INTRODUCTION
Colorectal cancer is the third most common cancer in men and the second in women. Worldwide, an estimated 1.2 million cases of colorectal cancer occur annually[1]. The highest incidence rates have previously been in North America, Australia, New Zealand, Europe, and Japan. In recent years some of these incidences has stabilised and even began to reduce, e.g., United States and this may, in some part, be related to the introduction of national screening programmes (Figure 1).
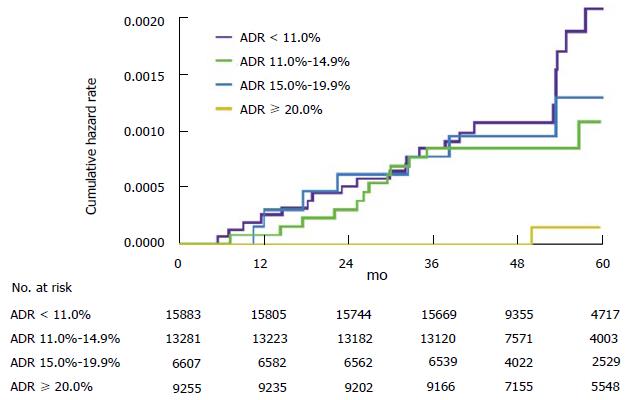
Figure 1 Cumulative hazard rates for interval colorectal cancer, according to the endoscopists adenoma detection rate.
The graph shows cumulative hazard rates for interval colorectal cancer among subjects who underwent screening colonoscopy that was performed by an endoscopist with an ADR in one of the following categories: less than 11.0%, 11.0% to 14.9%, 15.0% to 19.9%, and 20.0% or more. ADR: Adenoma detection rate.
Worldwide, colonoscopy forms the basis of colorectal cancer screening programmes and has been shown to reduce the risk of death from colorectal cancer through detection of tumours at an earlier, more treatable stage and through removal of precancerous adenomas[2]. There are a number of quality assurance measures for colonoscopy in screening programmes include caecal intubation rate, bowel preparation quality, complications, cancer detection and adenoma detection rate (ADR, the proportion of colonoscopies performed by a physician that detect at least one histologically confirmed colorectal adenoma). However, ADR is now established as the most important quality indicator due to 2 landmark studies. The first demonstrated increased risk of interval cancer when the colonoscopy is performed by an endoscopist with a ADR below 20%[3]. As a result professional societies recommend a detection rate of > 25% in order to be deemed adequate[4]. The second demonstrated an inverse relationship between ADR and the risks of interval colorectal cancer, advanced-stage interval cancer, and fatal interval cancer. With each 1.0% increase in ADR was associated with a 3.0% decrease in the risk of cancer[2].
There are a number of techniques and technologies, both established and emerging that provide an exciting and promising potential to improve ADR. This article will discuss effective technique and behaviours, developments in image enhancement, advancement in endoscope design and developments in accessories that may improve ADR.
EFFECTIVE TECHNIQUE AND BEHAVIOURS
Bowel preparation
Good bowel preparation is vital for effective lesion recognition at colonoscopy. Consequently, guidance from the United States multi-society task force for colorectal cancer recently published strong recommendations for adequate bowel preparation with split-dose regimes in order to optimise ADR[5]. Poor bowel preparation has been associated with a adenoma miss rate of 43%[6]. Studies have demonstrated a clear improvement in ADR (35%) with split dose preparation (P≤ 0.001). They also showed a clear improvement in caecal intubation rate (95.5%) and preparation quality[7]. Attempts to implement further measures to improve bowel preparation have also been studied. One such scheme studied telephone education relating to the bowel preparation prior to colonoscopy. There was a improvement in compliance, preparation quality and ADR[8].
