INTRODUCTION
Cholangiocarcinoma (CC) is an epithelial malignancy with markers of cholangiocyte differentiation arising within the biliary tree. It is characterized by a marked genetic heterogeneity which explains its high therapeutic resistance[1]. CC is rare but related mortality is high because it is most often diagnosed at a locally advanced stage, not amenable to curative surgery.
Although the incidence of CC is rapidly increasing it remains a rare disease. Data about endoscopic therapeutic options are often comprised into large databases of malignant obstructive jaundice mainly due to pancreatic head cancer. This may have influenced the reported outcomes and benefits of endoscopic treatment modalities[2].
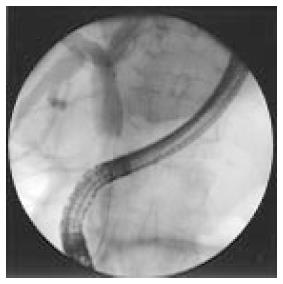
Currently, classification of CC is based on anatomical site, defining intrahepatic, perihilar and distal CCs[2]. Intrahepatic CC is defined as a tumor located proximally to the branch of the right and left lobe bile ducts; the extrahepatic and perihilar cholangiocarcinoma is localized to the area between the second branches bile ducts and the insertion of the cystic duct into the common bile duct (Figure 1); whereas distal CC is confined to the area between the origin of the cystic duct and the ampulla of Vater[3].
Figure 1 Distal cholangiocarcinoma during endoscopic retrograde cholangiography.
Several progresses in the management (diagnosis, treatment and palliation) of CC have befallen in the last decades. However, surgical resection or liver transplantation represents the only potential curative alternative for all subtypes of CC[2]. Unfortunately, involvement of the vascular structures and lymphnodes is associated with very low 5-year survival rates even after curative-intent surgery[2] and, overall the clinical results of patients undergoing liver resection are disappointing with a survival rate of 20%-35% within 5-year[4-9]. Palliative therapy, in patients not amenable of surgical intervention includes systemic chemotherapy and loco regional therapies (TACE, RFA) to reduce masses but increased survival rate has not yet been shown[2].
The main onset of CC is unpainful icterus in > 90% of patients and preoperative biliary drain (endoscopic or percutaneous), has been introduced because jaundice is thought to increase the risk of postoperative complications, but the advantages of this procedure are still unclear[10]. Moreover, in patients who will undergo neo-adjuvant therapy the work-up preceding chemotherapy includes biliary stenting. In the last decades advances in stenting materials and acknowledgement of the benefits in the post-surgical outcome due to pre-operative biliary drainage has led endoscopic retrograde cholangiography (ERC) to a pivotal role in the work up of CC, both in patients amenable to surgical intervention and in those unfit for surgery.
EPIDEMIOLOGY
The reported incidence in the United States is one or two cases per 100000 person/year, also in Europe is 1.5 per 100000 person/year, and it accounts for approximately 3% of all gastrointestinal malignancies. CC is the most common primary malignancy of the liver after hepatocellular carcinoma. An increase in intrahepatic CC mortality has been registered worldwide particularly in western compared with central and northern Europe. The increased incidence of intrahepatic CCs may in part be attributed to new diagnostic methods for obstructive jaundice allowing to identify biliary malignancies which previously would have gone undetected. In spite of this, the rising incidence of intrahepatic CC has not been associated with an increased proportion of early stage or small size lesions[11-15].
Perihilar disease represents about 50%, distal disease 40% and intrahepatic disease less than 10% of CC cases. Age-adjusted rates of CC are reported to be the highest in Hispanic and Asian populations (2.8-3.3 per 100000 person/year) and lowest in non-Hispanic white people and black people (2.1 per 100000 person/year)[2].
