Published online Jun 10, 2016. doi: 10.4253/wjge.v8.i11.425
Peer-review started: November 16, 2015
First decision: January 4, 2016
Revised: February 16, 2016
Accepted: March 7, 2016
Article in press: March 9, 2016
Published online: June 10, 2016
Processing time: 205 Days and 21.2 Hours
AIM: To assess pediatric patients for choledocholithiasis. We applied current adult guidelines to identify predictive factors in children.
METHODS: A single-center retrospective analysis was performed at a tertiary children’s hospital. We evaluated 44 consecutive pediatric patients who underwent endoscopic retrograde cholangiography (ERCP) for suspected choledocholithiasis. Patients were stratified into those with common bile duct stones (CBDS) at ERCP vs those that did not using the American Society of Gastrointestinal Endoscopy (ASGE) guidelines (Very Strong and Strong criteria) for suspected CBDS.
RESULTS: CBDS were identified in 84% at the time of ERCP. Abdominal ultrasound identified CBDS in 36% of patients. Conjugated bilirubin ≥ 0.5 mg/dL was an independent risk factor for CBDS (P = 0.003). The Very Strong (59.5%) and Strong (48.6%) ASGE criteria identified the majority of patients (P = 0.0001). A modified score using conjugated bilirubin had a higher sensitivity (81.2% vs 59.5%) and more likely to identify a stone than the standard criteria, odds ratio of 25.7 compared to 8.8. Alanine aminotransferase and gamma-glutamyl transferase values identified significant differences in a subset of patients with odds ratio of 4.1 and 3.25, respectively.
CONCLUSION: Current adult guidelines identified the majority of pediatric patients with CBDS, but specific pediatric guidelines may improve detection, thus decreasing risks and unnecessary procedures.
Core tip: In pediatric patients with gallstones, biliary obstruction has been reported in up to 30% of patients with limited data to predict need for endoscopic retrograde cholangiography for choledocholithiasis. In this single-center retrospective study we evaluated 44 consecutive pediatric patients and used the American Society of Gastrointestinal Endoscopy guidelines for suspected choledocholithiasis. We found that the Very Strong and Strong criteria identified the majority of patients. Conjugated bilirubin was also identified as an important predictor. Current adult guidelines can be used in the majority of patients, but specific pediatric guidelines may improve detection, thus decreasing risks.
- Citation: Fishman DS, Chumpitazi BP, Raijman I, Tsai CMW, Smith EO, Mazziotti MV, Gilger MA. Endoscopic retrograde cholangiography for pediatric choledocholithiasis: Assessing the need for endoscopic intervention. World J Gastrointest Endosc 2016; 8(11): 425-432
- URL: https://www.wjgnet.com/1948-5190/full/v8/i11/425.htm
- DOI: https://dx.doi.org/10.4253/wjge.v8.i11.425
Choledocholithiasis can complicate symptomatic gallstones in up to 10% of adults at cholecystectomy[1]. Children may be at higher risk with recent studies demonstrating up to 30% of patients evaluated for pediatric gallbladder disease having some form of complicated bile duct obstruction as evidenced by jaundice, pancreatitis, or imaging with a visualized stone or dilated bile duct[2-4]. As in adult patients with choledocholithiasis, management options in children include both endoscopic and surgical methods. However, normal laboratory value differences and differences in bile duct size between pediatric and adults patients pose further challenges to appropriate patient selection for the management of pediatric choledocholithiasis.
Multiple studies in adult patients have evaluated specific keys in identifying common bile ducts stones at endoscopic retrograde cholangiography (ERCP)[5-9]. Algorithms and scoring systems have been developed in order to identify patients with a high likelihood of having common bile duct stones (CBDS) that would benefit from treatment with ERCP, or other modalities such as laparoscopic cholecystectomy with intraoperative cholangiogram.
Current American Society of Gastrointestinal Endoscopy (ASGE) guidelines stratify adult patients using several predictors[10]. A probability of stone identification of greater than 50% at ERCP is set as an appropriate level of detection of CBDS. These conditions are met if any of the following were identified: The value of total bilirubin (measured in mg/dL) was greater than 4, a CBDS is visualized by trans-abdominal ultrasound, or the presence of cholangitis. If both the CBD diameter was greater than 6 mm by ultrasound and the total bilirubin was greater than 1.8 mg/dL, ERCP was recommended and considered to meet the 50% threshold. When present these factors were useful for CBDS prediction, while other factors such as age greater than 55 years old, presence of gallstone pancreatitis and abnormal markers of liver and biliary inflammation [e.g., alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (γGT), and alkaline phosphatase] were less likely to predict CBDS. In contrast, limited data and recommendations are available for the management of suspected CBDS or gallstone pancreatitis in children[11-13]. The aim of our study was to determine the applicability of the current ASGE guidelines in pediatric patients with suspected CBDS and to identify other factors that may be predictive in the pediatric population.