Insertion and withdrawal polypectomy
Colonic configuration during insertion phase and withdrawal phase is different and some polyps seen during insertion are difficult to find during withdrawal and vice versa[9]. It is typical practice to perform the formal mucosal examination and polypectomy on withdrawal, noting any pathology on insertion for subsequent intervention. One study suggested this may not be preferable, finding that polyp < 10 mm identified during insertion are frequently missed on withdrawal, suggesting polypectomy during insertion[10]. A more recent study compared 610 colonoscopies where patients were randomised to either polypectomy during insertion and withdrawal or just withdrawal. In both arms, mean number of adenomas detected per patient were similar. With the only significant difference being that of insertion time[9]. Overall, the evidence suggests neither technique is superior over the other.
Retroflexion in the caecum
Rectal retroflexion forms part of the required standards for colonoscopy completion. Retroflexion in the right colon is not routinely performed but has been reported to improve ADR. A prospective cohort study conducted in the United States examined the potential impact of caecal retroflexion on ADR. One thousand consecutive adults undergoing colonoscopy were studied. A standard forward viewing colonoscopy of the right colon was performed and polyps were removed. There was then repeated examination in retroflexion from the caecum to the hepatic flexure. Retroflexion was successful in 94.4% of the patients. The subsequent examination in retroflexion demonstrated a 9.3% miss rate for the forward viewing method[11]. However, safety concerns have been raised due to the risk of perforation of using this technique.
Dynamic position change
Randomised controlled trials examining dynamic position changes have produced conflicting results regarding ADR, but predominate positive findings. It is clear that position change aids caecal intubation rate and patient comfort. Such position changes result in better distension with less insufflation of air, shifting of fluids and residues, and opening tight angles at flexures[12]. Specifically during withdrawal, such position changes have repeatedly been shown to improve ADR[13,14].
Antispasmodics
Hyoscine butylbromide is a relatively safe antispasmodic anticholinergic agent that blunts the response of colonic neurons to muscarinic and nicotinic stimulation which leads to inhibition of smooth muscle contraction in the colon[15]. A recent meta-analysis assessed the results of 8 Randomised control trials (RCTs) conducted in Europe, Asia and Australia concluded hyoscine use in patients undergoing colonoscopy does not appear to significantly increase the detection of adenomas[16]. However, a recent study has shown that within the bowel cancer screening programme (BCSP) in England, it does improve ADR when used[17]. Recently, another antispasmodic topically applied: L-menthol (an organic compound found in peppermint oil, has been shown to improve ADR when sprayed on to the colonic mucosa during colonoscopy[18]. Whilst promising further studies are need to corroborate these findings.
Procedural factors-withdrawal time, use of sedation, colonoscopist and time of day
Variable factors inherent to colonoscopy have been shown to affect ADR. Time spent during the withdrawal phase is one such factor. A recent study within the BCSP in England demonstrated a plateau effect after approximately ten minutes. The lowest ADR was demonstrated if the withdrawal was less than 7 min, with the maximum ADR, seen with a withdrawal time of 9-11 min[17]. A multi centred RCT assessed multiple factors that may affect ADR, namely, bowel cleansing, sedation, withdrawal time in normal colonoscopies, and caecal intubation rates. They concluded a mean withdrawal time of > 8 min was the only modifiable factor related to the ADR in colorectal cancer screening colonoscopies[19].
Sedation use in one study found that larger amounts of sedation improved many aspects of colonoscopy quality. ADR increased (25.9% to 35%), early complications rate decreased (3.4% to 0.3%) and completion rates increased (88.3% to 96.4%)[20]. The mode of sedation that is used also appears to influence the quality of colonoscopy and particularly ADR. Again the literature reports conflicting results. A study which compared 843 colonoscopies found that deep sedation was associated with improved caecal intubation rates, and increased ADR. There were more immediate complications reported in the deep sedation group[21]. Another study suggested the type of sedation used during colonoscopy does not affect the number of patients in whom adenomatous polyps are detected. This followed a retrospective study that examined 3252 colonoscopies across two centres. ADR was the comparable for those receiving propofol and conscious sedation (midazolam and fentanyl)[22].