RISK FACTORS
The main risk factors are considered primary sclerosing cholangitis (PSC) and choledochal cysts. The per-year cumulative risk of CC in patients with PSC is 1.5% after the development of jaundice and the prevalence of CC in patients with PSC ranges between 8% and 40%. A recent study from the Netherlands showed that the risk of CC for patients with PSC is 9% after 10 years from the time of the diagnosis[13]. However for the majority of patients a specific risk factor has not been identified. Recently, cirrhosis and viral hepatitis have also been proposed as potential risk factor, particularly for intrahepatic CCs[2]. Another risk factor for the development of CC are choledochal cysts (incidence of CC is between 10% and 20%), significantly reduced by early diagnosis and surgical ablation[15]. The carcinogenetic pathway is not clear although biliary stasis and reflux of pancreatic fluids are suspected through chronic inflammation way[1]. Unfortunately, CC can also occur years after resection of the cyst suggesting some genetic abnormality predisposing to the development of biliary neoplasia[16].
MANAGEMENT
CC have an remarkably poor five-year survival rate estimated from 5% to 10%. Some difference could be detected if survival is stratified by location of the lesion: the percentage of patients amenable of surgical resection is higher if the location is distal CCs compared to proximal (intrahepatic and perihilar) tumors. Nakeeb et al[17] published a large series about resectability rates for distal, intrahepatic, and perihilar lesions: 91%, 60%, and 56%, respectively[17]. Moreover patients who undergo a potentially curative resection, at pathology examination achieve tumor-free margins barely in 20% to 40% of proximal and 50% of distal location[18]. These percentage are even lower if a proximal tumor-free margin of at least 5 mm is requested as a curative criteria.
Surgery data for CCs have increased over year, largely owing to more aggressive surgery strategies and extended criteria for resectability.
Criteria for resectability of CC in the United States include[19]: (1) absence of retro-pancreatic and celiac nodal metastases or distant liver metastases[20]; (2) absence of portal vein or main hepatic artery involvement; (3) absence of extrahepatic adjacent organ invasion; (4) absence of spread disease; however, resectability is finally determined at surgical exploration, particularly with perihilar tumors[21]. Due to their location within the upper hepatoduodenal ligament, these tumors often extend into the liver and major vascular structures, and preoperative evaluation of resectability is often difficult. Thus, surgical exploration is indicated for proximal bile duct carcinomas whenever feasible.
Whether preoperative biliary decompression using an endoscopically or percutaneously placed stent should be carried out in patients who present with obstructive jaundice is still controversial and will be discussed below. Obstructive jaundice is the most common presenting symptom of CC. If biliary drainage is advantageous or not is still under debate. Cholestatic malabsorption, liver dysfunction, and biliary cirrhosis develop rapidly with unresolved obstruction and severe liver dysfunction is one of the main factors that increase postoperative morbidity and mortality following surgical resection[21].
The European Society of Gastrointestinal Endoscopy (ESGE) focused his attention on the treatment options in order to select the most appropriate procedure (with or without sphincterotomy) and stent choice (plastic or metal, short or long) on the basis of patient’s disease stage and tumor location.
ENDOSCOPIC TREATMENT IN PATIENTS ELIGIBLE FOR SURGERY
Preoperative biliary drainage was introduced to improve the postoperative outcome, for the reason that patients with jaundice had an increased risk of postoperative complications[10-22]. In various experimental studies and retrospective case series, preoperative biliary drainage reduced morbidity and mortality after surgery[23-25]. Nevertheless, two meta-analyses of randomized trials and a systematic review of descriptive series showed that the overall complication rate in patients undergoing preoperative biliary drainage was higher than in those who were referred straight to surgery[26]. In patients, fit for surgery for malignant common bile duct (CBD) obstruction, introduction of a plastic biliary stent followed by postponed surgery was associated with a higher morbidity compared with surgery within 1 wk. This was partly explained by complications associated with the biliary drainage procedure itself. Nevertheless, in many institution preoperative biliary drainage has been incorporated into the work-up of cancer of the pancreatic head or distal CBD[27]. In 2010 van der Gaag et al[10] conducted a large multicenter randomized trial in which 202 patients were randomized to receive whether preoperative biliary drainage followed by surgery within 4-6 wk, or surgery alone within 1 wk of diagnosis. Serious complications were registered in 39 percent in the immediate surgery group and 74 percent in the group with biliary drainage (RR = 0.54, P < 0.001)[28]. Neither mortality nor length of hospital stay were reduced in patients who underwent preoperative drainage. Moreover, the presence of a stent within the biliary tree could decrease the accuracy of diagnostic imaging to predict tumor resectability and the surgeon’s ability to determine the proximal tumor extent during intervention.
