Published online Sep 16, 2016. doi: 10.4253/wjge.v8.i17.572
Peer-review started: April 16, 2016
First decision: May 19, 2016
Revised: May 25, 2016
Accepted: July 14, 2016
Article in press: July 18, 2016
Published online: September 16, 2016
Processing time: 152 Days and 13.1 Hours
Capsule endoscopy (CE) currently plays an important role in Crohn’s disease (CD). It is a noninvasive technique that has led to a breakthrough in the endoscopic diagnosis of diseases of the small intestine. Its superior diagnostic performance and excellent safety profile lead to its considerable acceptance on the part of the patient. This paper reviews current indications of CE in three stages of clinical practice: Suspected CD, unclassified colitis and its extensive role in diagnosed CD. The diagnostic and therapeutic impact of the results of CE on the monitoring of this disease is also reviewed. Knowledge of its applications, the interpretation of its results in an appropriate context and the existence of a validated endoscopic activity index could change the way in which these patients are managed. The definition of mucosal healing and postoperative recurrence by means of endoscopic scoring systems will endow CE with new applications in the management of CD in the near future.
Core tip: We expose current indications and practical uses of capsule endoscopy in Crohn’s disease based on the most relevant published evidence. Likewise, we describe the diagnostic and therapeutic impact on this disease and an exhaustive summary of where it plays an extensive role.
- Citation: Luján-Sanchis M, Sanchis-Artero L, Larrey-Ruiz L, Peño-Muñoz L, Núñez-Martínez P, Castillo-López G, González-González L, Clemente CB, Albert Antequera C, Durá-Ayet A, Sempere-Garcia-Argüelles J. Current role of capsule endoscopy in Crohn’s disease. World J Gastrointest Endosc 2016; 8(17): 572-583
- URL: https://www.wjgnet.com/1948-5190/full/v8/i17/572.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i17.572
Early diagnosis of inflammatory bowel disease (IBD) is crucial, as the progression of inflammatory activity leads to irreversible damage[1-4]. There is currently no test for the diagnosis of Crohn’s disease (CD)[5,6]; therefore, the techniques used must be interpreted in the appropriate context[7]. Since its approval by the Food and Drug Administration (FDA) in 2001, capsule endoscopy (CE) has revolutionized the diagnostic imaging of diseases of the small bowel (SB). The endoscopic capsule is a small instrument that takes hundreds of photographs while moving naturally with intestinal movements, thus facilitating direct, noninvasive visualization of the intestinal mucosa. CE is currently the most important indicator of CD in children between 10 and 18 years age[8,9]; in adults and young children, its importance as an indicator is second only to bleeding of unknown origin[8].
This review presents the principal indications of CD based on the available evidence[10-17] in three scenarios: Suspected CD (SCD), unclassified colitis (UNC) and diagnosed CD (DCD). This is the best procedure for viewing mucosal lesions attributable to CD in the SB[11] and of identifying superficial lesions that go unnoticed by other endoscopic and radiological techniques[7,11,14,18-20].
These characteristics establish its indication as the technique of choice in the evaluation of the SB with CD in the absence of stenosis or fistulas[14,21], and particularly when it will lead to a change in patient management[6,10,14,15].
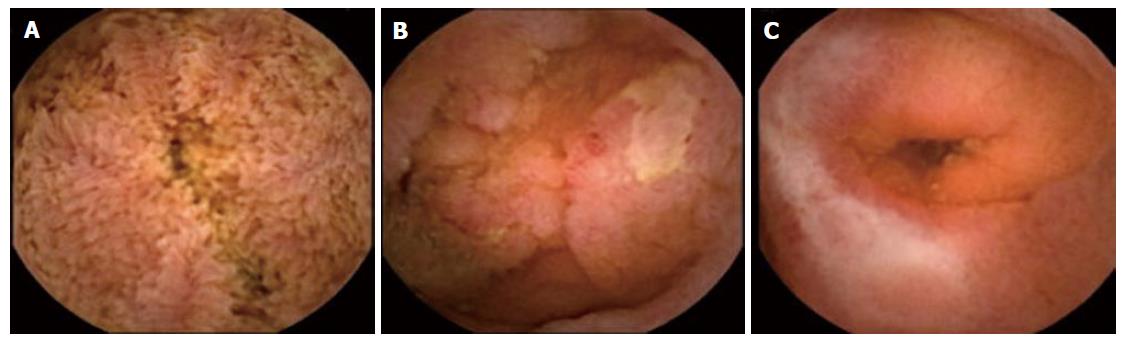
Lesions consistent with CD should be described according to a structured and standardized terminology called Capsule Endoscopy Structured Terminology, which was described in 2005[22]. The terminology is based on the presence of stenosis, ulcers, erosions, cankers, pseudopolyps and fistulas (Figure 1), and it enables the use of a common language to interpret lesions consistent with CD. These lesions are not specific; therefore, other diseases with the same endoscopic features (infections, ischemia, vasculitis, iatrogenesis, tumors, lymphoma and Behcet’s disease, among others) need to be ruled out. Other lesions such as erythema, nodularity, denudation or petechiae are not considered to be related to inflammation of the mucous membranes. Most studies have used the diagnostic criteria for CD by means of CE, defined by Mow et al[23] in 2004, as the existence of more than three diffuse or multiple ulcerations when nonsteroidal anti-inflammatory drugs (NSAIDs) are not being taken. This criterion provides a sensitivity (S) of 77%, specificity (SP) of 89%, positive predictive value (PPV) of 55% and a negative predictive value (NPV) of 96% for the diagnosis of CD in relation to clinical, endoscopic, radiological and histological findings. The rate of mucosal lesions missed by CE is minimal (0.5%); therefore, CD can be excluded after two years of monitoring[24].
Other authors have described criteria used less commonly in clinical practice such as the presence of multiple aphthous or erosive lesions (> 10), whether distributed continuously or discontinuously[25], or the presence of four or more ulcers, erosions, or a region with exudate, hyperemia and edema[26].
The current guidelines of both ASGE[27] and ECCO[14] recommend the use of two endoscopic indices that quantify the inflammatory activity of the CD by means of CE. Both have been prospectively validated[28,29] and enable the objective assessment of severity of the disease. They focus more on the presence or absence of inflammatory activity than on its extent and location. The first is the Niv or Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) score (Table 1), which was published by Gal et al[30] and defines the size of ulcers and the extent of inflammation and stenosis, dividing the SB into two segments, proximal and distal. The total score (from 0 to 36) is the sum of both segments. The CECDAI does not have a specific threshold; however, an increase in its value indicates more severe mucosal inflammation.
| Inflammation score |
| 0: None |
| 1: Mild to moderate edema/hyperemia/denudation |
| 2: Severe edema/hyperemia/denudation |
| 3: Bleeding, exudate, aphtha, erosion, small ulcer (< 0.5 cm) |
| 4: Moderate ulcer (0.5-2 cm), pseudopolyp |
| 5: Large ulcer (> 2 cm) |
| Disease extension score |
| 0: No disease - normal exploration |
| 1: Focal disease (single segment involvement) |
| 2: Patchy disease (2-3 segments involved) |
| 3: Diffuse disease (> 3 segments involved) |
| Stricturing score |
| 0: None |
| 1: Single - passed |
| 2: Multiple - passed |
| 3: Obstructing (not passed) |
| Segmentary score (proximal or distal): (A × B) + C |
| Total score: Proximal [(A × B) + C)] + distal [(A × B) + C] |
The second is the Lewis score described in 2008 by Gralnek et al[31] (Table 2). It divides the SB into three equal parts and also quantifies the edema of the villi, the ulcer and the stenosis. A score of < 135 indicates a normal mucosa or insignificant inflammation, a score of between 135 and 790 represents mild inflammation, and a score of ≥ 790 represents moderate or severe inflammation[32]. This index has been more widely used in clinical practice than the CECDAI, because there is an automatic calculation tool in a CE reading program (Rapid Reader® workstation of PillCam® capsules). It has been demonstrated that, the more lesions that are detected, the greater the endoscopic score and the more specific the diagnosis of CD by means of CE[33]. Similarly, with a Lewis score of < 135, the probability of it being a case of CD is unlikely[29,32,34]. In healthy patients (who do not take NSAIDs, have not had an intestinal resection, and do not have ankylosing spondylitis or digestive symptoms), only 9% may exhibit mucosal lesions similar to CD, and in all cases, the Lewis score would indicate mild activity (< 450)[33].