Retrospective analysis was performed on consecutive ERCPs in children ages 6-18 years of age, performed over 24 mo. Cases were reviewed for patients with suspected common bile duct stones with gallbladder in situ evaluated in the hospital or emergency department. Patients were excluded if they were status post-cholecystectomy, had ERCP for another indication, or ultrasound (US) results were not available. Presence of CBDS by US and bile duct diameter (measured in millimeters) were recorded. Bilirubin (unconjugated and conjugated) and other laboratory values were captured pre-procedure (within 24 h). This study approved by the Institutional Review Board at Baylor College of Medicine, Houston, Texas.
For the purposes of this study, total bilirubin was calculated as the sum of unconjugated and conjugated bilirubin levels. Normal values for unconjugated bilirubin at our institution is 0-1.0 mg/dL, and for conjugated bilirubin is 0-0.3 mg/dL. Biliary cannulation and sphincterotomy was performed in all patients at the time of the procedure. Patients were classified into two groups; Group 1, patients with CBDS found at ERCP and Group 2 those without CBDS at ERCP.
ASGE guidelines to predict the likelihood of detecting CBDS at ERCP were used to classify patients[10]. Predictors per ASGE guidelines were: Very Strong (VS) if CBDS was identified on abdominal US or total bilirubin > 4 mg/dL or Strong (S) if both CBD diameter ≥ 6 mm on US and bilirubin ≥ 1.8-4 mg/dL.
Patients were assessed on each of the following ASGE factors: (1) Visualized CBDS on ultrasound imaging; (2) CBD diameter > 6 mm on ultrasound imaging; and (3) Total bilirubin level.
For subset analysis, patients were divided into one of two groups: VS: Either CBDS on US or total bilirubin > 4, or those meeting S criteria, with the combination of having both a total bilirubin > 1.8 and a CBD diameter of > 6 mm.
SPSS (Statistical Package for the Social Sciences, IBM, Armonk, NY) Version 19.0 was used for statistical calculations. χ2 with McNemar’s test to compare correlated groups was used with interquartile range (IQR) and medians and percentiles calculated for continuous data. Similarly, Mann-Whitney test was used to compare groups with non-parametric data and the Mantel-Haenzsel test was used to calculate a Common Odds Ratio Estimate. A P-value of < 0.05 was considered to be statistically significant. Unless otherwise specified, values are presented as median with interquartile range in parentheses. Confidence intervals were calculated using http://vassarstats.net/clin1.html. The statistical methods of this study were reviewed by Dr. Smith, biostatistician, Baylor College of Medicine.
Forty-four consecutive children with gallbladder in situ hospitalized for evaluation of suspected CBDS were evaluated. The median age was 15.4 years (ages 6-18 years old) (Table 1). Eight of 44 patients (18.2%) had hemolytic disease. Gallstone pancreatitis was the initial presentation in 15 patients (34%). Forty-three/forty-four patients had general anesthesia, and the remaining patients received deep sedation with intravenous midazolam and propofol. Magnetic resonance cholangiopancreatography (MRCP) was performed in 14/44 patients, and identified choledocholithiasis in 9 of 14. ERCP identified stones in 84% (n = 37), referred to as Group 1. In Group 2, (n = 7) that did not have CBDS at ERCP, common bile duct dilation > 6 mm was evident in 85.7% (n = 6), and all had endoscopic or radiographic findings suspicious for papillary stenosis, suprapapillary stricture from stone passage or recent pancreatitis. All patients had a native papilla, and sphincterotomy was performed at time of the procedure. No patients had a clinical picture of cholangitis. Adverse event rates in both groups were similar, with one case of mild pancreatitis in each group.
| Group number n | 1 | 2 | Total | |
| 37 | 7 | 44 | ||
| Mean age | 14.5 (± 3.8) | 14.5 (± 2.0) | 14.5 (± 3.5) | |
| Sex | Male | 14 | 0 | 14 |
| Female | 23 | 7 | 30 | |
| Ethnicity | White | 4 | 2 | 6 |
| African-American | 12 | 0 | 12 | |
| Latino-Hispanic | 20 | 5 | 25 | |
| Other | 1 | 0 | 1 | |
| Imaging | CBDS on US | 16 | 0 | 16 |
| No CBDS on US | 21 | 7 | 28 | |
| CBDS on MRCP | 6 of 8 | 3 of 6 | 9 of 14 | |
| Clinical | Gallstone pancreatitis | 11 | 4 | 15 |
All patients had abdominal ultrasound performed and a portion of the common bile duct was visualized in all but one patient (43/44). CBDS were identified by US in 36% (n = 16), and this differed from the 85% (n = 37) found to have CBDS by ERCP (P = 0.029). Sensitivity of US for CBDS was poor, 43% (95%CI: 28%-60%), with specificity 100% (95%CI: 56%-100%), positive predictive value (PPV) of 100% (95%CI: 76%-100%) and a negative predictive value (NPV) of 25% (95%CI: 11%-45%).