A variety of different studies have questioned whether the individual colonoscopist, i.e., the person performing the examination, influences ADR. A study that assessed factors that influence the quality of 12000 screening colonoscopy found that annual case volume and life experience did not affect ADR but continued medical education (CME) was found to be most influential, with those who attended most CME meetings having the highest ADR[23]. These findings were supported by a study from the Mayo clinic that formally assessed the impact of a colonoscopy education program. An additional training program, known as Endoscopic Quality Improvement Program (EQUIP) was used. ADRs were measured at baseline, then half of the 15 colonoscopist were randomly assigned to EQUIP. Baseline and post training ADRs were then compared, a total of 1200 procedures were completed in each of the two study phases. In the post-training phase, the group of endoscopists randomized to EQUIP training had an increase in ADR to 47%, whereas the ADR for the group of endoscopists who were not trained remained unchanged at 35%[24].
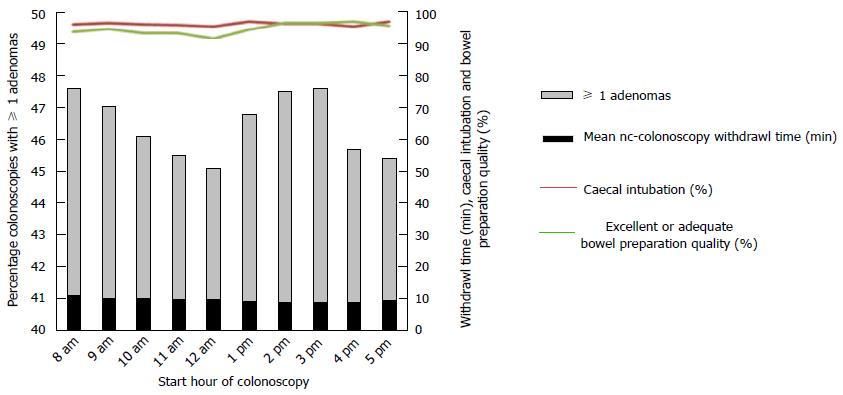
The procedural start time may also affect ADRs as suggested by a study of > 31000 colonoscopies. Procedures starting in the second half of a session (11:00-14:00 or 16:00-18:00) were associated with a reduction in detection of adenomas and advanced adenomas compared with procedures starting between 08:00 and 11:00 or 14:00 and 16:00[17]. Having assistance from the entire technical team to spot abnormalities during the examination has also been shown to improve ADR. In one such study the process was termed “all eyes on screen”, increasing ADR from 34% to 51% in 2 years[25]. A central visual gaze pattern on the colonoscopist has also been shown to improve ADR[26] (Figure 2).
Figure 2 Relationship between start time of colonoscopy and adenoma detection, withdrawal time, caecal intubation, and bowel preparation quality.
Water infusion techniques
The original goal of this novel technique was to facilitate caecal intubation, reduce colonic spasms, lower patient discomfort and need for sedation, for which it performs well[27,28]. It works by combining or replacing air-insufflation with water infusion. Concerns have been raised about an impaired ability to detect lesions due to contaminated water impairing visibility[27]. A systematic review performed in 2012 reported no difference in ADR when comparing water infusion to conventional insufflations[29]. A similar technique is known as Water Exchange. The water-exchange method is a technique in which water containing residual faeces is removed and “exchanged” for clean water in lieu of air-insufflation. The exchange of large volumes of water during the insertion of the colonoscope results in additional cleansing of the mucosa[27]. A study in 2009 exploring this technique failed to reach statistical significance for an improved ADR[30]. Improved ADR was demonstrated in one study when they combined the water exchange technique with cap-assisted colonoscopy (P = 0.002)[31].
The prolonged insertion time, colonoscopist experience and general technicalities of these techniques including expense are likely to limit their introduction into routine practice.