| Lesions in the proximal, mid, and distal small bowel thirds |
| Villous appearance |
| 0: Normal |
| 1: Edema |
| 8: Short segment |
| 12: Long segment; 20: The whole third |
| 1: Single; 14: Patchy |
| Ulcers |
| 0: None; 3: One; 5: Few; 10: Multiple |
| 5: Short segment; 10: Long segment; 15: The whole third |
| 9: 1⁄4; 12: 1⁄4-1⁄2; 18: > 1⁄2 |
| Strictures |
| 0: None; 14: One |
| 2: Non ulcerated; 24: Ulcerated |
| 7: No retention; 10: Capsule retention |
| Score calculation: Stricture score is added to the sum total for highest scoring villous edema and segment ulcers |
It is important to remember that the endoscopic findings themselves are not diagnostic of CD, and there is no cutoff value above which the diagnosis can be firmly established[35]. Moreover, endoscopic activity shows no correlation with the clinical evidence; consequently, in a symptomatic patient, CE detects lesions in only half of the cases[36,37] and conversely, when the patient is in clinical remission (Crohn’s disease activity index < 150), CE will show signs of inflammation in 62%[38]. This means that, once the objective assessment of CD activity has been performed by means of CE, decisions can be made regarding the management of the patient.
There is no gold standard for the diagnosis of CD; therefore, all techniques are complementary and should be interpreted with an appropriate degree of skepticism. Thus, CE and enteroscopy are useful for the early diagnosis and assessment of the extent and activity of the disease; radiology is better for studying the progression of damage and extraintestinal complications; and serological and fecal markers of inflammation are generally used to decide on the indication of radiological and endoscopic techniques. The selection of these will depend on the availability at the center, operator experience, its practical usefulness and cost[39].
The appropriate indication of CE for SCD was defined at the International Conference on CE through the selection of the following criteria: Existence of consistent symptoms, associated or not associated with extraintestinal manifestations and laboratory and/or radiological abnormalities[7]. In these cases, an ileocolonoscopy (IC) with biopsies should be performed, and regardless of the outcome, it would be advisable to assess the proximal extension of the disease into the stomach and/or intestine for its prognostic implications[5,14,15,27,40].
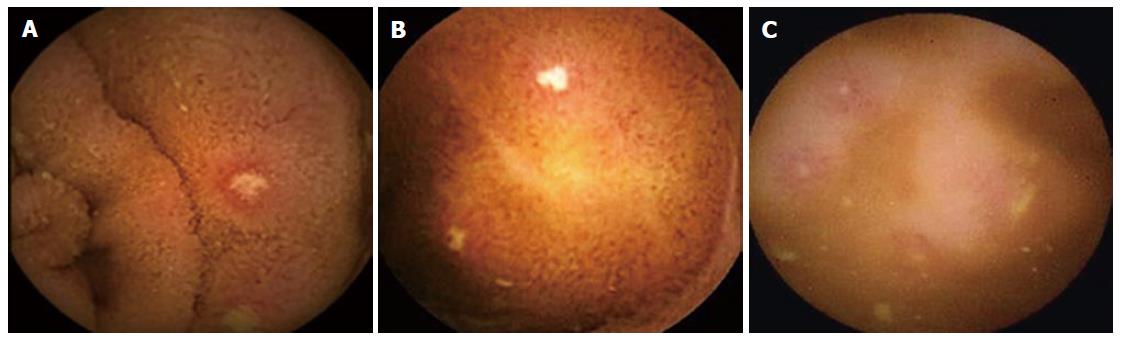
CE is the diagnostic technique of first resort when the IC and radiology are negative or inconclusive[14,15,27,41], because it detects subtle inflammatory changes that go unnoticed by radiological techniques or are unachievable by conventional endoscopy (Figure 2)[42,43]. Thus, two broad meta-analyses[44,45] show that its performance in cases of SCD is superior to that of IC, barium follow-through examinations (BFT) and computerized tomography (CT) at 22%, 32% and 47%, respectively. Faced with lesions consistent with CD, enteroscopy may be useful for taking biopsies, but its routine performance is not indicated according to the ASGE[27] and ECCO[14] guidelines.
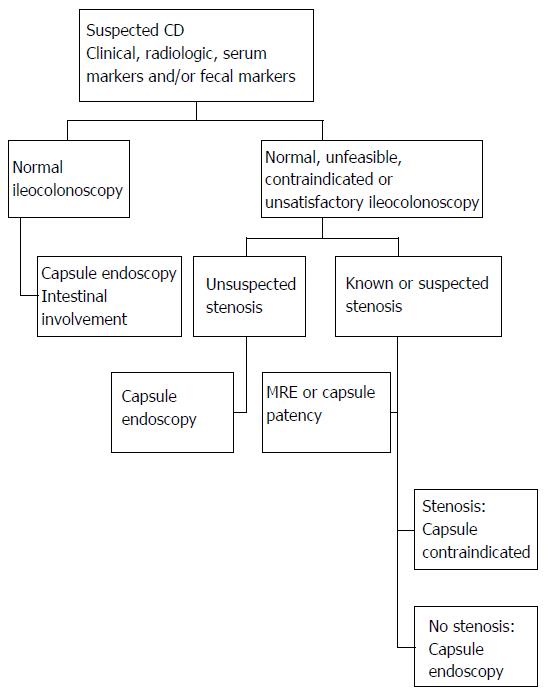
The capsule’s diagnostic performance with respect to CD varies as a function of how early the disease is suspected as well as the extension, activity and distribution of the disease[46,47]. The findings of CE have diagnostic value when they are interpreted with an adequate degree of skepticism. Overall performance is higher when additional data besides clinical evidence such as intestinal manifestations and/or serum or fecal markers of inflammation[7,14,26,32,48-50], are presented. Thus, when the disease is suspected based on one criterion, CE shows mild activity, and the diagnosis is confirmed in 20% of cases; however, when it is based on three criteria, activity will be more severe, and the diagnosis is confirmed in 80%[32]. In accordance with the above, Figure 3 sets out a proposal to focus on the diagnosis of SCD.
In the context of DCD, the indication of CE should be considered when providing for a change in the management of the disease[6,10,11,40]. It has been demonstrated that the investigation of proximal extension into the SB using CE has prognostic and therapeutic implications in disease progression[14,51]. Therefore, given its superior diagnostic performance in DCD (85.7%), its findings can influence a change in the management and clinical follow-up for 64% of these patients[52].
As with SCD, several meta-analyses[44,45] show that performance in cases of DCD is superior to that of push enteroscopy, BFT and CT at 57%, 38% and 32% respectively. The identification of mucosal lesions in the SI is better than with BFT (78% vs 32%) and can be better than enterotomography (ETC) (68% vs 38%) or enterography with nuclear magnetic resonance (MRE) (93% vs 79%), although the clinical significance of these differences is not defined in prospective studies. The primary role of CE in cases of DCD is when there are symptoms or signs which cannot be explained by the normal or inconclusive result of radiology and/or IC, as it can detect lesions between the duodenum and terminal ileum which are inaccessible with conventional endoscopy or imperceptible with radiology which substantiate the clinical picture[14,40,53]. The applications of CE for DCD in habitual clinical practice are set out below.
