Published online Sep 10, 2015. doi: 10.4253/wjge.v7.i12.1055
Peer-review started: May 1, 2015
First decision: June 1, 2015
Revised: June 18, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 10, 2015
Processing time: 136 Days and 17.2 Hours
Various procedure-related adverse events related to colonoscopic treatment have been reported. Previous studies on the complications of colonoscopic treatment have focused primarily on perforation or bleeding. Coagulation syndrome (CS), which is synonymous with transmural burn syndrome following endoscopic treatment, is another typical adverse event. CS is the result of electrocoagulation injury to the bowel wall that induces a transmural burn and localized peritonitis resulting in serosal inflammation. CS occurs after polypectomy, endoscopic mucosal resection (EMR), and even endoscopic submucosal dissection (ESD). The occurrence of CS after polypectomy or EMR varies according previous reports; most report an occurrence rate around 1%. However, artificial ulcers after ESD are largely theoretical, and CS following ESD was reported in about 9% of cases, which is higher than that for CS after polypectomy or EMR. Most cases of post-polypectomy syndrome (PPS) have an excellent prognosis, and they are managed conservatively with medical therapy. PPS rarely develops into delayed perforation. Delayed perforation is a severe adverse event that often requires emergency surgery. Since few studies have reported on CS and delayed perforation associated with CS, we focused on CS after colonoscopic treatments in this review. Clinicians should consider delayed perforation in CS patients.
Core tip: Few studies have reported on coagulation syndrome (CS) and delayed perforation associated with CS. Thus, in this review, we focused on CS after colonoscopic treatments. CS is found in around 1% of cases after polypectomy and endoscopic mucosal resection and in 7%-8% of cases after endoscopic submucosal dissection. The prognosis for CS is excellent. However, clinicians should be mindful of delayed perforation in CS patients.
- Citation: Hirasawa K, Sato C, Makazu M, Kaneko H, Kobayashi R, Kokawa A, Maeda S. Coagulation syndrome: Delayed perforation after colorectal endoscopic treatments. World J Gastrointest Endosc 2015; 7(12): 1055-1061
- URL: https://www.wjgnet.com/1948-5190/full/v7/i12/1055.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i12.1055
Various endoscopic treatments such as polypectomy, endoscopic mucosal resection (EMR), and endoscopic submucosal dissection (ESD) have been used to treat colorectal neoplasms[1-14]. Colonoscopic polypectomy reduces the incidence of colorectal cancer by 70%-80%, and it has been used worldwide[1,2]. EMR is indicated for the treatment of colorectal adenomas, intramucosal, and submucosal superficial cancers (SM1; invasion of < 1000 μm from the muscularis mucosae) because of the negligible risk of lymph-node metastasis and excellent clinical outcomes; however, there is a limit to the size of en bloc resection[3-11].
Recently, ESD is another procedure used to remove large colorectal lesions according to the EMR curative criteria. This procedure is frequently used for removing large lesions by en bloc fashion, which includes lesions that would require piecemeal EMR for removal[11-14].
With respect to these aforementioned procedures, various procedure-related adverse events have been reported. Serious complications included delayed bleeding and perforation. Previous studies on the complications of colonoscopic polypectomy or EMR have primarily focused on perforation or bleeding[15-17]. Coagulation syndrome (CS) following endoscopic treatment, which was first reported as post-polypectomy syndrome (PPS)[18], is another typical complication. This has syndrome has a variety of names, including post-polypectomy CS, post-polypectomy electrocoagulation syndrome, and transmural burn syndrome[17-33]. Recognizing CS is important to avoid unnecessary exploratory laparotomy, because the syndrome can resolve with conservative treatment in most patients[19,21,23,26,30,31]. However, little is known about the clinical characteristics, clinical outcomes, and risk factors associated with CS, and the frequency of CS varies in previous studies[17-33]. Additionally, CS occurs after polypectomy, EMR, and even ESD[26,34]. With the evolution of treatment, the occurrence of this syndrome is thought to increase. Furthermore, there is a possibility that CS causes delayed perforation, which is a very severe complication[25,26,33,35-42]. In this review, we clarify the present status of CS following endoscopic treatment for colorectal neoplasms.