The ESGE recommends preoperative biliary drainage only in patients who will undergo neo-adjuvant therapies or in patients with biliary sepsis, or in patients with troublesome itching or predicted delay in surgical intervention[29-50].
How to achieve biliary drainage: endoscopically or via a percutaneous approach? Retrospective series and at least two prospective trials conducted in patients with obstructive jaundice from a malignant hilar obstruction (mainly proximal CCs or gallbladder cancer) suggest that successful palliation of jaundice is more likely and the incidence of post-procedure cholangitis may be lower with the percutaneous as compared to the endoscopic approach[31-33].
Endoscopic biliary drainage can be obtained using either plastic or self-expandable metal stents (SEMSs). Many stents (plastic and metal, both covered and uncovered), are available and both produce similar short-term results with respect to clinical success, morbidity, mortality, and improvement in quality of life[50]. A systematic review concluded that neither stent type offered a survival advantage[34]. Accordingly, in patient candidate for surgery the choice of stent should be guided by tumor location and extension.
The use of a plastic stent is inexpensive and effective, and the stent can be easily removed or replaced. Plastic stents, however, eventually develop occlusion by sludge and/or bacterial biofilm, and maintaining biliary drainage with plastic stents usually requires repeated endoscopic procedures. Plastic stents are available in multiple diameters ranging from 7 to 11.5 French, though 10 French stents are the most commonly used for distal common bile duct obstruction[35]. SEMSs provide a larger opening diameter than plastic one thus enabling prolonged patency and rapid biliary drainage[50]. However, the cost of metal stents is considerably higher and their removal may be challenging. The indications for using SEMSs in patients candidate to surgery is not well established yet. The main reason for the preferential use of plastic stents in patients with pancreatic cancer was the notion that uncovered SEMS could hinder pancreatoduodenectomy by interfering with transection of the bile duct proximal to the neoplasia[36]. With growing experience it has been shown that, when 2 cm or more of the common hepatic duct can be exposed proximally to the SEMS, the surgical procedure is not more complex than in the presence of a plastic stent[35].
Which kind of metal stent? SEMS models have been significantly developed and changed in the last decade: out of five types in use ten years ago, only single one is still available[29-37]. The distinguishing features of the various available SEMSs are prices, shortening ratio, radio-opacity, covering, radial force, flexibility, size of open cells of the mesh, anchoring mechanism and design of the tip[29-37]. In vitro measurements of radial expansion force and of flexibility have shown markedly different results between the various SEMSs, including covered and uncovered models of otherwise identical SEMSs[38]. The opening procedure shorten SEMSs by 0%-50%: different models with different shortening ratio are available. If the stricture is long and narrow the deployment could be difficult and irregular. Large open cells in the mesh may allow tissue to ingrow into the stent lumen, getting an inefficacious biliary drainage either immediately after the insertion or during follow-up[39-41]. Some special SEMS models, studied for hilar strictures, have a section with larger mesh cells in order to allow the introduction through the mesh of a new stent to reach another biliary branch[29]. In case of covered SEMSs, anti-migration mechanisms are particularly important: these may include flared ends or external fins, but some complications have been registered like bleeding of the bile duct wall caused by decubitus ulcers[42]. Recently models with soft ends and slip-knot to facilitate removal have been commercialized reducing the risk of bleeding or perforation if the wires are sharp and not fused.
ENDOSCOPIC TREATMENT IN PATIENTS WITH LOCALLY ADVANCED DISEASE
The long-term prognosis in CC patients who have undergone potentially curative surgical resection remains poor: these discouraging results have prompted interest in the use of neo-adjuvant therapy in patients amenable to surgery in order to improve survival. Such a strategy has also been proposed in locally advanced cases aiming to downstage the disease to allow surgical resection. This topic is valid for distal as well as for hilar CC. Recently in case of bilateral extension beyond the secondary radicles curative resection has been proposed after application of neoadjuvant therapy PDT oRFA (its applications and results will be discussed later).