Currently, at the time of the initial diagnosis of CD, it is advisable to assess the extent throughout the entire gastrointestinal tract[14,54]. The SB is affected in 80% of patients with CD[51]. In general, in more than half of the patients with ileal CD, the proximal SB is also involved, with the most frequent distribution being in the proximal ileum (67%) followed by the proximal jejunum (53%) and/or proximal duodenum (32%)[36,55]. After the entire SB was able to be accessed with CE, it was observed that this location could coexist with the ileal and the colonic. Therefore, the Vienna classification was replaced by Montreal in 2005, adding the involvement of the upper digestive tract through to the proximal ileum (that which is called L4)[56] to the rest of the locations. The advantage of the phenotypic classification of DCD using the Montreal classification is important for predicting the progression of the disease and the selection of the best management strategy.
Flamant et al[51] found that jejunal (L4) involvement was 40% when the ileum (L1) was affected and 12% when the colon (L2) was affected. Isolated jejunal involvement occurred in 17% of the cases, and this figure has been corroborated by other authors[57]; however, other authors have observed jejunal involvement in a third of patients with normal IC[58]. In the pediatric population, these figures are superimposable, with L4 involvement in 24% of patients with DCD, 30% being associated with L1, 18% with L2 and 21% if the phenotype is ileocolonic (L3)[59].
Recent findings published by Lazarev et al[60] have been decisive in understanding the involvement of the SB in CD. Of the 2015 patients analyzed, 14% exhibited proximal involvement, and this location is associated with younger age groups, non-smoking patients, coexistence with ileal involvement and a pattern of stenosis. Specifically, jejunal involvement is associated with patterns of stenosis which necessitate further surgery. Based on these findings, this author proposes revising the Montreal classification, as jejunal involvement should be viewed as a separate phenotype due to the prognostic implications of this location. The behavior of the proximal location is similar to that of the ileal location and most frequently develops into a pervasive, stenotic pattern in contrast with the colonic location[61].
The diagnosis of isolated CD in the SB is a true challenge, and as it occurs with colonic involvement, it is not correlated with endoscopic activity. Population-based epidemiological studies show that more than 50% of Western patients with CD and 77%-87% of Asian patients exhibit involvement of the SB at the time of diagnosis[62-65]. The use of CE with DCD is currently considered to be complementary to other techniques, and the selection thereof will depend on the experience of each center[66].
As for radiology, ETC and MRE evaluate the progression of transmural damage and the complications (transmural extension, abscesses, fistulas, stenosis and collections); therefore, studies are preceded or completed with CE when there is interest in identifying these[10]. Its primary advantage over radiological techniques is its elevated sensitivity for the detection of superficial mucosal lesions[42], as there are few series, which provide sensitivity similar to that of MRE (75% vs 77.8%, respectively)[67]. The advantage of CE over MRE focuses principally on jejunal lesions, as the jejunum has a larger mucosal surface than the ileum as well as more numerous and redundant folds and a relative minor distension, which leads to false positives and negatives with MRE in this section[68]. Similarly, it has been observed that its diagnostic performance when combined with IC and CE is 97.3% vs 57.3% when IC and BFT[69] are performed, so the use of the BFT in this context is currently controversial in addition to its being rejected due to the radiation which it involves[70].
As regards inflammation markers, fecal calprotectin (FC) studies inflammatory activity noninvasively and indirectly but does not differentiate the location thereof in the SB or colon[71]. Some authors have observed a good correlation with the results of CE with a S of 83%, SP of 100%, PPV of 100% and NPV of 80%[37]; however, more recent studies have demonstrated that the elevation of C-reactive protein, FC, or a combination of the two are poorly correlated with significant inflammation of the SB[72]. In general, the Lewis score has demonstrated a good correlation with FC in cases of mild inflammation, so when it is < 100 μg/g, the Lewis score is normal, but it is less useful when the CBF is elevated[73]. For SCD with a normal IC, a FC of > 100 mg/g may suggest the indication of CE, and a value of approximately 200 μg/g is associated with a diagnostic performance of 65%[74].
CE enables the assessment of both the extent and the inflammatory activity in the SI. When CD is suspected based on the presence of anemia, thrombocytosis, weight loss and/or fecal inflammatory markers which are not justified by the findings of the IC or radiology, the performance of CE is indicated in order to look for activity in the SB[40,46]. In this context, the Lewis score diagnoses CD with a PPV of 82.6%, NPV of 87.9%, S of 82.6% and SP of 87.9% for the diagnosis of CD with respect to the clinical, analytical, radiological, endoscopic and/or histological evaluation[32]. Endoscopic score systems maintain a good correlation with each other, with CECDAI levels of 3.8 and 5.8 proportional to Lewis scores of 135 and 790 respectively, with the first values for mild activity and the last values for moderate to severe activity[73]. Recently, other authors have identified a higher CECDAI threshold of 23.5 for severe inflammation, which may be helpful for guiding clinical management[75]. The use of these indices in the therapeutic algorithm decision, requires prospective studies[14]; therefore, the findings should currently be seen as complementary to the rest of the panel of diagnostic tests[66].
Achieving deep remission (clinical, biological and mucosal healing) improves the prognosis for CD[3], with mucosal healing being an objective of treatment[76]. The various radiological modalities, as opposed to endoscopic modalities, cannot provide direct visualization of the mucosa of the SB; consequently, they have an inherent limitation in the objective assessment of mucosal healing.
Mucosal healing is considered the initial event in the suppression of inflammation of the deeper layers of the intestinal wall[77] and, as occurs with colonic lesions, this healing is not correlated with the clinical evidence[78]; therefore, it is necessary to evaluate it endoscopically in order to detect it. In this sense, endoscopic evaluation of the whole intestinal mucosa should be crucial for measuring the treatment response and establishing a treatment strategy.
In the few studies that have focused on mucosal healing of the SB using CE for CD (not fistulizing or pervasive), it has been observed, paradoxically, that ulcers improve one month after immunosuppressive treatment and cankers can take up to 6 mo[79]. Current recommendations on the monitoring of mucosal healing indicate first conducting an IC in patients with involvement of the ileum and/or colon; in those with SB involvement that cannot be reached by IC, MRE would probably be the standard test. However, given the modest NPV of MRE to exclude mucosal lesions, CE should be considered if symptoms persist despite normal MRE results, or if there is suspicion of activity[80].
Currently, there is no agreed definition for mucosal healing through CE. It has been suggested that it could be the resolution of all active inflammatory lesions[37] or the absence of all visible ulcers (according to the International Organization for the Study of Inflammatory Bowel Diseases)[81]. In both cases, quantification of inflammatory activity by means of the validated Lewis score and CECDAI index is recommended[14].
CE detects SB involvement in 24% of cases involving perianal disease patients with a normal IC, and these findings lead to a change in therapeutic management in all patients. In these cases, the predictors of a positive outcome from the CE are not associated with laboratory abnormalities, family history of IBD or age[82].
According to the recommendations of the ASGE[8], there are other indications of CE such as suspected intestinal tumors and malabsorption syndromes, and both can be associated during the progression of DCD.