Most previous studies have investigated CS after polypectomy and EMR. Thus, in this review, we defined PPS as CS associated with only polypectomy and EMR, while CS included ESD.
CS is the result of an electrocoagulation injury to the bowel wall that induces a transmural burn and localized peritonitis resulting in serosal inflammation[17-33]. Patients with CS are diagnosed when they present with abdominal pain (sometimes tenderness with rebound); fever; leukocytosis; an elevated C-reactive protein level; or peritoneal irritation symptoms and signs that occur after colonoscopic treatment (polypectomy, EMR, and ESD) with electrocoagulation, in the absence of visualized perforation by abdominal radiography and/or computed tomography (CT)[26,34]. It is important to recognize that CS can be misleading, as it can resemble a true rupture of the colon and present with pain, a low fever, and mild leukocytosis. Typically, patients with CS present within a few hours to 7 d after colonoscopic treatment with fever, localized abdominal pain, and localized peritoneal signs[19,20,26,30]. It is important to recognize this condition, because it does not require surgical treatment in most cases[19,21,23,26,30,31]. There is a range in severity of PPS between admission to the intensive care unit and post-discharge, as it can lead to shock, additional surgery, or death from possible follow-up on an outpatient basis.
The occurrence rate of PPS varies widely from 0%-7.6% in previous reports; however, most studies report a rate around 1%. It is considered some reports had high percentages of occurrence due to small patient populations[18-21,23-26,28-33].
Some previous reports have investigated the risk factors of PPS Nivatvongs[18] showed that 83% of PPS patients had polyps in the right side of the colon, and all were sessile polyps. Choo et al[24] also showed that right-colon polypectomies had a statistically significantly higher tendency for developing PPS[24]. Lee et al[20] reported that a polyp size > 2 cm (OR = 1.08) and hypertension (OR = 14.40) were associated with a significantly increased risk of PPS. The most recent report showed that hypertension, a large lesion size, and non-polypoid configuration of the lesion were independently associated with PPS according to multivariate analysis[19].
PPS develops when the electrical current applied during colonoscopic polypectomy extends past the mucosa into the muscularis propria and serosa, resulting in a transmural burn without perforation[17-33]. Therefore, larger lesions and non-polypoid configuration are logical risk factors, as they usually require a large amount of thermal energy for a longer duration. However, the mechanism of hypertension to promote PPS is unclear. Patients with hypertension are more likely to have endothelial dysfunction[43] and atherosclerosis[44,45], which may be contributing factors.
However, with thinness of the wall, there is also concern regarding the frequency of PPS. The right colon wall is thin, and a large study that addressed major post-polypectomy complications reported barotraumatic perforations, and all of them were caused by cecal blow-out[34,46-48]. Regarding colonic perforation, it has been suggested that air insufflation during colonoscopy generates a higher pressure in the cecum than in the rest of the colon, increasing vulnerability to injury. In addition, Rutter et al[49] hypothesized that a more perpendicular approach to polypectomy in the cecum may increase the risk of complications. However, scientific evidence in support of these theories is lacking.
Loffeld et al[47] also reported that barotrauma caused by insufflated air occurs more often than therapeutic perforation due to polypectomy or coagulation.
Theoretically, submucosal saline injections of large, non-polypoid lesions prior to EMR may reduce the risk of PPS. The rationale for this is that a submucosal saline injection may increase the thickness of the submucosal layer and consequently reduce the risk of PPS[32]. However, no studies have supported this assumption. Sethi et al[17] hypothesized that submucosal injection itself leads to serosal irritation and localized peritonitis, and then patients present with PPS symptoms. Therefore, the protective role of the saline “cushion” for PPS should be considered in future studies.
The improvement of devices would likely reduce PPS. Galloro et al[50] reported that steel snares induced significantly deeper tissue injury than tungsten snares in the pure cut mode; therefore, tungsten snares may reduce the risk of PPS[32]. Another way to reduce the risk of PPS is dependent on skill. Using lower risk procedures when clinically appropriate or referring patients to high-volume endoscopists can reduce the complication rates[51].