The choice of the best stent to be used in this selected patient is less controversial than in those eligible for surgery. The efficacy of plastic stents is generally poor: more than one half of patients treated with plastic stents during neo-adjuvant therapy requires repeated stent replacement owing to stent occlusion or cholangitis[43]. Several studies have demonstrated that the use of SEMSs leads to improved outcome during neo-adjuvant therapy. Aadam et al[44] reported a 7 times higher complications rate and a 3 times higher hospitalization rate in patients treated with plastic stents as compared with patients treated with metal stents.
Uncovered and covered SEMSs are available. Uncovered SEMSs have a mesh design that allows them to be embedded in the biliary duct wall but it also makes them susceptible to tissue in-growth, which can lead to occlusion in as many as 20% of cases. Covered SEMSs were designed to prevent tissue in-growth but, as expected their use is associated with an increased rates of migration[45]. In an effort to guarantee patency and decrease rates of migration, partially covered SEMSs have been developed. In a recent meta-analysis, Saleem et al[46] concluded that covered SEMSs supply a significantly longer patency than uncovered SEMSs (average 60 d), but at the price of a higher migration rate[46-48]. Similar rates of cholecystitis were also found (approximately 2% in each group). Through subgroup analysis, Saleem et al[46] did not find any difference in rates of migration or stent patency comparing partially covered SEMSs to fully covered SEMSs. Contrastingly, in a retrospective cohort study analyzing the outcome of 749 patients by Lee et al[47] no difference in stent obstruction was found (covered SEMSs 35%, uncovered SEMSs 38%) . While obstruction due to tumor in-growth was more frequent in patients treated with uncovered SEMSs (76% vs 9%, P < 0.001), other mechanisms of obstruction occurred in patients treated with covered SEMSs, including sludge formation and food debris. Conversely, higher rates of migration (36% vs 2%, P < 0.001) and of acute pancreatitis (6% vs 1%, P < 0.001) were found in patients treated with covered SEMSs[47]. This study was retrospective and open, and follow-up was not standardized. In a recent study, Kitano et al[48] used a covered SEMS modified to reduce migration. The anti-migration characteristics consisted of low axial forces and uncovered flare ends, and was compared to uncovered SEMSs of similar design. One hundred and twenty patients were included in this prospective randomized multicenter study and the covered SEMS group had a substantial longer stent patency (mean of 219.3 d vs 166.9 d, P = 0.047) and less need for re-intervention (23% vs 37%, P = 0.08) compared to uncovered SEMSs. The tumor ingrowth was also lower in the covered SEMS group (0% vs 25%, P < 0.01)[47,48].
Even if a lower complication rate and a lower hospitalization staying has been described in patients with SEMS compared with plastic stents, the management of long standing metallic stent is challenging due to ingrowth of neoplastic tissue. Usually patients with positioned SEMS underwent neoadjuvant therapy to achieve a tumor downstaging and even if a 5-year survival rate is not influenced a prolonged survival is described and stent obstruction occurs frequently. Management of stent obstruction is challenging especially in hilar CC when previous bilateral SEMS have been positioned, due to the difficulties in bypassing the stent with the guidewire without enter the stent mesh. If not possible an option could be the ballon dilation of stent mesh.
ENDOSCOPIC TREATMENT IN PATIENTS WITH ADVANCED DISEASE
Placement of a stent is currently considered the treatment of choice for palliation of malignant obstructive jaundice in patients with advanced CC since it is associated with similar rates of jaundice relief and survival but less morbidity compared to the surgical approach[49-59]. Successful endoscopic deployment of a stent (or multiple stents as needed to span the malignant stricture) is possible in 70% to 100% of patients. Pre-procedure CT and/or MRI is often used in an attempt to identify the dominant biliary system in the event that only one side can be drained endoscopically.
Endoscopic stenting has been compared to the percutaneous approach. Retrospective series and trials conducted in patients with obstructive jaundice from a malignant hilar obstruction (mainly proximal CCs or gallbladder cancer) suggest that successful palliation of jaundice is more likely and rates of early cholangitis may be lower with the percutaneous as compared to the endoscopic approach[60,61]. However, other complications may be more frequent (e.g., bile leaks and bleeding), potentially increasing morbidity and mortality. Furthermore, percutaneous stents usually imply an open external drainage, at least initially, and this is often inconvenient to the patient. As a result, in most institutions an initial endoscopic attempt at drainage is usually preferred whenever possible.