IMAGE ENHANCEMENT TECHNIQUES AND TECHNOLOGY
Standard white light, high definition and zoom endoscopy
There is conflicting evidence when assessing the superiority of high definition colonoscopy vs standard white light. A meta-analysis involving 4422 patients provided data on ADR. There was no significant difference in detection of high risk ADR. The detection of small adenomas was slightly better in the high definition group, but overall the analysis concluded here were marginal differences between high definition colonoscopy and SVE for the detection of colonic polyps/adenomas[32]. A more recent study showed improved ADR with high definition colonoscopy, when used by endoscopists with a low ADR (< 20%). For those with an ADR already > 20% there was no improvement in detection of high risk polyps, flat polyps or proximal lesions[33]. In contrast, other studies that have directly compared high definition colonoscopy to standard video endoscopy have shown significant improvements in ADR. On such study did so without compromising procedure duration, caecal intubation or levels of sedation. The additional polyps detected were mainly flat and sessile[34]. A further study with similar design also showed a lower adenoma miss rate with high definition colonoscopy[35]. Interestingly, a study assessing the multiple factors that influence the quality of colonoscopy identified advancing generations of colonoscope technology as a positive effector over ADR[23].
In summary, it would appear there are gains to be made from the use of high definition colonoscopy, but these may be limited, but the use of new generation colonoscopes (compared to older ones) may be the important factor.
Chromoendoscopy
Chromoendoscopy refers to the topical application of stains or dyes at the time of endoscopy in an effort to enhance tissue characterization, differentiation, or diagnosis[36]. The stains that are used for chromoendoscopy are classified as absorptive, contrast, or reactive. Indigo carmine is an example of a contrast stain and is most commonly used to improve ADR. Indigo carmine staining combined with magnification endoscopy appears to be a useful technique for the detection of aberrant crypt foci in the rectum, a potential biomarker for proximal flat colonic neoplasia[36,37].
A number of studies have examined whether the use of chromoendoscopy can improve ADR when compared to conventional white light colonoscopy, many of which have demonstrated an increase in the yield of neoplasia detection[38-40]. Many of these studies examine its use in high risk groups[37], One study compared high-definition chromocolonoscopy with high-definition white light colonoscopy for the detection of colorectal adenomas in average-risk United States persons undergoing screening colonoscopy. They compared the colonoscopy results of 660 patients, finding no significant difference in the number of small adenomas, advanced adenoma or carcinoma. Concluding that their results do not support the routine use of high-definition chromo-colonoscopy for colorectal screening in average-risk patients[41]. These conflicting results and the time consuming nature of dye spray may limit its adoption into routine screening of average risk patients.
New promising techniques are emerging, with stains incorporated into bowel preparation. One such formulation uses methylene blue (MB MMX, Cosmos Technolgies). This has been designed as a modified release device which ensures colonic release. The methylene blue is taken up by normal mucosa and poorly by neoplasia resulting in unstained areas where the lesions are present. A preliminary study has been promising on the efficacy of MB MMX 25 mg for the detection of polyps involved 96 patients undergoing routine colonoscopy. Polyps were detected in 61 patients, resulting in a 63.5% polyp detection rate[42].
Digital chromoendoscopy
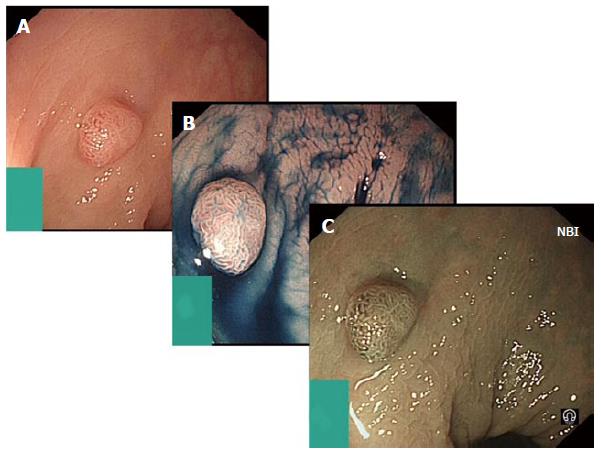
Digital chromoendoscopy refers to advances in endoscope technology that manipulate wavelengths of the light source to create an effect similar to chromoendoscopy by accentuating lesion characteristics (Figure 3).