PPS is considered different from infection from a local mucosal defect. Min et al[52] reported that blood cultures at baseline and 5 min after the procedure were all negative, and a blood culture at 30 min after the procedure showed a positive result in only 1 of 40 patients (2.5%). However, this one positive sample was considered contamination. None of the 40 patients showed any signs or symptoms associated with infection. Therefore, the prior administration of antibiotics is considered controversial for preventing PPS.
Most cases of PPS have an excellent prognosis, and they are managed conservatively with medical therapy. In some reports, all patients were admitted to the hospital, while in other reports, some cases underwent outpatient observation[19,21,23,26,30,31]. Treatment of PPS requires bowel rest and the administration of intravenous fluids and broad-spectrum parenteral antibiotics to cover the colonic bacterial flora. Nothing is taken by mouth until the symptoms subside. Patients with mild symptoms and adequate outpatient follow-up can be managed with oral antibiotics and a clear liquid diet for 1-2 d.
In contrast, to diffuse peritoneal signs, there is an indication for immediate surgical intervention. Within the spectrum of post-polypectomy cautery injury, “mini-perforation” falls between a “serosal burn” and frank perforation (with diffuse peritonitis). It is a minimal defect that can be quickly covered by peri-intestinal fat and omentum[16]. Its clinical features include pneumoperitoneum without signs and symptoms of diffuse or spreading peritonitis, and with local tenderness that is characteristic of a full-thickness burn. The patient usually improves within 24 h, and the symptoms should resolve within 96 h with conservative treatment. The dilemma as to whether the conservative or surgical approach is more appropriate for managing this kind of perforation still exists[19-22,26,30,31].
Although conservative treatment can generally be performed in most patients, it is important to adopt careful measures such as prolonging the fasting period and considering the possibility of delayed perforation[26,35-39].
Immediate perforation is diagnosed by endoscopy during resection and by the presence of free air on plain abdominal film or abdominal CT scan[15-17,35,51,53]. This is very rare; however, delayed perforation, which is considered to be caused by an electrical or thermal injury after electrocoagulation, was reported in these cases. Delayed perforation after colonoscopic resection can begin as PPS, which can evolve into a perforation or as a free perforation with air and fluid leakage, resulting in pneumoperitoneum and peritonitis[35-39].
Japan Gastroenterological Endoscopy Society guidelines for colorectal ESD/EMR defined delayed perforation as an intestinal perforation that develops over a certain period postoperatively (i.e., intestinal perforation that is detected after the scope has been withdrawn following completion of ESD/EMR during which perforation did not occur). This is diagnosed based on abdominal pain, abdominal findings, the presence of a fever, and an inflammatory response that is consistent with PPS. Most cases of delayed perforation occur within 14 h after endoscopic resection. However, approximately one-third of delayed perforation cases are confirmed within 24 h after treatment. Free air, which cannot be detected by simple radiographic imaging, is sometimes found on abdominal CT. Therefore, in cases where delayed perforation is suspected, abdominal CT should be performed. Surgeons must be called for emergency surgery, because it is essential in cases of delayed perforation[26].
In 1994, Lo et al[54] reported that 43.8% of therapeutic perforations were managed conservatively with a mortality rate of 4.1%. This means that perforation is still a severe condition that reduces patients’ quality of life[25,35-39]. Thus, prevention of PPS and its potential sequelae are most important, and clinicians must always consider the potential for delayed perforation due to PPS.
Only two studies have reported on the incidence of delayed perforation. Taku et al[39] reported delayed perforation in 7 of 15070 cases, while Waye et al[25] reported it in 1 of 777 cases. This is still not sufficient evidence. For ESD, the incidence of delayed perforation ranges from 0.1% to 0.4%[26,40-42].
ESD has been a reliable method for en bloc resection of colorectal tumors regardless of the lesion size for years. Although colorectal ESD has been established as a procedure with reproducible safety and efficacy, complications such as intestinal perforation and delayed bleeding remain to be problematic. Similarly, few studies have reported CS after ESD[11-14,26].