The relative risk of intestinal tumors presented by IBD in the long term (10-25 years) is low (0.2%-2%), although this is higher than in the general population[83,84]. According to ECCO’s recommendations, CE is recommended for suspected intestinal tumors. In CD with a long-term, pervasive stenotic pattern, the abrupt onset of symptoms after a prolonged remission or with refractory strictures should be suspected to medical treatment[85].
Moreover, celiac disease and its complications can be associated with DCD. CE has shown lesions consistent with CD in 6% of doubtful cases of celiac disease with negative antibodies and signs of atrophy in the duodenal biopsy[85].
The management of postsurgical recurrence of DCD by means of endoscopic monitoring and its management is determined by the risk factors among which is extension into the SB[86,87]. IC is currently the reference technique for evaluating postoperative recurrence, which is measured using the Rutgeerts index[86,88]. Although the clinical relevance of the findings has not been studied, CE exhibits a S of 62%-76% and a SP of 100% over ileoscopy for this indication[10]. CE is performed when endoscopy is contraindicated or unsatisfactory[40], and it is selected with anastomosis that is difficult to access or when preferred by the patient[10,15,40,89,90].
It is recommended to perform it six months to one year after surgery depending on the association with other risk factors[89] in order to identify the recurrence and the proximal lesions not attainable with ileoscopy[40,53,91]. Some authors have used the Buchmann activity index[92] to classify lesions, but the use of the Lewis score is currently recommended in the context of clinical trials[35].
However, prospective studies are lacking in this context for evaluating the prognosis and clinical significance of the results of CE for this indication. Recurrence has only been assessed in one study using CE at one month and six months after surgery, and recurrence in the SB is defined as being when the residual lesions at one month after surgery have progressed after 6 mo, with an increase of 100 points in the Lewis score[93].
Population-based studies have shown that, for up to 10% of adult patients and 30% of children with IBD and the exclusive involvement of the colon, it is difficult to distinguish between CD and ulcerative colitis (UC). This entity is called unclassified or UNC, and in most cases, the final diagnosis is established during the first 8 years of development[94-96]. In these cases, CE can identify lesions consistent with CD in 17%-70% of the cases[96], which is better than BFT or enteroclysis. There are no comparative data for ETC or MRE. Similarly, when the CE is normal, a future diagnosis is not excluded[14], and its repetition can be recommended in the medium term[10].
Several retrospective studies have suggested that CE produces a definitive diagnosis of CD, has resulted in management changes, or has had a potential impact on prediction of the prognosis with this fact being particularly significant in young patients. In one pediatric study, 50% of UC or UNC were ultimately diagnosed as CD[97].
It has been demonstrated that the extension of CD into the SB and/or its proximal location are two poor prognostic factors and determine therapeutic decisions through early indication of immunosuppression[6,51,98-100].
The management changes that CE findings prompt are related to the initiation of a new treatment, the change or suspension thereof, or the indication of surgery[52,101,102]. On a practical level, the impact on management of the disease depends on the reason why CE is indicated. This impact is particularly relevant in the pediatric age group, as CE reclassifies 50% of ulcerative colitis and UNC as CD, as it detects proximal lesions undetected by other techniques; in 78% of these cases, there is a change in the therapeutic decision[101].
In general, current publications report the diagnostic performance of CE for CD at 60%-85%[52,103], which gives rise to an overall therapeutic impact of 50% (40%-67%)[27]. In long-term studies (6 years), this will lead to changes in decision-making based on the indication: 90% of patients when CE is requested for SCD, 88% for UNC and 73% for DCD[104].
In the case of DCD, therapeutic management is modified in 64% of patients[52]. In studies involving more than 900 patients with CD[102], the decision to change the medication is made three months after the CE for 61.6%, and for 39.5%, a new treatment is initiated. Pathologic findings of CE compared with none or minimal findings, resulted in significant differences in treatment modifications (73.2% vs 51.1%, P = 0.04), the addition of drugs (58.5% vs 22.2%, P < 0.01), and the indication of surgery (21.9% vs 4.4%, P = 0.01). Treatment is intensified after CE when activity of the lesions is more severe: In 14.5%, 48% and 87% of patients with Lewis score < 135, 135-790 and ≥ 790, respectively[72].
The most significant complication of CE, and almost the only one, is retention, which is still very rare with this disease, as the exploration of the entire SB is achieved in 85.4% (from 79% to 90.8%) of the cases[105]. DCD is considered a risk factor for retention with CE, although the overall figures in long series are low at 2.6% (1.6-3.9) and very similar to other indications[105]. Currently, when intestinal stenosis is suspected, the recommended approach is to assess the contraindication of CE in a test of intestinal permeability with the degradable capsule Patency (PC) (Given Imaging, Yoqneam, Israel), approved by the FDA in 2006 for this purpose, or to perform radiology depending on its local availability and the experience of the center[14,106-108]. For pediatric patients, the choice is between the PC and MRE due to the safety of both types of exploration for this age range[109].
It has been observed that, in most capsule retention cases with CE, radiology was not adequate to suspect its risk[110]; otherwise, for suspected radiation stenosis (CT or BFT) the retention rate is low (21%). Therefore, it is proposed that radiology be avoided (especially in young patients), unless the permeability test is abnormal[111]. For some authors, it is a “therapeutic” complication, because it diagnoses stenoses that have gone unnoticed by other techniques and results in a change in patient management[112]. The treatment of retention depends on the diameter and nature of the stenosis and provides for the wait-and-see approach with monitoring for the expulsion of the capsule and medical or endoscopic treatment if there is not complete obstruction, in which case surgery is indicated[113]. Most cases are resolved conservatively[114]. Medical treatment includes the administration of laxatives or corticosteroids depending on the etiology of the retention. Enteroscopy indicates whether to recover the endoscopic capsule, biopsy the stenosis and/or treat with dilation.
The risk of retention in DCD and SCD are not the same. Accordingly, the highest percentage was published in a single retrospective study of 102 patients, with the risk for DCD being 13% (5.6%-28%), whereas in cases of SCD, the figure dropped to 1.6% (0.2%-10%)[115], and that was a decade ago, when the PC did not exist. However, a multicenter Japanese study was recently published which shows no difference between retention in DCD (7.4%) and SCD (6.4%)[116].
In general, the retention rates with SCD are low and vary from 0% to 5%[105,112,117-119]. In 22 of the 1000 patients of the series of Li et al[120] CE was performed for SCD (2.2%), and of those, there were only 3 retentions.
In a retrospective study involving 78 patients with SCD, there were 3 retentions (5%)[121], and similar data were obtained in the study of Cheon, with retention rates of 5.4% (2/37)[113].
In patients with DCD, the retention rate oscillates between 1.8% and 13%[23,102,105,112,113,116,122]. The first publications, such as Cheifetz et al[115], estimate higher retention figures while in more recent publications, the figures have dropped considerably[116]. Cotter et al[99] presented a retention rate of 6% and Dussault et al[47] rates of 4%. However, in studies with active CD, where mucosal healing is assessed, retentions account for only 1.8%[123].
In CD, a rigorous selection of the indication of CE is required due to the risk of retention in patients with known intestinal stenosis[8,10]. It should be noted that, in the preliminary studies in which tests with the PC were not available, retention rates in this context were 21%[112]. However, in a more recent study involving 19 patients with active CD in which 43 sequential scans were performed, no retentions were recorded despite the inclusion of patients with multiple stenosis and intestinal surgery[124]. This study confirms that the PC is an excellent predictor of intestinal permeability with respect to CE for these patients[14,125]. However, the latest reports indicate that the retention rate is not affected by the selective use of the PC, as the retention rate is 2.3%, which is similar to when it is not performed (1.5%) as well as when the PC is negative (2.1%). When the PC is positive, the retention rate is 11.1%[126].