The median CBD diameter in Group 1 was 9.0 mm (7.0, 11.0) and 8.0 mm (6.1-10.0) in Group 2 (Table 1). A CBD greater than 6 millimeters was demonstrated in 36 (81.8%) patients, 30 in Group 1 and 6 in Group 2 (P = NS). The combination of ultrasound findings of CBDS and a dilated bile duct > 6 mm was seen in 15 patients (34.1%). Twenty-two patients had one or the other, 16 in Group 1 and 6 in Group 2. Seven patients had a CBD diameter of less than 6 mm or CBDS by ultrasound, and the majority (84%) were in Group 1. Conversely, all 6 patients in Group 2 had a bile duct diameter > 6 mm. Although it would be expected that in the presence of a larger bile duct, a greater chance for CBDS would be found but this was not the case emphasizing the importance of using multiple parameters in making the clinical assessment.
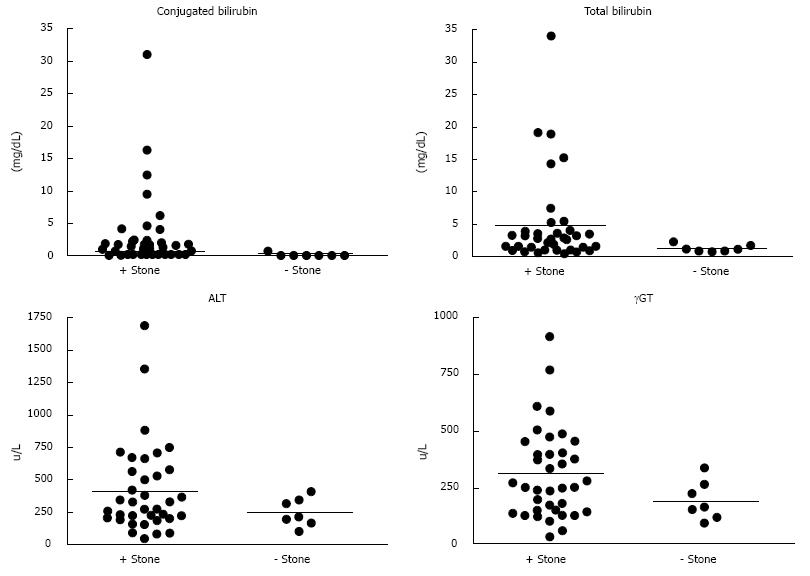
Serum bilirubin was measured in all patients (Table 2 and Figure 1). There were significant differences between Group 1 and 2 for mean values of total bilirubin (P = 0.004) and conjugated bilirubin P = 0.02 (0.004 including patients with hemolytic disease). In Group 1, 8 (22%) patients had a total bilirubin greater than 4 mg/dL, while none did in Group 2 (P = 0.0001). Twenty-one (58%) patients in Group 1 and 1 patient in Group 2 had total bilirubin > 1.8 mg/dL (P = 0.0001). In comparison, 25 (68%) patients in Group 1 had a conjugated bilirubin ≥ 0.5 mg/dL, and none in Group 2 (P = 0.003). Sensitivity was also higher using conjugated bilirubin ≥ 0.5 mg/dL than cut-offs of total bilirubin of 4 or 1.8 mg/dL (Table 3). Multivariate logistic regression identified conjugated bilirubin ≥ 0.5 mg/dL as an independent risk factor for detection of CBDS.