Hong et al[48] reported that 8.6% showed CS after colorectal ESD. There were no differences in the demographic and endoscopic characteristics (age, sex, underlying disease, procedure time, tumor size, macroscopic type, location, and pathologic findings) between patients with CS and those without CS. The mean hospitalization stay was statically significantly longer in the CS group than that in the non-CS group. All patients with CS were treated with conservative (non-surgical) management (e.g., fasting and intravenous antibiotics). CS showed a favorable progression even after ESD, and delayed perforation was not reported. See comment in pubmed commons below.
CS is reported even after ESD, and its frequency is clearly higher than polypectomy or EMR[26,34,48]. The procedure time and ulcer bed to energization that largely affects the characteristics of ESD procedures is evident theoretically. Delayed perforation in ESD is also a great concern. The indications for ESD are markedly different from those for conventional EMR, and the overall perforation rate is higher compared to conventional EMR[55]. Delayed perforation after ESD reportedly ranges from about 0.1%-0.4%; however, this may be because of the small number of reports[26,40-42,55].
Saito et al[41] reported that delayed perforations occurred in another 4 patients (0.4%) after ESD. Two of the 4 patients with delayed perforations were successfully treated conservatively, because the abdominal findings and inflammatory changes based on laboratory data were slight. However, other patients with delayed perforation required emergency surgery because of the risk of peritonitis. Saito et al[41] also reported that 0.11% (1/900) showed delayed perforation that required emergency surgery. Previous studies have cautioned that clinicians must carefully follow patients with delayed perforation, and continually close communication with consulting surgeons is essential since the number of such cases has been quite limited to date.
Few studies have reported on delayed perforation after ESD. While previous reports have shown the success of endoscopic clip closure with over-tube[42], the treatment and prognosis often require emergent surgery.
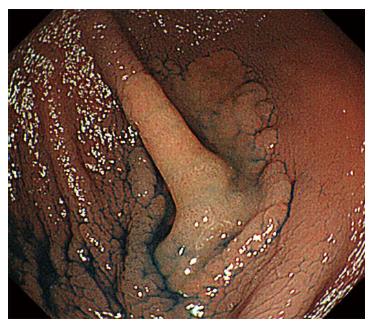
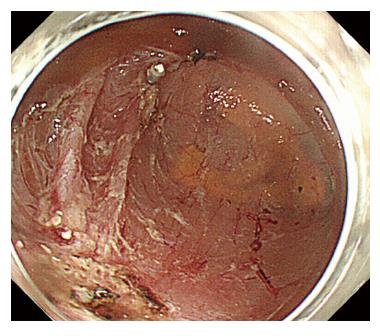
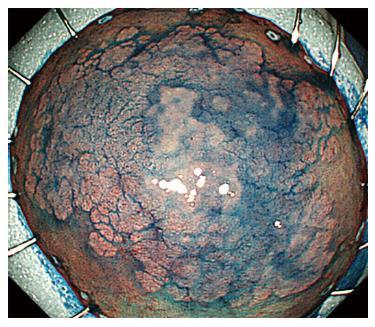
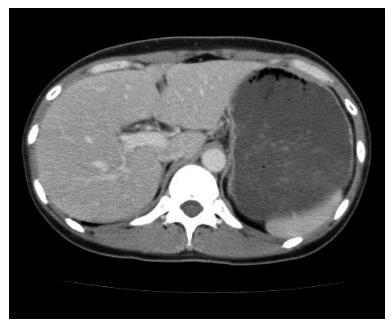
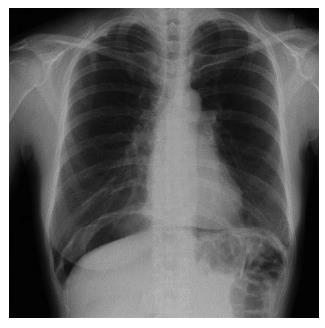
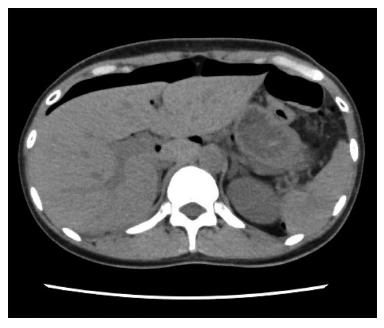
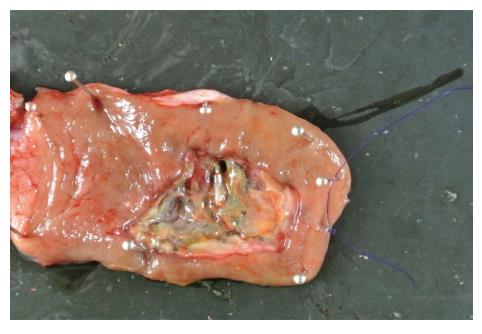
A 44-year-old woman underwent colonoscopy for surveillance of ulcerative colitis, and a 30 mm cecal sessile polyp was revealed (Figure 1). We diagnosed this tumor as a sessile serrated adenoma/polyp using the pit and narrow-band imaging patterns. Because of the size of the tumor and the tumor morphology, we chose ESD in order to perform en-bloc resection. ESD was performed safely without any perioperative complications (Figures 2 and 3), and she reported no symptoms. However, 24 h after ESD, she had a high fever (38.6 °C) with slight abdominal pain and leukocytosis. Subsequently, she was diagnosed with CS after ESD. She fasted and received antibiotics (cefmetazole). CT was obtained immediately, but no findings were suggestive of perforation (i.e., free air and ascites were not present) (Figure 4). Thirty hours after ESD, severe abdominal pain developed, and 36 h after ESD, free air appeared on radiography and CT (Figures 5 and 6). At this point, we diagnosed the patient with delayed perforation that developed after CS. Emergent laparoscopic surgery was performed, and a perforation site was found in the ESD ulcer at the bottom of the cecum (Figure 7). Partial cecum resection was performed, and the patient’s condition improved rapidly.