In summary, CE is a noninvasive technique, which plays a wide-ranging role in CD. Its principal advantages over other diagnostic techniques are the absence of invasiveness and irradiation and the direct study of the mucosa of the entire SB. It enables the early diagnosis of CD due to its ability to detect superficial mucosal lesions, which go unnoticed by radiology or cannot be accessed with IC. These characteristics, along with its excellent level of safety, define it as the best exploratory method for the study of inflammatory activity in the mucosa of the SI with CD. Its only contraindication is the objective presence of intestinal stenosis.
Its primary use is well defined in the early diagnosis of SCD, the assessment of the extent of DCD and the study of unclassifiable colitis. After ruling out intestinal stenosis, CE is the technique of first resort for patients with SCD who have had negative evaluations with radiology and IC. For patients diagnosed with CD, if cross-sectional imaging tests are normal or non-diagnostic, CE is performed if the result implies a change in patient management.
The systematic use of validated indices for scoring endoscopic activity enables the interpretation of lesions and monitoring of the developmental history of each patient to be standardized. Its use in future prospective studies will enable the definition of the criteria for mucosal healing and postoperative recurrence, which may suggest guidance for treatment. As is the case with other diagnostic tests and current treatments, the involvement of all these applications of CE in changing the natural history of this disease has yet to be established.
| 1. | Cosnes J, Cattan S, Blain A, Beaugerie L, Carbonnel F, Parc R, Gendre JP. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244-250. [PubMed] |
| 2. | Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, Chowers Y, D’Haens G, Feagan BG, Hibi T. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 471] [Article Influence: 31.4] [Reference Citation Analysis (1)] |
| 3. | Panaccione R, Hibi T, Peyrin-Biroulet L, Schreiber S. Implementing changes in clinical practice to improve the management of Crohn’s disease. J Crohns Colitis. 2012;6 Suppl 2:S235-S242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Peyrin-Biroulet L, Loftus EV, Colombel JF, Sandborn WJ. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut. 2010;59:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 5. | Stange EF, Travis SP, Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Feakins R, Fléjou JF, Herfarth H, Hommes DW. European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Definitions and diagnosis. J Crohns Colitis. 2008;2:1-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 378] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 6. | Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 798] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 7. | Mergener K, Ponchon T, Gralnek I, Pennazio M, Gay G, Selby W, Seidman EG, Cellier C, Murray J, de Franchis R. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy. 2007;39:895-909. [PubMed] |
| 8. | Early DS, Ben-Menachem T, Decker GA, Evans JA, Fanelli RD, Fisher DA, Fukami N, Hwang JH, Jain R, Jue TL. Appropriate use of GI endoscopy. Gastrointest Endosc. 2012;75:1127-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 187] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Fritscher-Ravens A, Scherbakov P, Bufler P, Torroni F, Ruuska T, Nuutinen H, Thomson M, Tabbers M, Milla P. The feasibility of wireless capsule endoscopy in detecting small intestinal pathology in children under the age of 8 years: a multicentre European study. Gut. 2009;58:1467-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Bourreille A, Ignjatovic A, Aabakken L, Loftus EV, Eliakim R, Pennazio M, Bouhnik Y, Seidman E, Keuchel M, Albert JG. Role of small-bowel endoscopy in the management of patients with inflammatory bowel disease: an international OMED-ECCO consensus. Endoscopy. 2009;41:618-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 12. | Doherty GA, Moss AC, Cheifetz AS. Capsule endoscopy for small-bowel evaluation in Crohn’s disease. Gastrointest Endosc. 2011;74:167-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Lucendo AJ, Guagnozzi D. Small bowel video capsule endoscopy in Crohn’s disease: What have we learned in the last ten years? World J Gastrointest Endosc. 2011;3:23-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 601] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 15. | Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E, Adler SN, Albert J, Baltes P. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 574] [Article Influence: 52.2] [Reference Citation Analysis (1)] |
| 16. | Luján-Sanchis M, Sanchis-Artero L, Suárez-Callol P, Medina-Chuliá E. Indications of capsule endoscopy in Crohn´s disease. Rev Esp Enferm Dig. 2014;106:37-44. [PubMed] |
| 17. | Wang A, Banerjee S, Barth BA, Bhat YM, Chauhan S, Gottlieb KT, Konda V, Maple JT, Murad F, Pfau PR. Wireless capsule endoscopy. Gastrointest Endosc. 2013;78:805-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (2)] |
| 18. | Leighton JA, Shen B, Baron TH, Adler DG, Davila R, Egan JV, Faigel DO, Gan SI, Hirota WK, Lichtenstein D. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558-565. [PubMed] |
| 19. | Rey JF, Ladas S, Alhassani A, Kuznetsov K. European Society of Gastrointestinal Endoscopy (ESGE). Video capsule endoscopy: update to guidelines (May 2006). Endoscopy. 2006;38:1047-1053. [PubMed] |
| 20. | Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57:125-136. [PubMed] |
| 21. | Pohl J, Delvaux M, Ell C, Gay G, May A, Mulder CJ, Pennazio M, Perez-Cuadrado E, Vilmann P. European Society of Gastrointestinal Endoscopy (ESGE) Guidelines: flexible enteroscopy for diagnosis and treatment of small-bowel diseases. Endoscopy. 2008;40:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Delvaux M, Friedman S, Keuchel M, Hagenmüller F, Weinstein M, Cave D, de Franchis R, Gay G, Korman LY. Structured terminology for capsule endoscopy: results of retrospective testing and validation in 766 small-bowel investigations. Endoscopy. 2005;37:945-950. [PubMed] |
| 23. | Mow WS, Lo SK, Targan SR, Dubinsky MC, Treyzon L, Abreu-Martin MT, Papadakis KA, Vasiliauskas EA. Initial experience with wireless capsule enteroscopy in the diagnosis and management of inflammatory bowel disease. Clin Gastroenterol Hepatol. 2004;2:31-40. [PubMed] |
| 24. | Hall B, Holleran G, Costigan D, McNamara D. Capsule endoscopy: High negative predictive value in the long term despite a low diagnostic yield in patients with suspected Crohn’s disease. United European Gastroenterol J. 2013;1:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Voderholzer WA, Beinhoelzl J, Rogalla P, Murrer S, Schachschal G, Lochs H, Ortner MA. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369-373. [PubMed] |
| 26. | Fidder HH, Nadler M, Lahat A, Lahav M, Bardan E, Avidan B, Bar-Meir S. The utility of capsule endoscopy in the diagnosis of Crohn’s disease based on patient’s symptoms. J Clin Gastroenterol. 2007;41:384-387. [PubMed] |
| 27. | Shergill AK, Lightdale JR, Bruining DH, Acosta RD, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Evans JA, Fanelli RD. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015;81:1101-1121.e1-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 269] [Article Influence: 24.5] [Reference Citation Analysis (1)] |
| 28. | Niv Y, Ilani S, Levi Z, Hershkowitz M, Niv E, Fireman Z, O’Donnel S, O’Morain C, Eliakim R, Scapa E. Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): a multicenter prospective study. Endoscopy. 2012;44:21-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 29. | Cotter J, Dias de Castro F, Magalhães J, Moreira MJ, Rosa B. Validation of the Lewis score for the evaluation of small-bowel Crohn’s disease activity. Endoscopy. 2015;47:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Gal E, Geller A, Fraser G, Levi Z, Niv Y. Assessment and validation of the new capsule endoscopy Crohn’s disease activity index (CECDAI). Dig Dis Sci. 2008;53:1933-1937. [PubMed] |
