]. However, limitations have been reported to arise from the formation of air bubbles, which obscure the operative field, and the splashing of blood fluid, which could subject surgeons and nurses to cross infection[16]. These limitations may be resolved when using the instrument in a luminal organ such as the gastrointestinal tract or in laparoscopic or thoracoscopic surgery. In addition, the development of a treatment instrument with lower water consumption would help address the limitations. Endoscopic treatment such as ESD in a narrow surgical field requires the application of highly advanced techniques by the operator. A lack of instruments that can aid this procedure preventing the risk of potential complications (thermal injury and vascular damage) is a drawback of the current ESD technique using an electric scalpel[25]. Water jet technology, which is based on a conventional, pressure-driven continuous jet[15,26] or a laser/electrically-induced pulsed pressure jet[27-29], could provide an alternative method or novel procedure for the dissection of soft tissue without impairing small-diameter vessels or causing mechanical or thermal damage during endoscopic therapy.
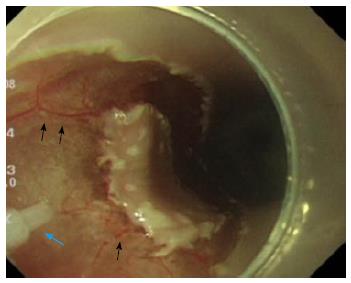
Endoscopic resection has become the standard of care for the treatment of early stage gastrointestinal tumors. EMR is performed on relatively small lesions. ESD enables the resection of large lesions in a single piece, and has low local recurrence rates[30,31]; however, operation time and the risk of complications are increased[31,32]. Various knives such as the dual knife (Olympus Medical Systems Co., Tokyo, Japan), B-knife (Zeon Medical, Tokyo, Japan), IT-knife, or Hook knife (Olympus Medical Systems Co., Tokyo, Japan) are used in ESD[33,34]; these are devised for safety and ease of use. As a preparation for safe EMR or ESD, it is useful either to inject fluids such as saline or hyaluronate or inject carbon dioxide into the submucosal layer to lift the lesion from the muscular layer[35,36]. Various water jet dissectors have been developed, such as the Flush knife (Fujifilm Medical, Tokyo, Japan), Splash needle (Pentax Co., Tokyo, Japan), HybridKnife (ERBE, Tübingen, Germany), and the ENKI-2 water-jet system (NESTIS, Lyon, France)[37-40]; these use continuous water flow to incise mucosa and inject fluid into the submucosal layer to lift the lesion. In contrast, the applying conventional pressure-driven continuous water jets endoscopically is limited to transmucosal injection of water into the submucosal layer for mucosal elevation prepare for EMR instead of tissue dissection[40,41]. This may be because of the continuous water jet. An advantage of these water jet devices is that washing of the surgical field or additional submucosal injection can be performed by flushing water through the knife without changing the instrument; this results in marked improvements pertaining to the efficiency and safety of the procedure[42]. Incision capability of these devices would be mostly due to the cooperation of water jet and electric cautery. Although Lesser et al[43] attempted to use a water jet dissector to cut polyp stalks clinically in the airway; the attempt to cut or dissect a submucosal layer under gastrointestinal endoscopy has been performed only in preclinical animal experiments. A continuous water jet flow of 30 kgf/cm2 (Angiomat 3000, Liebel-Flarsheim, United States) was necessary to cut mucosa and mucosal muscle; however, injection fluid was spread in the submucosal layer in the swine stomach[44]. Kaehler et al[41] reported that a continuous water jet dissector, the Helix Hydro-Jet (ERBE), is capable of penetrating the mucosa and creating highly selective fluid accumulation in the submucosal layer, using a water pressure of 50-70 bar and an application angle of 20°-90°[41]. Lepilliez et al[45] reported a porcine gastric ESD where continuous jet dissection using a WJ medical system (Eschmann Equipment, West Sussex, England) in vivo was technically difficult due to the lack of visual control. Using continuous water jet also poses a potential risk of obscuring the narrow endoscopic operative view due to the large amounts of water. To date, there has been no report of continuous water flow being used to dissect the submucosal layer effectively. It has been reported that a pulsed water jet was feasible at 120 mL/min of water supply, but pulsed dissection was slower than IT knife dissection in the porcine stomach[45]. That volume of water would interfere with the endoscope view in a narrow lumen such as the esophagus or large intestine. On the other hand, Sato et al[46] reported that laser-induced pulsed water jet dissection in the porcine esophagus was performed safely and effectively, and the dissection rate was not different from hook knife dissection. Preservation of the vessels by water jet, which could be treated with pin-point ablation by hemostatic equipment would contribute to reliable hemostasis (Figure 3). They reported the feasibility of ESD of the esophagus with very small amounts of water (1.6 mL/min) and preserved micro-vessels. The optimal conditions for submucosal dissection are still unclear for both continuous and pulsed water jets, including the best size or shape of the nozzle, water pressure of the jet, pulse rate or volume of water supply. Since the required condition of the jet also depends on the physical properties of the tissue to be dissected[47], the conditions may vary between the esophagus, stomach, and large intestine. Further study is needed to elucidate the optimal conditions for dissection by water jet.