CS is found in around 1% of cases after polypectomy and EMR and in 7%-8% of cases after ESD. Although the prognosis is excellent, clinicians should consider delayed perforation in CS patients.
| 1. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3173] [Article Influence: 96.2] [Reference Citation Analysis (1)] |
| 2. | Webb WA, McDaniel L, Jones L. Experience with 1000 colonoscopic polypectomies. Ann Surg. 1985;201:626-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Yokota T, Sugihara K, Yoshida S. Endoscopic mucosal resection for colorectal neoplastic lesions. Dis Colon Rectum. 1994;37:1108-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 6. | Saito Y, Fujii T, Kondo H, Mukai H, Yokota T, Kozu T, Saito D. Endoscopic treatment for laterally spreading tumors in the colon. Endoscopy. 2001;33:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Heldwein W, Dollhopf M, Rösch T, Meining A, Schmidtsdorff G, Hasford J, Hermanek P, Burlefinger R, Birkner B, Schmitt W. The Munich Polypectomy Study (MUPS): prospective analysis of complications and risk factors in 4000 colonic snare polypectomies. Endoscopy. 2005;37:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 214] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 8. | Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 9. | Watabe H, Yamaji Y, Okamoto M, Kondo S, Ohta M, Ikenoue T, Kato J, Togo G, Matsumura M, Yoshida H. Risk assessment for delayed hemorrhagic complication of colonic polypectomy: polyp-related factors and patient-related factors. Gastrointest Endosc. 2006;64:73-78. [PubMed] |
| 10. | Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2015;pii:gutjnl-2014-308481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 11. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 185] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Uraoka T, Parra-Blanco A, Yahagi N. Colorectal endoscopic submucosal dissection: is it suitable in western countries? J Gastroenterol Hepatol. 2013;28:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 13. | Yoshida N, Yagi N, Naito Y, Yoshikawa T. Safe procedure in endoscopic submucosal dissection for colorectal tumors focused on preventing complications. World J Gastroenterol. 2010;16:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Shiga H, Endo K, Kuroha M, Kakuta Y, Takahashi S, Kinouchi Y, Shimosegawa T. Endoscopic submucosal dissection for colorectal neoplasia during the clinical learning curve. Surg Endosc. 2014;28:2120-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, Ransohoff DF. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849-857, W152. [PubMed] |
| 16. | Christie JP, Marrazzo J. “Mini-perforation” of the colon--not all postpolypectomy perforations require laparotomy. Dis Colon Rectum. 1991;34:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 100] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Sethi A, Song LM. Adverse events related to colonic endoscopic mucosal resection and polypectomy. Gastrointest Endosc Clin N Am. 2015;25:55-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Nivatvongs S. Complications in colonoscopic polypectomy. An experience with 1,555 polypectomies. Dis Colon Rectum. 1986;29:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 89] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Cha JM, Lim KS, Lee SH, Joo YE, Hong SP, Kim TI, Kim HG, Park DI, Kim SE, Yang DH. Clinical outcomes and risk factors of post-polypectomy coagulation syndrome: a multicenter, retrospective, case-control study. Endoscopy. 2013;45:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Lee SH, Kim KJ, Yang DH, Jeong KW, Ye BD, Byeon JS, Myung SJ, Yang SK, Kim JH. Postpolypectomy Fever, a rare adverse event of polypectomy: nested case-control study. Clin Endosc. 