| 31. | Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146-154. [PubMed] |
| 32. | Rosa B, Moreira MJ, Rebelo A, Cotter J. Lewis Score: a useful clinical tool for patients with suspected Crohn’s Disease submitted to capsule endoscopy. J Crohns Colitis. 2012;6:692-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Lewis JR, Pashinsky Y, Tindley A, Lewis BS. Capsule endoscopy in healthy individu-als. Gastroenterology. 2012;142:52-53. [DOI] [Full Text] |
| 34. | Monteiro S, Boal Carvalho P, Dias de Castro F, Magalhães J, Machado F, Moreira MJ, Rosa B, Cotter J. Capsule Endoscopy: Diagnostic Accuracy of Lewis Score in Patients with Suspected Crohn’s Disease. Inflamm Bowel Dis. 2015;21:2241-2246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Rosa B, Pinho R, Mão de Ferrod S, Almeidae N, Cotter J, Mascarenhas M. Endoscopic Scores for Evaluation of Crohn’s Disease Activity at Small Bowel Capsule Endoscopy: General Principles and Current Applications. GE Port J Gastroenterol. 2016;23:36-41. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Mehdizadeh S, Chen GC, Barkodar L, Enayati PJ, Pirouz S, Yadegari M, Ippoliti A, Vasiliauskas EA, Lo SK, Papadakis KA. Capsule endoscopy in patients with Crohn’s disease: diagnostic yield and safety. Gastrointest Endosc. 2010;71:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 37. | De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2013;19:429-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (1)] |
| 38. | Aggarwal V, Day SD, Connor SJ, Leach ST, Brown GJ, Singh R, Friedman A, Grimm MC, Craig PI. Multicenter Capsule Endoscopy Study of Small Bowel Crohn’s Disease Pa-tients in Clinical Remission: Long-Term Follow-up and Correlation With Faecal Biomarkers and Clinical Outcome. Gastroenterology. 2012;142 suppl 1:169. [DOI] [Full Text] |
| 39. | Park SJ, Kim WH. A look into the small bowel in Crohn’s disease. Clin Endosc. 2012;45:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Papay P, Ignjatovic A, Karmiris K, Amarante H, Milheller P, Feagan B, D’Haens G, Marteau P, Reinisch W, Sturm A. Optimising monitoring in the management of Crohn’s disease: a physician’s perspective. J Crohns Colitis. 2013;7:653-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (36)] |
| 42. | Costamagna G, Shah SK, Riccioni ME, Foschia F, Mutignani M, Perri V, Vecchioli A, Brizi MG, Picciocchi A, Marano P. A prospective trial comparing small bowel radiographs and video capsule endoscopy for suspected small bowel disease. Gastroenterology. 2002;123:999-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 519] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 43. | Dubcenco E, Jeejeebhoy KN, Petroniene R, Tang SJ, Zalev AH, Gardiner GW, Baker JP. Capsule endoscopy findings in patients with established and suspected small-bowel Crohn’s disease: correlation with radiologic, endoscopic, and histologic findings. Gastrointest Endosc. 2005;62:538-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Triester SL, Leighton JA, Leontiadis GI, Gurudu SR, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with non-stricturing small bowel Crohn’s disease. Am J Gastroenterol. 2006;101:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 367] [Article Influence: 18.4] [Reference Citation Analysis (1)] |
| 45. | Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240-1248; quiz 1249. [PubMed] |
| 46. | de Melo SW, Di Palma JA. The role of capsule endoscopy in evaluating inflammatory bowel disease. Gastroenterol Clin North Am. 2012;41:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 47. | Dussault C, Gower-Rousseau C, Salleron J, Vernier-Massouille G, Branche J, Colombel JF, Maunoury V. Small bowel capsule endoscopy for management of Crohn’s disease: a retrospective tertiary care centre experience. Dig Liver Dis. 2013;45:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | De Bona M, Bellumat A, Cian E, Valiante F, Moschini A, De Boni M. Capsule endoscopy findings in patients with suspected Crohn’s disease and biochemical markers of inflammation. Dig Liver Dis. 2006;38:331-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 49. | Valle J, Alcántara M, Pérez-Grueso MJ, Navajas J, Muñoz-Rosas C, Legaz ML, Cuena R, Carrobles JM. Clinical features of patients with negative results from traditional diagnostic work-up and Crohn’s disease findings from capsule endoscopy. J Clin Gastroenterol. 2006;40:692-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 50. | Shim KN, Kim YS, Kim KJ, Kim YH, Kim TI, Do JH, Ryu JK, Moon JS, Park SH, Hee Park C. Abdominal pain accompanied by weight loss may increase the diagnostic yield of capsule endoscopy: a Korean multicenter study. Scand J Gastroenterol. 2006;41:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 51. | Flamant M, Trang C, Maillard O, Sacher-Huvelin S, Le Rhun M, Galmiche JP, Bourreille A. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1390-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Lorenzo-Zúñiga V, de Vega VM, Domènech E, Cabré E, Mañosa M, Boix J. Impact of capsule endoscopy findings in the management of Crohn’s Disease. Dig Dis Sci. 2010;55:411-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Bourreille A, Jarry M, D’Halluin PN, Ben-Soussan E, Maunoury V, Bulois P, Sacher-Huvelin S, Vahedy K, Lerebours E, Heresbach D. Wireless capsule endoscopy versus ileocolonoscopy for the diagnosis of postoperative recurrence of Crohn’s disease: a prospective study. Gut. 2006;55:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1577] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 55. | Petruzziello C, Onali S, Calabrese E, Zorzi F, Ascolani M, Condino G, Lolli E, Naccarato P, Pallone F, Biancone L. Wireless capsule endoscopy and proximal small bowel lesions in Crohn’s disease. World J Gastroenterol. 2010;16:3299-3304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 56. | Silverberg MS, Satsangi J, Ahmad T, Arnott ID, Bernstein CN, Brant SR, Caprilli R, Colombel JF, Gasche C, Geboes K. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19 Suppl A:5A-36A. [PubMed] |
| 57. | Pérez-Cuadrado Martínez E, Pérez-Cuadrado Robles E. Capsule endoscopy and deep enteroscopy. Gastrointest Endosc. 2014;80:396-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 58. | Hall B, Holleran G, McNamara D. Small bowel Crohn’s disease: an emerging disease phenotype? Dig Dis. 2015;33:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | de Bie CI, Paerregaard A, Kolacek S, Ruemmele FM, Koletzko S, Fell JM, Escher JC. Disease phenotype at diagnosis in pediatric Crohn’s disease: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis. 2013;19:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 60. | Lazarev M, Huang C, Bitton A, Cho JH, Duerr RH, McGovern DP, Proctor DD, Regueiro M, Rioux JD, Schumm PP. Relationship between proximal Crohn’s disease location and disease behavior and surgery: a cross-sectional study of the IBD Genetics Consortium. Am J Gastroenterol. 2013;108:106-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 61. | Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1600] [Article Influence: 106.7] [Reference Citation Analysis (3)] |
| 62. | Molinié F, Gower-Rousseau C, Yzet T, Merle V, Grandbastien B, Marti R, Lerebours E, Dupas JL, Colombel JF, Salomez JL. Opposite evolution in incidence of Crohn’s disease and ulcerative colitis in Northern France (1988-1999). Gut. 2004;53:843-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 63. | Lashner B. Clinical features, laboratory findings, and course of Crohn’s disease. Inflamm Bowel Dis. Philadelphia: Saunders 2000; 305-314. |