Palliative endoscopic biliary decompression can be achieved using either plastic or SEMSs. In the last two decades, SEMSs have been increasingly used and have been demonstrated to be more effective than plastic stents allowing a more rapid biliary drainage and consequently a lower incidence of septic complications since the first procedure[51,52]. A systematic review concluded that none stent improves survival rate however uncovered metal stents have a lower risk of causing cholecystitis and pancreatitis and migration rate is significantly lower than in covered group[31].
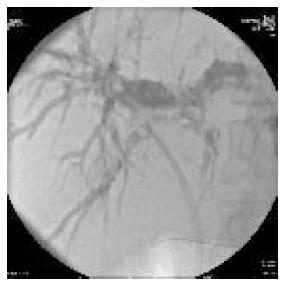
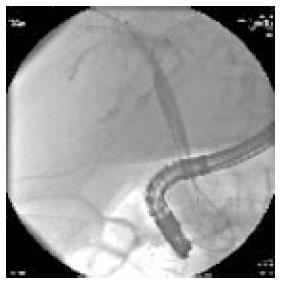
Whether to use unilateral or bilateral stents in patients with hilar obstruction is debated. The issue should be to drain as much as possible but this does always mean that you need to put a stent in every single duct. In many cases, unilateral stent placement will be sufficient to relieve jaundice and frequently, a dominant duct could be identified during ERC procedure, as the more effective to be drained (Figure 2)[32]. However, unilateral drainage alone may not relieve jaundice completely and may increase the risk of cholangitis especially if contrast medium have been injected and not drained. Studies comparing these approaches have reached variable and dubious conclusions. Many endoscopists place bilateral stents (plastic or metal); certainly a minimum of two stents (left and right branches) is need in an attempt to maximize biliary drainage (Figure 3). The choice to use more than two stents is linked to patient disease features and endoscopist skill.
Figure 2 Endoscopic retrograde cholangiography with a plastic stent in the right hepatic duct.
However the left hepatic duct remains dilated.
Figure 3 Use of covered self-expandable metal stent in patients with hylar cholangiocarcinoma.
RADIOFREQUENCY ABLATION
Radiofrequency ablation (RFA) has been used to treat liver malignancies since the early 1990s[61-65]. More recently this technique has been applied in malignant biliary strictures[62].
Habib TM Endo-HPB EMcision is an endoscopic bipolar catheter studied to be introduced through biliary malignant strictures, so that radiofrequency energy can be delivered locally before stent positioning. Potential advantages of the device use could be longer stent patency by ease down tumor growth. Endo-HPB is a 8 F, 1.8 m coaxial over the wire catheter that is designed to be inserted through a 3.2 mm working channel of the endoscope. At the distal end of the catheter, two ring electrodes spaced 8 mm apart produces a heating zone length of approximately 25 mm.
RFA in bile duct appears to be safe however its efficacy in long term and its role, alone or combined with SEMS is unclear. Sharaiha et al[64] recently compared RFA combined with SEMS with SEMS alone in 66 patients. Twenty-six were treated with RFA and SEMS and 40 only with stent placement. The author confirms a statistically significant improvement in malignant strictures diameter after RFA treatment[63-65]. Randomized controlled trials are needed.
ALTERNATIVE STENT DESIGN AND STRATEGIES
Recently Shah[65], proposed drug-eluting stents designed to improve SEMS patency by delivering a chemotherapeutic agent such as paclitaxel to prevent tumor in-growth and stent occlusion[66]. Unfortunately, in a multicenter prospective study comparing drug-eluting covered SEMSs with covered SEMSs no significant difference in stent patency was found[67].
PHOTODYNAMIC THERAPY AND DRUG ELUTING STENT
Photodynamic therapy (PDT) is a new palliative technique for malignant bile duct stenosis that seems to improve pain relief, increase biliary patency and increase survival.