2014;47:236-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Anderloni A, Jovani M, Hassan C, Repici A. Advances, problems, and complications of polypectomy. Clin Exp Gastroenterol. 2014;7:285-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Raju GS, Saito Y, Matsuda T, Kaltenbach T, Soetikno R. Endoscopic management of colonoscopic perforations (with videos). Gastrointest Endosc. 2011;74:1380-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Benson BC, Myers JJ, Laczek JT. Postpolypectomy electrocoagulation syndrome: a mimicker of colonic perforation. Case Rep Emerg Med. 2013;2013:687931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Choo WK, Subhani J. Complication rates of colonic polypectomy in relation to polyp characteristics and techniques: a district hospital experience. J Interv Gastroenterol. 2012;2:8-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Waye JD, Lewis BS, Yessayan S. Colonoscopy: a prospective report of complications. J Clin Gastroenterol. 1992;15:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 237] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 26. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 456] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 27. | Fisher DA, Maple JT, Ben-Menachem T, Cash BD, Decker GA, Early DS, Evans JA, Fanelli RD, Fukami N, Hwang JH. Complications of colonoscopy. Gastrointest Endosc. 2011;74:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 28. | Parra-Blanco A, Kaminaga N, Kojima T, Endo Y, Tajiri A, Fujita R. Colonoscopic polypectomy with cutting current: is it safe? Gastrointest Endosc. 2000;51:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Chan AO, Lee LN, Chan AC, Ho WN, Chan QW, Lau S, Chan JW. Predictive factors for colonoscopy complications. Hong Kong Med J. 2015;21:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 30. | Mönkemüller K, Neumann H, Malfertheiner P, Fry LC. Advanced colon polypectomy. Clin Gastroenterol Hepatol. 2009;7:641-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 31. | Luigiano C, Consolo P, Scaffidi MG, Strangio G, Giacobbe G, Alibrandi A, Pallio S, Tortora A, Melita G, Familiari L. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy. 2009;41:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Ferrara F, Luigiano C, Ghersi S, Fabbri C, Bassi M, Landi P, Polifemo AM, Billi P, Cennamo V, Consolo P. Efficacy, safety and outcomes of ‘inject and cut’ endoscopic mucosal resection for large sessile and flat colorectal polyps. Digestion. 2010;82:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004;60:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Toyonaga T, Man-i M, Fujita T, East JE, Nishino E, Ono W, Morita Y, Sanuki T, Yoshida M, Kutsumi H. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for laterally spreading tumors of the colorectum. Endoscopy. 2010;42:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Kang HY, Kang HW, Kim SG, Kim JS, Park KJ, Jung HC, Song IS. Incidence and management of colonoscopic perforations in Korea. Digestion. 2008;78:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Sagawa T, Kakizaki S, Iizuka H, Onozato Y, Sohara N, Okamura S, Mori M. Analysis of colonoscopic perforations at a local clinic and a tertiary hospital. World J Gastroenterol. 2012;18:4898-4904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Macrae FA, Tan KG, Williams CB. Towards safer colonoscopy: a report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut. 1983;24:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 316] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 38. | Panteris V, Haringsma J, Kuipers EJ. Colonoscopy perforation rate, mechanisms and outcome: from diagnostic to therapeutic colonoscopy. Endoscopy. 2009;41:941-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 39. | Taku K, Sano Y, Fu KI, Saito Y, Matsuda T, Uraoka T, Yoshino T, Yamaguchi Y, Fujita M, Hattori S. Iatrogenic perforation associated with therapeutic colonoscopy: a multicenter study in Japan. J Gastroenterol Hepatol. 2007;22:1409-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 40. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 609] [Article Influence: 38.1] [Reference Citation Analysis (5)] |