| Combined group data median (IQR) | Group 1 median (IQR) | Group 2 median (IQR) | |
| Age (yr) | 15.8 (12.5, 17.3) | 16.1 (12.2, 17.3) | 14.8 (12.5, 15.4) |
| Time to procedure (d) | 2 (1.0, 2.3) | 2 (1.0, 2.0) | 2 (1.0-3.0) |
| US CBD diameter (mm) | 8.8 (6.8, 10.5) | 9 (7.0, 11.0) | 8 (6.1-10.0) |
| ERCP CBD diameter (mm) | 11 (9.0, 13.0) | 11 (9.3, 13) | 9 (7.0, 10.0) |
| Total bilirubin (mg/dL) | 2 (0.8, 3.6) | 2.5 (0.9, 3.8) | 0.9 (0.6-1.5) |
| Conjugated bilirubin (mg/dL) | 1 (0.0-2.1) | 1.3 (0.0, 2.4) | 0 (0, 0) |
| ALT (u/L) | 242 (142.5, 386.5) | 253 (145.0, 403.0) | 166 (122.0-166.0) |
| AST (u/L) | 128 (86.0, 188.0) | 129 (89.0, 215.0) | 119 (53-150) |
| γGT (u/L) | 259 (177.0, 453.5) | 259 (181.0, 521.0) | 203 (159.0-333) |
| Alkaline phosphatase (u/L) | 252 (179.0, 349.0) | 254 (182.0, 405.0) | 208 (107.0-256.0) |
| Criteria | Sensitivity% (95%CI) | Specificity% (95%CI) | PPV% (95%CI) | NPV% (95%CI) | Odds ratio (95%CI) | P-value |
| VS-PM | 81.2 (64-91) | 85.7 (42-99) | 96.8 (81-100) | 46.2 (20-74) | 25.7 (2.65-249) | 0.07 |
| S-PM | 59.5 (42-75) | 85.7 (42-99) | 95.7 (76-100) | 28.6 (12-52) | 8.8 (0.96-80.7) | 0.001 |
| VS-Adult | 59.5 (42-75) | 100 (56-100) | 100 (81-100) | 31.8 (15-55) | 18.8 (0.96-80.7) | 0.0001 |
| S-Adult | 48.6 (32-65) | 85.7 (42-99) | 94.7 (72-100) | 24 (10-45) | 5.68 (0.62-51.97) | 0.0001 |
| CBDS by US | 43.2 (28-60) | 100 (56-100) | 100 (76-100) | 25 (11-45) | 14.57 (0.50-41.9) | 0.0001 |
| CBD > 6 mm | 81.1 (64-91) | 14.3 (1-58) | 83.3 (67-93) | 12.5 (1-33) | 0.714 (0.074-6.92) | 1 |
| CBD > 8 mm | 91.7 (76-98) | 28.6 (5-70) | 86.8 (71-95) | 40 (7-83) | 4.4 (0.58-33.2) | 0.727 |
| TB > 4.0 | 21.6 (10-39) | 100 (56-100) | 100 (60-100) | 19.4 (8-37) | 11.66 (0.17-15.82) | 0.0001 |
| TB ≥ 1.8 | 56.8 (41-71) | 85.7 (49-97) | 95.5 (75-100) | 27.3 (12-50) | 7.88 (0.86-72.12) | 0.0001 |
| CB ≥ 0.5 | 67.6 (50-81) | 85.7 (42-99) | 96.2 (78-100) | 33.3 (14-59) | 12.5 (1-115) | 0.003 |
| ALT > 300 | 56.8 (40-72) | 14.3 (1-58) | 77.8 (57-91) | 5.9 (0-31) | 0.219 (0.024-2.00) | 0.052 |
| ALT > 350 | 40.5 (26-57) | 100 (56-100) | 100 (80-100) | 24.1 (11-42) | 14.1 (0.45-37.5) | 0.0001 |
| AST > 155 | 43.2 (28-60) | 85.7 (42-99) | 94 (69-100) | 22.2 (9-43) | 4.57 (0.499-41.9) | 0.0001 |
| γGT > 400 | 35.1 (21-53) | 100 (56-100) | 100 (72-100) | 22.6 (10-42) | 13.25 (0.352-30.0) | 0.0001 |
Determinations for each patient were made as to whether patients met the ASGE VS or S criteria (Table 3). As expected, there was a significant difference between patients in Group 1 and 2 using the VS criteria to stratify patients (P = 0.0001). The sensitivity for CBDS at the time of ERCP in our population using VS criteria was 59.5%, compared to 48.6% in the patients meeting S criteria (Table 3). Specificity ranged from 86%-100% for each of the VS and S categories.