Figure 3 Digital chromoendoscopy.
A: Represents sessile adenoma seen in standard white light; B: Shows the same adenoma after the use of indigo carmine applied for chromoendoscopy; C: Shows further assessment of the adenoma using narrow band imaging (NBI).
Narrow band imaging (NBI) is available on Olympus endoscopes. When used in colonoscopy, it allows potential improvement in ADR due to the enhanced appearance of certain mucosal and vascular features. A filter leads to the use of ambient light of wavelengths of 440 to 460 nm (blue) and 540 to 560 nm (green). Because the peak light absorption of haemoglobin occurs at these wavelengths, blood vessels will appear very dark, allowing for their improved visibility and the improved identification of other surface structures[43]. Compared with chromoendoscopy, classification of colorectal polyps by NBI appears to have a shorter learning curve. However, there is still substantial inter-observer variability, and classification of colorectal lesions based on vascular patterns is not objectively standardized yet[44]. A meta-analysis of 7 studies in 2936 patients showed no statistically significant difference in the overall adenoma detection rate with the use of NBI or white light (36% vs 34%, P = 0.4). They also showed no difference in the number of polyps detected between the two modalities (P = 0.2). A second met-analysis performed again failed show a significant difference in ADR between NBI and conventional white light. Concluding, NBI does not increase the yield of colon polyps, adenomas, or flat adenomas, nor does it decrease the miss rate of colon polyps or adenomas in patients undergoing screening/surveillance colonoscopy[45]. A further, larger, meta-analysis examined 11 RCTs evaluated NBI and ADR in a screening population of average- and higher-risk individuals and found limited benefit compared with white light colonoscopy[46]. These results were supported by a recent Cochrane review of 3673 patients in 8 randomized trials [relative risk (RR), 0.94; 95%CI: 0.87-1.02][47].
As with narrow-band imaging, the Fujinon intelligent colour enhancement (FICE) also narrows the bandwidth of conventional white-light colonoscopy to improve visualization, but it creates this effect electronically. Dedicated computer algorithms are used to generate the image. FICE enables the endoscopist to choose between different wavelengths for optimal examination of the colon mucosa[48]. It is reported to allow inspection of microvascular patterns as well as pit patterns and circumvents some limitations in conventional chromoendoscopy[49]. Back to back studies have examined FICE and its impact upon ADR. Neither study demonstrated an improvement in ADR or adenoma miss rate when compared to NBI and white light[50,51].
The Pentax technology equivalent is i-Scan, for which there are limited RCT with most of the literature focusing on lesion characterisation. Some studies have compared high definition scopes coupled with i-Scan against standard resolution scopes. One such study demonstrated significantly more neoplastic lesions and more flat adenomas could be detected using high definition endoscopy with surface enhancement. Histology could be predicted with high accuracy (98.6 %) within the HD+ group[52].
Digital-auto-fluorescence
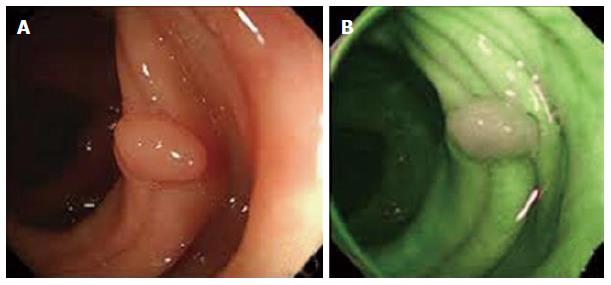
Digital-auto-fluorescence (AFI) is technology available only in Olympus endoscopes where rotating filter wheel in front of the light source sequentially generates blue light (390-470 nm) and green light (540-560 nm)[53]. The exposure of tissue to this specific light leads to the excitation of some endogenous substances and subsequently the emission of fluorescent light. The reflected blue light is blocked by a second filter while the reflected red light and the emitted green autofluorescence from the tissue are used to obtain an image. AFI colonoscopy colours neoplastic lesions red-purple while non-neoplastic mucosa appears green[27] (Figure 4).