| 41. | Saito Y, Yamada M, So E, Abe S, Sakamoto T, Nakajima T, Otake Y, Ono A, Matsuda T. Colorectal endoscopic submucosal dissection: Technical advantages compared to endoscopic mucosal resection and minimally invasive surgery. Dig Endosc. 2014;26 Suppl 1:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Xiao YF, Bai JY, Yu J, Lin XL, Zhao XY, Yang SM, Fan CQ. Endoscopic treatment of delayed colon perforation: the enteroscopy overtube approach. Endoscopy. 2014;46:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Egashira K, Inou T, Hirooka Y, Yamada A, Maruoka Y, Kai H, Sugimachi M, Suzuki S, Takeshita A. Impaired coronary blood flow response to acetylcholine in patients with coronary risk factors and proximal atherosclerotic lesions. J Clin Invest. 1993;91:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 269] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 44. | Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986;314:488-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3689] [Cited by in RCA: 3408] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 45. | MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, Abbott R, Godwin J, Dyer A, Stamler J. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2832] [Cited by in RCA: 2757] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 46. | Gimeno-García AZ, Quintero E. Post-polypectomy complications: high risk in the cecum. Endoscopy. 2014;46:88-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | Loffeld RJ, Engel A, Dekkers PE. Incidence and causes of colonoscopic perforations: a single-center case series. Endoscopy. 2011;43:240-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Hong MJ, Kim JH, Lee SY, Sung IK, Park HS, Shim CS. Prevalence and clinical features of coagulation syndrome after endoscopic submucosal dissection for colorectal neoplasms. Dig Dis Sci. 2015;60:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 49. | Rutter MD, Nickerson C, Rees CJ, Patnick J, Blanks RG. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy. 2014;46:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 50. | Galloro G, Magno L, Ruggiero S, Iovino P, Formisano C, Cortese L, Fusco F, Meola C, Carlomagno GM. Comparison between tungsten and steel polypectomy snares: evaluation of depth of colonic thermal wall injury in a pig model. Endoscopy. 2013;45:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Chukmaitov A, Bradley CJ, Dahman B, Siangphoe U, Warren JL, Klabunde CN. Association of polypectomy techniques, endoscopist volume, and facility type with colonoscopy complications. Gastrointest Endosc. 2013;77:436-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 52. | Min BH, Chang DK, Kim DU, Kim YH, Rhee PL, Kim JJ, Rhee JC. Low frequency of bacteremia after an endoscopic resection for large colorectal tumors in spite of extensive submucosal exposure. Gastrointest Endosc. 2008;68:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Lüning TH, Keemers-Gels ME, Barendregt WB, Tan AC, Rosman C. Colonoscopic perforations: a review of 30,366 patients. Surg Endosc. 2007;21:994-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 54. | Lo AY, Beaton HL. Selective management of colonoscopic perforations. J Am Coll Surg. 1994;179:333-337. [PubMed] |
| 55. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Kozarek R S- Editor: Qiu S L- Editor: A E- Editor: Jiao XK