In gastrointestinal endoscopy, using a water jet to maintain a clear endoscopic view is useful for reducing time and the discomfort of examination; furthermore, water jets contribute to endoscopic therapy such as ESD or EMR. Using the water jet as an operative instrument is a recent development. A continuous water jet is used to lift up the mucous layer to pretreat EMR or ESD. Hybrid products combining water jet and electric scalpel have also been developed, and their results reported. It may be difficult to dissect the submucosal layer directly using continuous flow due to its nature, but use of a pulsed water jet is feasible, with a lower volume of water consumption. Although the research reported is mostly based on animal studies limited, further research is expected in the future.
| 1. | Lee SH, Park YK, Lee DJ, Kim KM. Colonoscopy procedural skills and training for new beginners. World J Gastroenterol. 2014;20:16984-16995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 40] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (2)] |
| 2. | Lee SH, Park YK, Cho SM, Kang JK, Lee DJ. Technical skills and training of upper gastrointestinal endoscopy for new beginners. World J Gastroenterol. 2015;21:759-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (11)] |
| 3. | Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Development of a lavage solution associated with minimal water and electrolyte absorption or secretion. Gastroenterology. 1980;78:991-995. [PubMed] |
| 4. | Nagatani K, Mitsushima T, Yokouchi K, Nakamoto K, Abe Y, Arima N, Yokota T, Minamihara Y, Ikuma H; Tsuda S, Ohashi S. Evaluation of Colonic Lavage For The Screening Total Colonoscopy [in Japanese]. Gastroenterological Endoscopy. 1989;31:856-865. [DOI] [Full Text] |
| 5. | Bhandari P, Green S, Hamanaka H, Nakajima T, Matsuda T, Saito Y, Oda I, Gotoda T. Use of Gascon and Pronase either as a pre-endoscopic drink or as targeted endoscopic flushes to improve visibility during gastroscopy: a prospective, randomized, controlled, blinded trial. Scand J Gastroenterol. 2010;45:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Chang CC, Chen SH, Lin CP, Hsieh CR, Lou HY, Suk FM, Pan S, Wu MS, Chen JN, Chen YF. Premedication with pronase or N-acetylcysteine improves visibility during gastroendoscopy: an endoscopist-blinded, prospective, randomized study. World J Gastroenterol. 2007;13:444-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Campo R, Montserrat A, Brullet E. Transnasal gastroscopy compared to conventional gastroscopy: a randomized study of feasibility, safety, and tolerance. Endoscopy. 1998;30:448-452. [PubMed] |
| 8. | Christensen M, Achiam M, Trap R, Støckel M, Rosenberg J, Schulze S. Transnasal gastroscopy. Ugeskr Laeger. 2000;162:3464-3467. [PubMed] |
| 9. | Dumortier J, Napoleon B, Hedelius F, Pellissier PE, Leprince E, Pujol B, Ponchon T. Unsedated transnasal EGD in daily practice: results with 1100 consecutive patients. Gastrointest Endosc. 2003;57:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Roy JF, Duforest D, Marek TA. Prospective comparison of nasal versus oral insertion of a thin video endoscope in healthy volunteers. Endoscopy. 1996;28:422-424. [PubMed] |
| 11. | Komazawa Y, Amano Y, Yuki M, Fukuhara H, Mishiro T, Mishiro T, Shizuku T, Kinoshita Y. Oolong tea is useful for lens cleansing in transnasal small-caliber esophagogastroduodenoscopy. Endoscopy. 2010;42:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Abe K, Miyaoka M. Trial of Transnasal Esophagogastroduodenoscopy. Digest Endosc. 2006;18:212-217. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Takahashi S, Nagata H, Kamata H, Takano T, Uchida J, Inada M, Asada M, Akabane A, Kon H, Wada R. Usefulness of water jet cleaning for observation area transnasal upper gastrointestinal endoscopy: mechanize versus manual cleaning [in Japanese]. Official Journal of Japan Society of Ningen Dock. 2012;27:743-747. [DOI] [Full Text] |