Recently Bae et al[2] proposed a photosensitizer-embedded self-expanding metal stent (PDT-stent) which provides a photodynamic treatment without the need of systemic injection of photosensitizer and the treatment could be repeated more than one time due to the incorporation of the polymeric photosensitizer into the mesh of the stent. Photo-fluorescence imaging of the PDT-stent demonstrated homogeneous distribution of polymeric Pheo-A (PPA) on stent surface and the stent maintained its photodynamic power at least for 8 wk, for repeated PDT procedure if necessary after stent positioning. The PDT-stent after light exposure created cytotoxic free radical such as singlet oxygen in the close tissues, inducing destruction of neoplastic cells on animal models[66,67].
EUS GUIDED BILIARY DRAINAGE
Endoscopic biliary drainage with stent positioning is technically successful in > 90% of procedure. In the case of failure, endoscopic ultrasound (EUS)-guided biliary drainage has recently emerged as an effective alternative method providing technical success in > 80% of cases[49]. EUS-guided biliary drainage was first reported in 2001 by Giovannini et al[67] and can be approached into 3 different ways: (1) EUS-guided transluminal biliary drainage including choledoco-duodenostomy and hepatico-gastrostomy; (2) EUS-rendezvous technique; and (3) EUS-antegrade approach[67,68].
For EUS-guided transluminal biliary drainage, the biliary duct is punctured from the proximal duodenum with a 19 G fine needle aspiration (FNA) under EUS guidance followed by cholangiography. Progressively a guidewire is driven into the biliary system and dilation of the needle way is carried out. After fistula creation with a cystotome, or a bougie dilator, the stent is deployed between the biliary duct and the duodenal lumen for biliary drainage.
In EUS-rendezvous technique, the biliary duct is approached under EUS and X-ray guidance via 19 G FNA needle. Progressively, a guidewire is driven into the biliary system then through the bile duct, through the ampulla within the duodenum. After guidewire positioning, ERCP is performed using guidewire and the guidewire is retrieved, once biliary cannulation is carried out or the stenosis has been exceeded. Therefore, EUS- rendezvous technique is feasible only in patients in which the endoscopic access to the ampulla is preserved[68].
In EUS-anterograde approach, the intra-hepatic biliary duct is accessed from the small bowel with creation of a temporary fistula between the small bowel and the intra-hepatic biliary duct then the stent placement is achieved through the fistula. This technique is appropriate for patients with surgically altered anatomy or duodenal obstruction which prevent ampullary access.
Published studies regarding choledoco-duodenostomy and hepatico-gastrostomy show technical success rates of 94% and 87% with early complication rates of 19% and 27%, respectively, despite the fact that different biliary access and fistula dilation methods have been utilized. Regarding stent type, covered SEMS have generally been preferred over plastic stents, especially in more recent studies. Radial expansion of covered SEMSs can reduce risk of complications such as bile peritoneal leak or pneumoperitoneum because the fistula is immediately plugged by the covered SEMS. On the other hand, stent migration is reported after endoscopic procedure. For this purpose, the development of stents specifically designed for these procedures could further improve the results.
One of the most challenging aspects of EUS-rendezvous technique is the guidewire manipulation, which requires skill, tact sensitivity and good cooperation with a second operator[66]. Similarly, EUS-antegrade approach requires careful guidewire manipulation, however a major care is the risk of bile leak into the peritoneal cavity through the dilated fistula even if no report of biliary peritonitis have been issued, and the overall success and complication rates are 77% and 5 %, respectively[69-73].
NEW TECHNIQUES
Cholangioscopy
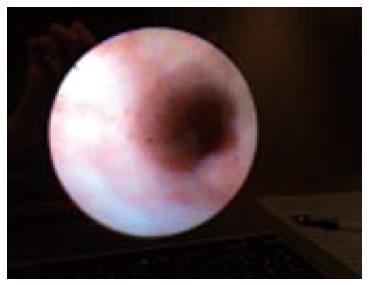
Peroral cholangioscopy, “mother-baby” technique, was utilized in the mid-1970s for the diagnosis and definition of bile duct narrowing. Nevertheless this technique revealed many limitations in visualization of the wall and required the cooperation of two skilled operators[74,75]. The “SpyGlass system” (Boston Scientific Corp, Natick, MA, United States) introduced in 2006 has enlarged the role of cholangioscopy from a diagnostic to a therapeutic one. The new system has overcome the need of two endoscopists and it has been launched as a single endoscopist cholangioscope. It allows the direct visualization of biliary tree (Figure 4) and consequently its use in the diagnostic work up of CC is well established. Sethi et al[74] reported a diagnostic accuracy of SpyGlass around 57% and these data were confirmed also in other series with an overall diagnostic accuracy, in differentiating neoplastic vs non neoplastic lesions, varied from 77% to 90%[75-79]. Although it is considered limitative to banish a cholangioscope to a diagnostic role in CC work up, more data are needed about its role in therapeutic endoscopy and biliary drainage. One of the main indications is the lithotripsy for difficult to remove, biliary stones[80]. Recently Dong Choon Kim described the use of an ultraslim endoscope (GIF-XP260N; Olympus, Tokyo, Japan) for intraductal stones fragmentation under endoscopic visualization[81].