| 64. | Rameshshanker R, Arebi N. Endoscopy in inflammatory bowel disease when and why. World J Gastrointest Endosc. 2012;4:201-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | Yang DH, Keum B, Jeen YT. Capsule Endoscopy for Crohn’s Disease: Current Status of Diagnosis and Management. Gastroenterol Res Pract. 2016;2016:8236367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Gurudu SR, Leighton JA. Correlation of two capsule endoscopy scoring systems with fecal calprotectin: does it really matter? Dig Dis Sci. 2012;57:827-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Kovanlikaya A, Watson E, Hayward J, Beneck D, Sockolow R, Solomon A, Christos P, Brill PW. Magnetic resonance enterography and wireless capsule endoscopy in the evaluation of patients with inflammatory bowel disease. Clin Imaging. 2013;37:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Lee SM, Kim WS, Choi YH. Pediatric Magnetic Resonance Enterography: Focused on Crohn’s Disease. Pediatr Gastroenterol Hepatol Nutr. 2015;18:149-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Leighton JA, Gralnek IM, Cohen SA, Toth E, Cave DR, Wolf DC, Mullin G, Ketover S, Legnani P, Seidman E. Capsule endoscopy is superior to small-bowel follow-through and equivalent to ileocolonoscopy in suspected Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:609-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Cave D, Legnani P, de Franchis R, Lewis BS. ICCE consensus for capsule retention. Endoscopy. 2005;37:1065-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 71. | Jensen MD, Kjeldsen J, Nathan T. Fecal calprotectin is equally sensitive in Crohn’s disease affecting the small bowel and colon. Scand J Gastroenterol. 2011;46:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 72. | Kopylov U, Nemeth A, Koulaouzidis A, Makins R, Wild G, Afif W, Bitton A, Johansson GW, Bessissow T, Eliakim R. Small bowel capsule endoscopy in the management of established Crohn’s disease: clinical impact, safety, and correlation with inflammatory biomarkers. Inflamm Bowel Dis. 2015;21:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 73. | Koulaouzidis A, Douglas S, Plevris JN. Lewis score correlates more closely with fecal calprotectin than Capsule Endoscopy Crohn’s Disease Activity Index. Dig Dis Sci. 2012;57:987-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 74. | Koulaouzidis A, Douglas S, Rogers MA, Arnott ID, Plevris JN. Fecal calprotectin: a selection tool for small bowel capsule endoscopy in suspected IBD with prior negative bi-directional endoscopy. Scand J Gastroenterol. 2011;46:561-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 75. | Holleran G, Hall B, Hussey M, Thornton O, Dobson M, McNamara D. How accurate are capsule endoscopy scoring sys-tems in Crohn′s disease. 8th Congress ECCO; 2013 Feb 14; Vienna, Austria. Abstract 233. . |
| 76. | Bouguen G, Levesque BG, Feagan BG, Kavanaugh A, Peyrin-Biroulet L, Colombel JF, Hanauer SB, Sandborn WJ. Treat to target: a proposed new paradigm for the management of Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13:1042-1050.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 77. | Laughlin DM, Friedmacher F, Puri P. Total colonic aganglionosis: a systematic review and meta-analysis of long-term clinical outcome. Pediatr Surg Int. 2012;28:773-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 676] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 78. | Efthymiou A, Viazis N, Mantzaris G, Papadimitriou N, Tzourmakliotis D, Raptis S, Karamanolis DG. Does clinical response correlate with mucosal healing in patients with Crohn’s disease of the small bowel? A prospective, case-series study using wireless capsule endoscopy. Inflamm Bowel Dis. 2008;14:1542-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (36)] |
| 79. | Tsibouris P, Periklis A, Chrissostomos K, Antonios Z, Panagiota M, Erasmia V, Georgios A. When Crohn’s disease is in remission, more patients complete capsule endoscopy study but less lesions are identified. Saudi J Gastroenterol. 2013;19:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 80. | Dulai PS, Levesque BG, Feagan BG, D’Haens G, Sandborn WJ. Assessment of mucosal healing in inflammatory bowel disease: review. Gastrointest Endosc. 2015;82:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 81. | D'Haens GR, Fedorak R, Lémann M, Feagan BG, Kamm MA, Cosnes J, Rutgeerts PJ, Marteau P, Travis S, Schölmerich J. Endpoints for clinical trials evaluating disease modification and structural damage in adults with Crohn’s disease. Inflamm Bowel Dis. 2009;15:1599-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Adler SN, Yoav M, Eitan S, Yehuda C, Eliakim R. Does capsule endoscopy have an added value in patients with perianal disease and a negative work up for Crohn’s disease? World J Gastrointest Endosc. 2012;4:185-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 83. | Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn’s disease. Aliment Pharmacol Ther. 2006;23:1097-1104. [PubMed] |
| 85. | Kurien M, Evans KE, Aziz I, Sidhu R, Drew K, Rogers TL, McAlindon ME, Sanders DS. Capsule endoscopy in adult celiac disease: a potential role in equivocal cases of celiac disease? Gastrointest Endosc. 2013;77:227-232. [PubMed] |
| 86. | Buisson A, Chevaux JB, Bommelaer G, Peyrin-Biroulet L. Diagnosis, prevention and treatment of postoperative Crohn’s disease recurrence. Dig Liver Dis. 2012;44:453-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 87. | De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, Liew D, Prideaux L, Lawrance IC, Andrews JM. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015;385:1406-1417. [PubMed] |
| 88. | Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956-963. |
| 89. | De Cruz P, Kamm MA, Prideaux L, Allen PB, Desmond PV. Postoperative recurrent luminal Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2012;18:758-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 90. | Goenka MK, Majumder S, Goenka U. Capsule endoscopy: Present status and future expectation. World J Gastroenterol. 2014;20:10024-10037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (2)] |
| 91. | Pons Beltrán V, Nos P, Bastida G, Beltrán B, Argüello L, Aguas M, Rubín A, Pertejo V, Sala T. Evaluation of postsurgical recurrence in Crohn’s disease: a new indication for capsule endoscopy? Gastrointest Endosc. 2007;66:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 92. | Buchman AL, Miller FH, Wallin A, Chowdhry AA, Ahn C. Videocapsule endoscopy versus barium contrast studies for the diagnosis of Crohn’s disease recurrence involving the small intestine. Am J Gastroenterol. 2004;99:2171-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 93. | Kono T, Hida N, Nogami K, Iimuro M, Ohda Y, Yokoyama Y, Kamikozuru K, Tozawa K, Kawai M, Ogawa T. Prospective postsurgical capsule endoscopy in patients with Crohn’s disease. World J Gastrointest Endosc. 2014;6:88-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 94. | Vind I, Riis L, Jess T, Knudsen E, Pedersen N, Elkjaer M, Bak Andersen I, Wewer V, Nørregaard P, Moesgaard F. Increasing incidences of inflammatory bowel disease and decreasing surgery rates in Copenhagen City and County, 2003-2005: a population-based study from the Danish Crohn colitis database. Am J Gastroenterol. 2006;101:1274-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 95. | Stewénius J, Adnerhill I, Ekelund G, Florén CH, Fork FT, Janzon L, Lindström C, Mars I, Nyman M, Rosengren JE. Ulcerative colitis and indeterminate colitis in the city of Malmö, Sweden. A 25-year incidence study. Scand J Gastroenterol. 1995;30:38-43. [PubMed] |