Because conjugated bilirubin levels are a prominent finding in obstruction and a component in the liver panel/biochemistries at many pediatric facilities, conjugated bilirubin was substituted for total bilirubin. Thus, ≥ 0.5 mg/dL was substituted into both the VS and S categories. A VS “Pediatric Modified” (VS-PM) criteria was defined as either a stone visualized on US or a conjugated bilirubin ≥ 0.5 mg/dL. To meet the Strong “Pediatric modified” criteria (S-PM), a patient needed to have a bile duct diameter > 6 mm and a conjugated bilirubin ≥ 0.5 mg/dL. In comparing patients in Group 1 and 2 using the VS-PM there was not a significant difference (P = 0.07) but significant using the S-PM criteria, (P = 0.001). An imputed odds ratio for a child meeting VS-PM criteria was calculated to be 25.7 times more likely to have a stone at ERCP, and 8.8 times more likely in those meeting S-PM criteria. The VS-PM and S-PM criteria also had improved sensitivity when compared to the respective adult criteria, up to 81.2% for identifying a CBDS at time of ERCP. The S-PM performed as well as the adult VS criteria, both with sensitivities of 59.5% (Table 3).
Use of aminotransferases andγGT in diagnosis of CBDS
Both ALT and AST levels were collected (Table 2 and Figure 1). The mean ALT and AST were not significantly different between Group 1 vs Group 2 (P = 0.127 and 0.149, respectively). When an arbitrary cut-off for ALT of 350 u/L was used, the differences between the two groups was significant (P = 0.0001), but not at 300 u/L (P = 0.052). Given that aminotransferases are elevated in hemolysis, when patients with hemolytic disease (n = 7) were excluded there was still a significant difference between means in Group 1 and 2 (P = 0.027).
Additionally, γGT is known to be elevated during biliary obstruction as a surrogate marker of biliary obstruction. The median γGT in patients with CBDS was 259 u/L (181-521) compared to 203 u/L (159-333) in those without CBDS at ERCP (P = 0.268). When a γGT cut-off level of 400 u/L was used, a high sensitivity and positive predictive value were seen (P = 0.0001). These findings suggest that aminotransferases and γGT may be of value in the prediction of CBDS in children.
While several groups have reported their experience using ERCP in pediatric patients, to our knowledge this is the first series to evaluate the management of choledocholithiasis using current clinical practice guidelines[13-15]. The ASGE guidelines published in 2010 utilize ultrasound findings of CBD stones or common bile duct diameter, total bilirubin, age, and presence of cholangitis to identify patients at highest risk for CBDS[10]. We classified a series of pediatric patients with suspected choledocholithiasis that underwent ERCP using these criteria at an acceptable sensitivity of 59.5 (VS) and 48.6% (S). However, we found that using conjugated bilirubin instead of total bilirubin improved the sensitivity for CBDS identification to 81%. However in practice, deciding on ERCP in those without a visualized stone on initial imaging and mild elevations or normal bilirubin is quite challenging. In this setting both the standard and modified pediatric strong criteria are important. In our subset of patients, the S-PM had a higher sensitivity than the standard criteria, and the same specificity. These criteria are dependent on both abnormal bilirubin and ductal dilatation, but in both criteria the major driver is the bilirubin level as even in children ductal dilatation is quite common in stone related disease.
The majority of published series and accepted guidelines in adults use identification of CBDS and bile duct diameter by trans-abdominal ultrasound as critical determinants[5-10]. The sensitivity of ultrasound for CBDS is reported up to 55% in adults, whereas in our series only 43% of patients had CBDS identified by ultrasound[16]. Additionally, the sensitivity of the modified VS criteria exceeded the lower limit of sensitivity for CBDS detection by ultrasound. Normal common bile duct diameters in adults are reported to be 4-6 mm, with small increases with advancing age[17]. A common bile duct diameter greater than 6 mm suggests obstruction and is used in the current ASGE guidelines. Early studies of pediatric common bile duct diameter using intravenous cholangiography, demonstrated an upper limit of 6 to 7 mm in children and that they were more distensible than adult bile ducts[18,19]. By ultrasound, the common bile duct in early adolescence should not exceed 2.5-3.0 mm, although values for teenagers are largely based on adult normative values[14,16,18-20]. In our series, patients in Group 2, had a median common bile duct diameter of 8 mm, suggesting some discrepancy in what should be considered abnormal or inflammatory change from a recently passed stone. For this reason and in keeping with current guidelines, a 6 mm cut-off was used for data analysis. Using a CBD diameter of 6 mm in the scoring is reasonable for older pediatric patients and likely to improve sensitivity of CBDS detection children compared to adults. However, an 8 mm cut-off compared to 6 mm for CBD diameter had improved sensitivity, with modest increase in PPV, NPV and specificity.