Figure 4 Visualization of biliary epithelium during SpyGlass.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) assesses the presence of chromosomal aberrations, in number or structures, and uses fluorescence-labeled probes to evaluate increases or decreases in chromosome number if referred to numerical abnormalities or to specific structural abnormalities in case of clonal diversity[82,83]. This technique is performed on ERC brushing smears.
Previous studies have demonstrated that FISH polysomy combined with cytology improves sensitivity. Some studies have considered the positive FISH results based on polysomy only, whereas some have considered trisomy or tetrasomy as a positive test results as well. Recently a review was published by Navaneethan et al[82] with a pooled sensitivity and specificity was 51% and 93% in detection of CC in patients with PSC. Vasilieva et al[83] in 2013 published data about the use of structural abnormalities as markers of clonal diversity and different clinical features of the disease. However more data are needed, the use of fish does not increase sensitivity significantly. A future role of the FISH will be the possibility to delineate the oncogenesis, to understand the response or not to chemotherapy[83,84].
CONCLUSION
Cholangiocarcinoma and bile duct tumors are an heterogeneous group of tumor with different biological behavior and prognosis according to their location and growth pattern. CC presents a special challenge in gastroenterology, oncology, and visceral surgery because of the difficulty in establishing the diagnosis, local complications in the biliary pathways, and a high recurrence rate after resection. Diagnosis is usually defined in advanced disease stage, due to paucisintomaticity of tumor and to low sensitivity of imaging technique for detection of lesions at early stage. The only curative treatment for CC is surgery, but 40%-85% of all patients have recurrent disease even after radical excision. Because of this high recurrence rate and because the majority of patients undergo palliative therapy (chemotherapy or endoscopic therapy) to try to downstage the tumor and adjuvant treatments are now under intense discussion. Moreover because of the low prevalence of the disease, there have been only a few studies of palliative chemotherapy for CC. On the basis of one positive phase 3 study, chemotherapy with gemcitabine and cisplatin is considered the standard and now plays an established role in palliative care[84].
Endoscopy, as explained in this review has gained in the last decades a key role in the work up of CC, both in patients amenable to surgical intervention as well as in those unfit for surgery or not amenable to immediate surgical curative resection owing to locally advanced disease. Endoscopy allows successful biliary drainage and stenting in more than 90% of cases. The development of new stents, metallic, covered, with different mesh materials, different mesh shape is a constant work in progress to reduce complications in patients with advanced disease, to avoid repeated endoscopic procedure and to improve long term results. Moreover in the last two years new stent prototype able to release drugs and/or photodynamic therapy have been commercialized with promising results but very few data are available, not enough to be validated. When endoscopy fails, endoscopic ultrasound-guided biliary drainage represents an effective alternative method affording successful biliary drainage in more than 80% of cases. Also in this field new dedicated stents fit for trans-duodenal biliary drainage or trans-hepatic biliary drainage are under construction.
This a new field that need constant updating and future studies should address the efficacy of combined local and systemic treatments.
In conclusion the final messages are: (1) The benefit of adjuvant chemotherapy has not yet been confirmed and require further investigation; and (2) Endoscopic biliary drainage by means of ERC is an integral component of the treatment of CC.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Cheon YK, Kouraklis G, Mais V, Skok P, Vynios D, Vasilieva LE S- Editor: Ji FF L- Editor: A E- Editor: Wu HL