| 14. | Hosoi H, Sazaki N, Tokoi S, Endo S, Saito Y, Kajiura K, Yamanaka A, Fujiki K, Tamura K, Takashimizu I. An automatic lavage pump for cleaning colon on colonoscopy [in Japanese]. Gastroenterological Endoscopy. 1992;34:1101-1103 1099. [DOI] [Full Text] |
| 15. | Papachristou DN, Barters R. Resection of the liver with a water jet. Br J Surg. 1982;69:93-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 110] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Aroussi AA, Sami IM, Leguerrier A, Verhoye JP. The blower: a useful tool to complete thrombectomy of the mechanical prosthetic valve. Ann Thorac Surg. 2006;81:1911-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Terzis AJ, Nowak G, Rentzsch O, Arnold H, Diebold J, Baretton G. A new system for cutting brain tissue preserving vessels: water jet cutting. Br J Neurosurg. 1989;3:361-366. [PubMed] |
| 18. | Izumi R, Yabushita K, Shimizu K, Yagi M, Yamaguchi A, Konishi K, Nagakawa T, Miyazaki I. Hepatic resection using a water jet dissector. Surg Today. 1993;23:31-35. [PubMed] |
| 19. | Rau HG, Duessel AP, Wurzbacher S. The use of water-jet dissection in open and laparoscopic liver resection. HPB (Oxford). 2008;10:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Oertel J, Gaab MR, Knapp A, Essig H, Warzok R, Piek J. Water jet dissection in neurosurgery: experimental results in the porcine cadaveric brain. Neurosurgery. 2003;52:153-159; discussion 159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Nakagawa A, Hirano T, Jokura H, Uenohara H, Ohki T, Hashimoto T, Menezes V, Sato Y, Kusaka Y, Ohyama H. Pulsed holmium: yttrium-aluminum-garnet laser-induced liquid jet as a novel dissection device in neuroendoscopic surgery. J Neurosurg. 2004;101:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Ohki T, Nakagawa A, Hirano T, Hashimoto T, Menezes V, Jokura H, Uenohara H, Sato Y, Saito T, Shirane R. Experimental application of pulsed Ho: YAG laser-induced liquid jet as a novel rigid neuroendoscopic dissection device. Lasers Surg Med. 2004;34:227-234. [PubMed] |
| 23. | Oertel J, Gaab MR, Schiller T, Schroeder HW, Warzok R, Piek J. Towards waterjet dissection in neurosurgery: experimental in-vivo results with two different nozzle types. Acta Neurochir (Wien). 2004;146:713-720. [PubMed] |
| 24. | Schurr MO, Wehrmann M, Kunert W, Melzer A, Lirici MM, Trapp R, Kanehira E, Buess G. Histologic effects of different technologies for dissection in endoscopic surgery: Nd: YAG laser, high frequency and water-jet. Endosc Surg Allied Technol. 1994;2:195-201. [PubMed] |
| 25. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. [DOI] [Full Text] |
| 26. | Oertel J, Gaab MR, Warzok R, Piek J. Waterjet dissection in the brain: review of the experimental and clinical data with special reference to meningioma surgery. Neurosurg Rev. 2003;26:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Hirano T, Komatsu M, Saeki T, Uenohara H, Takahashi A, Takayama K, Yoshimoto T. Enhancement of fibrinolytics with a laser-induced liquid jet. Lasers Surg Med. 2001;29:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Miller JM, Palanker DV, Vankov A, Marmor MF, Blumenkranz MS. Precision and safety of the pulsed electron avalanche knife in vitreoretinal surgery. Arch Ophthalmol. 2003;121:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Ogawa Y, Nakagawa A, Takayama K, Tominaga T. Pulsed laser-induced liquid jet for skull base tumor removal with vascular preservation through the transsphenoidal approach: a clinical investigation. Acta Neurochir (Wien). 2011;153:823-830. [PubMed] |
| 30. | Hotta K, Fujii T, Saito Y, Matsuda T. Local recurrence after endoscopic resection of colorectal tumors. Int J Colorectal Dis. 2009;24:225-230. [PubMed] |
| 31. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 535] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 32. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 609] [Article Influence: 38.1] [Reference Citation Analysis (5)] |