Both MRCP and endoscopic ultrasound (EUS) are commonly used in the pre-procedure management of choledocholithiasis[21]. MRCP use in pediatrics is common[22,23]. However, some patients require sedation or anesthesia, and access is sometimes limited. There is an expanding experience and accessibility of EUS in pediatric patients[24-27]. In a recent study by Adams et al[28], EUS and MRCP were used along with ERCP to identify the likelihood of CBDS in patients. Specific utilization of EUS and MRCP was not reported, however, using these modalities in addition to available guidelines and laboratory investigations, overall sensitivity and specificity were improved[28]. Despite the limited use of MRCP and no cases of EUS, in our population, CBDS at the time of ERCP were identified in 84% of patients.
One limitation of our study was the variation in timing of patient presentation to abdominal ultrasound to ERCP from 1 to 6 d. However, the majority of procedures occurred less than 48 h of presentation with a mean of 1.9 (± 1.3) d. Approximately one-fourth of patients had MRCP prior to ERCP, frequently extending time to definitive procedure by 12-24 h. In the patients with a positive MRCP, but negative CBDS, that variability was accentuated and likely contributed to the passage of stones during the interim period. Timing of MRCP and its relationship to ERCP should be considered when planning procedures. Due to restrictions or delays in either of these modalities, it can be expected to have some stone passage, but these should be mitigated by process improvement actions. Based on patient selection completed during routine clinical practice, and low rate of negative ERCP, our data is likely to represent a reasonable population in which to make predictions. Given the reported rates of stone migration (21% to 80%), we anticipate that data used within 24 h of ERCP, is applicable to optimize patient selection[14,29]. Another limitation of this study is the limited sample of patients that had ERCP in which CBDS were not identified. Although there was a clinical suspicion for a stone in those cases for which ERCP was considered (e.g., known gallstone disease), a passed stone, suprapapillary stricture or papillary stenosis from a stone was suspected. In the absence of stone this information was identified in the post-procedure note, but based on a normal appearing ampulla or post-sphincterotomy where the dilated bile duct is traced to a stenotic area above the ampulla (suprapapillary stricture) or tactile perception or visibly stenosed ampullary os (papillary stenosis).
Since the primary endpoint was the presence of a stone, this resulted in wide confidence intervals and did not allow for appropriate ROC curve representation. Similarly, due to the zero denominator in several calculations, imputed odds ratios were calculated for the following categories: Total Bilirubin > 4 mg/dL, VS-Adult criteria, and CBDS by US, but likely underestimating these factors.
There are also major differences in normal laboratory values and testing, such as alkaline phosphatase, typically several fold higher in pediatric patients compared to adults[30]. Similarly, conjugated bilirubin is more often utilized rather than total bilirubin in pediatric laboratory investigations of hepatobiliary inflammation and obstruction. Conjugated bilirubin is a thus a more sensitive marker of significant biliary obstruction, even when patients with hemolytic disease were separated from the analysis (P < 0.004 vs 0.02 respectively). Cholesterol stone disease is now more common in pediatric patients compared to pigmented stones from hemolytic disease, but the laboratory examinations in patients with hemolytic disease typically often have marked elevations in both total and conjugated bilirubin.
Our data is probably most applicable when the ASGE criteria are applied to adolescents, as they are more similar in mechanisms of disease and anatomy[2]. However, when consideration for bile duct size is taken into account, and with increases for advancing age, the use of imaging criteria (e.g., CBD diameter) may require a higher threshold for use in children and adolescents[18,31,32]. Management algorithms are highly dependent on patient population (e.g., rate of hemolytic disease or obesity), local expertise and availability of ERCP, surgical techniques, and different radiographic modalities. Although the current guidelines for adults use an accepted likelihood of stone identification of greater than fifty percent, a higher cut-off may be more appropriate for children[10,21,28]. It is our hope that the findings may serve as a clinical framework to pursue multi-center studies to identify optimal lab and imaging criteria in children in the management of CBDS prospectively.
Due to the relative variability in each of the available tests as well as the reported rates of both missed stones at ERCP and rates of stone passage, clinical experience should complement these tools and should take into consideration the inherent risks of the procedure with the risks of a retained stone (e.g., cholangitis, pancreatitis). It is also important to consider the possibility of an alternative diagnosis contributing to intraductal stones such as familial intrahepatic cholestasis or sclerosing cholangitis, both carrying malignancy risks. Intrahepatic stone disease has also been linked to cholangiocarcinoma[33].
Using ASGE guidelines in a series of pediatric patients with suspected CBDS, stones were appropriately identified in the majority of cases, while US was poorly predictive of a sensitivity of 42%. Modified criteria using conjugated bilirubin ≥ 0.5 mg/dL instead of total bilirubin performed better at identification of CBDS. Conjugated bilirubin, γGT, ALT and AST may improve specificity in identification of CBDS. Future studies are needed to assess pediatric specific criteria in children including both imaging (US, MRCP and EUS) and laboratory data. In the future, pediatric specific guidelines should be developed to optimize ERCP management in children with suspected CBDS.