| 96. | Mehdizadeh S, Chen G, Enayati PJ, Cheng DW, Han NJ, Shaye OA, Ippoliti A, Vasiliauskas EA, Lo SK, Papadakis KA. Diagnostic yield of capsule endoscopy in ulcerative colitis and inflammatory bowel disease of unclassified type (IBDU). Endoscopy. 2008;40:30-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Min SB, Le-Carlson M, Singh N, Nylund CM, Gebbia J, Haas K, Lo S, Mann N, Melmed GY, Rabizadeh S. Video capsule endoscopy impacts decision making in pediatric IBD: a single tertiary care center experience. Inflamm Bowel Dis. 2013;19:2139-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Park SK, Yang SK, Park SH, Park SH, Kim JW, Yang DH, Jung KW, Kim KJ, Ye BD, Byeon JS. Long-term prognosis of the jejunal involvement of Crohn’s disease. J Clin Gastroenterol. 2013;47:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 99. | Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn’s disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis. 2014;8:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 100. | Dias de Castro F, Boal Carvalho P, Monteiro S, Rosa B, Firmino-Machado J, Moreira MJ, Cotter J. Lewis Score--Prognostic Value in Patients with Isolated Small Bowel Crohn’s Disease. J Crohns Colitis. 2015;9:1146-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 101. | Gralnek IM, Cohen SA, Ephrath H, Napier A, Gobin T, Sherrod O, Lewis J. Small bowel capsule endoscopy impacts diagnosis and management of pediatric inflammatory bowel disease: a prospective study. Dig Dis Sci. 2012;57:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Long MD, Barnes E, Isaacs K, Morgan D, Herfarth HH. Impact of capsule endoscopy on management of inflammatory bowel disease: a single tertiary care center experience. Inflamm Bowel Dis. 2011;17:1855-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 103. | Kalla R, McAlindon ME, Drew K, Sidhu R. Impact of capsule endoscopy on management in patients with established Crohn’s disease-experience from a single tertiary centre. Gut. 2011;60:A216-A217. [DOI] [Full Text] |
| 104. | Kalla R, McAlindon ME, Drew K, Sidhu R. Clinical utility of capsule endoscopy in patients with Crohn’s disease and inflammatory bowel disease unclassified. Eur J Gastroenterol Hepatol. 2013;25:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 105. | Liao Z, Gao R, Xu C, Li ZS. Indications and detection, completion, and retention rates of small-bowel capsule endoscopy: a systematic review. Gastrointest Endosc. 2010;71:280-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 483] [Article Influence: 30.2] [Reference Citation Analysis (1)] |
| 106. | Herrerias JM, Leighton JA, Costamagna G, Infantolino A, Eliakim R, Fischer D, Rubin DT, Manten HD, Scapa E, Morgan DR. Agile patency system eliminates risk of capsule retention in patients with known intestinal strictures who undergo capsule endoscopy. Gastrointest Endosc. 2008;67:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 107. | Saurin JC, Maunoury V, Lapalus MG, Cellier C, Delvaux M, Favre O, Gay G, Heresbach D. [International consensus in Paris, 2006, on the indications and use of the endoscopic videocapsule test. Report of the SFED Capsule Commission]. Gastroenterol Clin Biol. 2007;31:798-805. [PubMed] |
| 108. | Postgate AJ, Burling D, Gupta A, Fitzpatrick A, Fraser C. Safety, reliability and limitations of the given patency capsule in patients at risk of capsule retention: a 3-year technical review. Dig Dis Sci. 2008;53:2732-2738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 109. | Nuutinen H, Kolho KL, Salminen P, Rintala R, Koskenpato J, Koivusalo A, Sipponen T, Färkkilä M. Capsule endoscopy in pediatric patients: technique and results in our first 100 consecutive children. Scand J Gastroenterol. 2011;46:1138-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 110. | Rondonotti E, Herrerias JM, Pennazio M, Caunedo A, Mascarenhas-Saraiva M, de Franchis R. Complications, limitations, and failures of capsule endoscopy: a review of 733 cases. Gastrointest Endosc. 2005;62:712-716; quiz 752, 754. [PubMed] |
| 111. | Leighton JA, Legnani P, Seidman EG. Role of capsule endoscopy in inflammatory bowel disease: where we are and where we are going. Inflamm Bowel Dis. 2007;13:331-337. [PubMed] |
| 112. | Cheifetz AS, Lewis BS. Capsule endoscopy retention: is it a complication? J Clin Gastroenterol. 2006;40:688-691. [PubMed] |
| 113. | Cheon JH, Kim YS, Lee IS, Chang DK, Ryu JK, Lee KJ, Moon JS, Park CH, Kim JO, Shim KN. Can we predict spontaneous capsule passage after retention? A nationwide study to evaluate the incidence and clinical outcomes of capsule retention. Endoscopy. 2007;39:1046-1052. [PubMed] |
| 114. | Richards J, Wass A. Small bowel obstruction after a capsule enteroscopy. BMJ Case Rep. 2013;2013:pii: bcr2013008606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 115. | Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, Lichtiger S, Lewis BS. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006;101:2218-2222. [PubMed] |
| 116. | Esaki M, Matsumoto T, Watanabe K, Arakawa T, Naito Y, Matsuura M, Nakase H, Hibi T, Matsumoto T, Nouda S. Use of capsule endoscopy in patients with Crohn’s disease in Japan: a multicenter survey. J Gastroenterol Hepatol. 2014;29:96-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 117. | Cheraskin E, Ringsdorf WM, Medford FH. Letter: Relationship of fatigue to smoking. South Med J. 1976;69:522. [PubMed] |
| 118. | Fireman Z, Mahajna E, Broide E, Shapiro M, Fich L, Sternberg A, Kopelman Y, Scapa E. Diagnosing small bowel Crohn’s disease with wireless capsule endoscopy. Gut. 2003;52:390-392. [PubMed] |
| 119. | Herrerías JM, Caunedo A, Rodríguez-Téllez M, Pellicer F, Herrerías JM. Capsule endoscopy in patients with suspected Crohn’s disease and negative endoscopy. Endoscopy. 2003;35:564-568. [PubMed] |
| 120. | Li F, Gurudu SR, De Petris G, Sharma VK, Shiff AD, Heigh RI, Fleischer DE, Post J, Erickson P, Leighton JA. Retention of the capsule endoscope: a single-center experience of 1000 capsule endoscopy procedures. Gastrointest Endosc. 2008;68:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 121. | Figueiredo P, Almeida N, Lopes S, Duque G, Freire P, Lérias C, Gouveia H, Sofia C. Small-bowel capsule endoscopy in patients with suspected Crohn’s disease-diagnostic value and complications. Diagn Ther Endosc. 2010;2010:pii: 101284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 123. | Hall B, Holleran G, Chin JL, Smith S, Ryan B, Mahmud N, McNamara D. A prospective 52 week mucosal healing assessment of small bowel Crohn’s disease as detected by capsule endoscopy. J Crohns Colitis. 2014;8:1601-1609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (37)] |
| 124. | Niv E, Fishman S, Kachman H, Arnon R, Dotan I. Sequential capsule endoscopy of the small bowel for follow-up of patients with known Crohn’s disease. J Crohns Colitis. 2014;8:1616-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 125. | Spada C, Riccioni ME, Costamagna G. Patients with known small bowel stricture or with symptoms of small bowel obstruction secondary to Crohn’s disease should not perform video capsule endoscopy without being previously tested for small bowel patency. Am J Gastroenterol. 2007;102:1542-1543; author reply 1543-1544. [PubMed] |
| 126. | Nemeth A, Kopylov U, Koulaouzidis A, Wurm Johansson G, Thorlacius H, Amre D, Eliakim R, Seidman EG, Toth E. Use of patency capsule in patients with established Crohn’s disease. Endoscopy. 2016;48:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 127. | Van de Bruaene C, De Looze D, Hindryckx P. Small bowel capsule endoscopy: Where are we after almost 15 years of use? World J Gastrointest Endosc. 2015;7:13-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 63] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (6)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Actis GC, Akyuz F, Koulaouzidis A, Sipahi AM S- Editor: Qiu S L- Editor: A E- Editor: Li D