| 33. | Oyama T, Tomori A, Hotta K, Morita S, Kominato K, Tanaka M, Miyata Y. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:S67-S70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 461] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 34. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 293] [Article Influence: 15.4] [Reference Citation Analysis (11)] |
| 35. | Uraoka T, Kawahara Y, Ohara N, Kato J, Hori K, Okada H, Yamamoto K. Carbon dioxide submucosal injection cushion: an innovative technique in endoscopic submucosal dissection. Dig Endosc. 2011;23:5-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Yamamoto H, Yube T, Isoda N, Sato Y, Sekine Y, Higashizawa T, Ido K, Kimura K, Kanai N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Ciocirlan M, Pioche M, Lepilliez V, Gonon N, Roume R, Noel G, Pinset C, Ponchon T. The ENKI-2 water-jet system versus Dual Knife for endoscopic submucosal dissection of colorectal lesions: a randomized comparative animal study. Endoscopy. 2014;46:139-143. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Fujishiro M, Kodashima S, Goto O, Ono S, Muraki Y, Kakushima N, Omata M. Technical feasibility of endoscopic submucosal dissection of gastrointestinal epithelial neoplasms with a splash-needle. Surg Laparosc Endosc Percutan Tech. 2008;18:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Toyonaga T, Man-I M, Morita Y, Sanuki T, Yoshida M, Kutsumi H, Inokuchi H, Azuma T. The new resources of treatment for early stage colorectal tumors: EMR with small incision and simplified endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S31-S37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Yahagi N, Neuhaus H, Schumacher B, Neugebauer A, Kaehler GF, Schenk M, Fischer K, Fujishiro M, Enderle MD. Comparison of standard endoscopic submucosal dissection (ESD) versus an optimized ESD technique for the colon: an animal study. Endoscopy. 2009;41:340-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Kaehler GF, Sold MG, Fischer K, Post S, Enderle M. Selective fluid cushion in the submucosal layer by water jet: advantage for endoscopic mucosal resection. Eur Surg Res. 2007;39:93-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Nonaka K, Kita H. Endoscopic Submucosal Dissection for Early Gastric Cancer. J Cancer Ther. 2013;4:26-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Lesser T. Atypical lung parenchyma resection with the Hydro-Jet--initial experimental and clinical experiences. Chirurg. 2000;71:592. [PubMed] |
| 44. | Nakamura N, Hayashi T, Arai T, Tokonabe S, Ito H, Tajiri H, Makoto K. Experimental study on water jet incision of gastric mucosa to support the treatment for early gastric cancer. JJMI. 1998;68:369-374. |
| 45. | Lepilliez V, Robles-Medranda C, Ciocirlan M, Lukashok H, Chemali M, Langonnet S, Chesnais S, Hervieu V, Ponchon T. Water-jet dissector for endoscopic submucosal dissection in an animal study: outcomes of the continuous and pulsed modes. Surg Endosc. 2013;27:2921-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Sato C, Nakano T, Nakagawa A, Yamada M, Yamamoto H, Kamei T, Miyata G, Sato A, Fujishima F, Nakai M. Experimental application of pulsed laser-induced water jet for endoscopic submucosal dissection: mechanical investigation and preliminary experiment in swine. Dig Endosc. 2013;25:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Yamada M, Nakano T, Sato C, Nakagawa A, Fujishima F, Kawagishi N, Nakanishi C, Sakurai T, Miyata G, Tominaga T. The dissection profile and mechanism of tissue-selective dissection of the piezo actuator-driven pulsed water jet as a surgical instrument: laboratory investigation using Swine liver. Eur Surg Res. 2014;53:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Iizuka T, Lee SH S- Editor: Ji FF L- Editor: A E- Editor: Wu HL