Gallstones are an increasingly reported problem in children and reported rates of choledocholithiasis may be higher in pediatric patients than adults. In patients with suspected choledocholithiais, criteria have been proposed for adults to help predict the likelihood of identifying and ultimately removing a stone at endoscopic retrograde cholangiography (ERCP). Limited data is available specific to children to guide management for this problem.
There is great interest in the study of choledocholithiasis and its related management. It offers opportunity to improve patient care by decreasing risks of a given procedure or related sedation. There is also great variability in the management in these patients despite guidelines due to numerous factors, which may impact both patients and endoscopists.
Using both a standard “adult” scoring system as well as a modified scoring system in a series of pediatric patients, the majority of patients could be identified. Specific laboratory tests such as bilirubin or findings on abdominal ultrasounds can assist in directing care for a pediatric patient with choledocholithiasis.
Using a combination of labs and imaging as well as clinical experience can help in identifying appropriate patients for ERCP. Utilization of newer applications such as endoscopic ultrasound or magnetic resonance cholangiopancreatography may improve our patient selection for ERCP. Multicenter studies may help to corroborate this data or identify other factors so that pediatric specific guidelines can be created.
ERCP: Endoscopic retrograde cholangiography, an endoscopic procedure used with X-ray to evaluate the biliary and pancreatic systems.
This manuscript applied the current adult guidelines from the American Society of Gastrointestinal Endoscopy in pediatric patients with suspected common bile duct stones to identify factors that may be predictive in the pediatric population. It is well designed and performed.
| 1. | Petelin JB. Laparoscopic common bile duct exploration. Surg Endosc. 2003;17:1705-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 2. | Mehta S, Lopez ME, Chumpitazi BP, Mazziotti MV, Brandt ML, Fishman DS. Clinical characteristics and risk factors for symptomatic pediatric gallbladder disease. Pediatrics. 2012;129:e82-e88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Herzog D, Bouchard G. High rate of complicated idiopathic gallstone disease in pediatric patients of a North American tertiary care center. World J Gastroenterol. 2008;14:1544-1548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Bogue CO, Murphy AJ, Gerstle JT, Moineddin R, Daneman A. Risk factors, complications, and outcomes of gallstones in children: a single-center review. J Pediatr Gastroenterol Nutr. 2010;50:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Tse F, Barkun JS, Barkun AN. The elective evaluation of patients with suspected choledocholithiasis undergoing laparoscopic cholecystectomy. Gastrointest Endosc. 2004;60:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Prat F, Meduri B, Ducot B, Chiche R, Salimbeni-Bartolini R, Pelletier G. Prediction of common bile duct stones by noninvasive tests. Ann Surg. 1999;229:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 74] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Abboud PA, Malet PF, Berlin JA, Staroscik R, Cabana MD, Clarke JR, Shea JA, Schwartz JS, Williams SV. Predictors of common bile duct stones prior to cholecystectomy: a meta-analysis. Gastrointest Endosc. 1996;44:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 150] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Tham TC, Lichtenstein DR, Vandervoort J, Wong RC, Brooks D, Van Dam J, Ruymann F, Farraye F, Carr-Locke DL. Role of endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis in patients undergoing laparoscopic cholecystectomy. Gastrointest Endosc. 1998;47:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Barkun AN, Barkun JS, Fried GM, Ghitulescu G, Steinmetz O, Pham C, Meakins JL, Goresky CA. Useful predictors of bile duct stones in patients undergoing laparoscopic cholecystectomy. McGill Gallstone Treatment Group. Ann Surg. 1994;220:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 174] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Maple JT, Ben-Menachem T, Anderson MA, Appalaneni V, Banerjee S, Cash BD, Fisher L, Harrison ME, Fanelli RD, Fukami N. The role of endoscopy in the evaluation of suspected choledocholithiasis. Gastrointest Endosc. 2010;71:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (3)] |
| 11. | Fox VL, Werlin SL, Heyman MB. Endoscopic retrograde cholangiopancreatography in children. Subcommittee on Endoscopy and Procedures of the Patient Care Committee of the North American Society for Pediatric Gastroenterology and Nutrition. J Pediatr Gastroenterol Nutr. 2000;30:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Mah D, Wales P, Njere I, Kortan P, Masiakos P, Kim PC. Management of suspected common bile duct stones in children: role of selective intraoperative cholangiogram and endoscopic retrograde cholangiopancreatography. J Pediatr Surg. 2004;39:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Guelrud M. ERCP in Pediatric Practice: Diagnosis and Management. USA: CRC Press 1988; 54. |
| 14. | Vrochides DV, Sorrells DL, Kurkchubasche AG, Wesselhoeft CW, Tracy TF, Luks FI. Is there a role for routine preoperative endoscopic retrograde cholangiopancreatography for suspected choledocholithiasis in children? Arch Surg. 2005;140:359-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Iqbal CW, Baron TH, Moir CR, Ishitani MB. Post-ERCP pancreatitis in pediatric patients. J Pediatr Gastroenterol Nutr. 2009;49:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Cronan JJ. US diagnosis of choledocholithiasis: a reappraisal. Radiology. 1986;161:133-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 78] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Chawla S, Trick WE, Gilkey S, Attar BM. Does cholecystectomy status influence the common bile duct diameter? A matched-pair analysis. Dig Dis Sci. 2010;55:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Hernanz-Schulman M, Ambrosino MM, Freeman PC, Quinn CB. Common bile duct in children: sonographic dimensions. Radiology. 1995;195:193-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 57] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Witcombe JB, Cremin BJ. The width of the common bile duct in childhood. Pediatr Radiol. 1978;7:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Parulekar SG. Ultrasound evaluation of common bile duct size. Radiology. 1979;133:703-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 68] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Maple JT, Ikenberry SO, Anderson MA, Appalaneni V, Decker GA, Early D, Evans JA, Fanelli RD, Fisher D, Fisher L. The role of endoscopy in the management of choledocholithiasis. Gastrointest Endosc. 2011;74:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (3)] |
| 22. | Tipnis NA, Dua KS, Werlin SL. A retrospective assessment of magnetic resonance cholangiopancreatography in children. J Pediatr Gastroenterol Nutr. 2008;46:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Fitoz S, Erden A, Boruban S. Magnetic resonance cholangiopancreatography of biliary system abnormalities in children. Clin Imaging. 2007;31:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Attila T, Adler DG, Hilden K, Faigel DO. EUS in pediatric patients. Gastrointest Endosc. 2009;70:892-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Scheers I, Ergun M, Aouattah T, Piessevaux H, Borbath I, Stephenne X, De Magnée C, Reding R, Sokal E, Veyckemans F. Diagnostic and Therapeutic Roles of Endoscopic Ultrasound in Pediatric Pancreaticobiliary Disorders. J Pediatr Gastroenterol Nutr. 2015;61:238-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Cohen S, Kalinin M, Yaron A, Givony S, Reif S, Santo E. Endoscopic ultrasonography in pediatric patients with gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2008;46:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Gordon K, Conway J, Evans J, Petty J, Fortunato JE, Mishra G. “EUS and EUS Guided Interventions Alter Clinical Management in Children with Digestive Diseases”. J Pediatr Gastroenterol Nutr. 2015;Dec 28; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Adams MA, Hosmer AE, Wamsteker EJ, Anderson MA, Elta GH, Kubiliun NM, Kwon RS, Piraka CR, Scheiman JM, Waljee AK. Predicting the likelihood of a persistent bile duct stone in patients with suspected choledocholithiasis: accuracy of existing guidelines and the impact of laboratory trends. Gastrointest Endosc. 2015;82:88-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 29. | Frossard JL, Hadengue A, Amouyal G, Choury A, Marty O, Giostra E, Sivignon F, Sosa L, Amouyal P. Choledocholithiasis: a prospective study of spontaneous common bile duct stone migration. Gastrointest Endosc. 2000;51:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 102] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Van Hoof VO, Hoylaerts MF, Geryl H, Van Mullem M, Lepoutre LG, De Broe ME. Age and sex distribution of alkaline phosphatase isoenzymes by agarose electrophoresis. Clin Chem. 1990;36:875-878. [PubMed] |
| 31. | Bruneton JN, Roux P, Fenart D, Caramella E, Occelli JP. Ultrasound evaluation of common bile duct size in normal adult patients and following cholecystectomy. A report of 750 cases. Eur J Radiol. 1981;1:171-172. [PubMed] |
| 32. | Bachar GN, Cohen M, Belenky A, Atar E, Gideon S. Effect of aging on the adult extrahepatic bile duct: a sonographic study. J Ultrasound Med. 2003;22:879-882; quiz 883-885. [PubMed] |
| 33. | Cai H, Kong WT, Chen CB, Shi GM, Huang C, Shen YH, Sun HC. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: a meta-analysis of observational studies. BMC Cancer. 2015;15:831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chen CH, Lai KH, Sergi CM, Sun LM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