Figure 4 Digital-auto-fluorescence.
A demonstrates polyp in white light, whilst B represents the same area in digital-auto-fluorescence. The normal mucosa appears green, with the adenoma appearing white.
Three of the most widely reported studies comparing AFI and white light describe a lower adenoma miss rate with AFI, with up to a 20% difference[54-56]. One study reported that the detection rate of flat and depressed adenoma, but not elevated adenoma; by AFI is significantly higher than that by white light. In less experienced hands, AFI dramatically increased the detection rate (30.3%) and reduced miss rate (0%) of colorectal adenoma in comparison to white light (7.7%, 50.0%); this was not seen with more experienced endoscopists. They did describe a significantly longer duration time in the AFI group[54]. Another study explored the use of AFI in those undergoing colonoscopy for Lynch syndrome surveillance or those with a family history of colorectal cancer (one first-degree relative with colorectal cancer diagnosed at a young age (< 50 years) or two first-degree relatives regardless of age). This study reported a significantly higher sensitivity of AFI compared with white light (92% vs 68%; P = 0.001). The additionally detected adenomas with AFI were significantly smaller than the adenomas detected by white light (mean 3.0 mm vs 4.9 mm, P < 0.01)[55]. AFI also achieved better diagnostic accuracy (77%) than white light (57%) or NBI (63%) for polyp differentiation in the evaluation of still images by inexperienced endoscopists (accuracy compared with white light, P = 0.001; with NBI, P = 0.016)[57].
At present, whilst evidence exists that digital chromoendoscopic techniques (NBI, FICE and i-Scan) aide’s lesion recognition, the evidence does not currently support that it improves ADR. There is some evidence to support of the positive effects of AFI, however, it is associated with added expense and poor image resolution, which are practical concerns for the widespread introduction of this technology.
ADVANCEMENTS IN ENDOSCOPE DESIGN
Extra-wide angle view colonoscopes
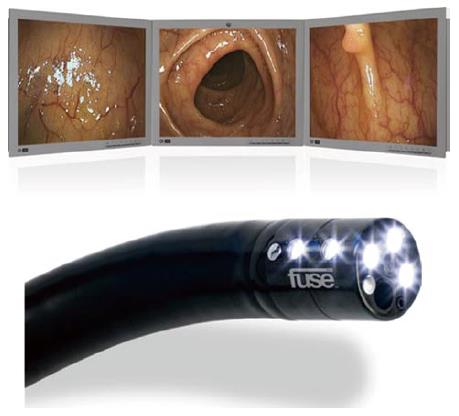
This may represent one of the few recent developments in the design of the colonoscope that aide ADR. The full spectrum endoscopy (FUSE) system (EndoChoice) is currently on the market. It allows for full-spectrum views of the colon lumen, comprising 330 degrees. The colonoscope in the Fuse system has 2 additional cameras, on the left and right side of the scope’s tip, to supplement the front camera. The video images transmitted from the cameras are displayed on 3 contiguous monitors corresponding to each camera. This array provides a comprehensive view of the total colonic lumen, including imaging of the traditionally encountered blind spots at the flexures or proximal edges of the mucosal folds (Figure 5).
Figure 5 Example of the display module of the full spectrum endoscopy system and the 330° view (top).
Bottom image is the full spectrum endoscopy scope demonstrating the side mounted camera and lights[27].
During its initial development, trials revolved around anatomical models with simulated polyps, some of which were purposely placed in the tradition blind spot, e.g., behind folds. In one such study 37 endoscopists performed colonoscopy by using the forward-viewing camera scope, followed by a colonoscopy with all 3 camera on; this increases the field of view to previously described 330 degrees. In total, 85.7% of the polyps were detected with the three cameras compared to 52.9% with only forward-viewing colonoscopy (P≤ 0.001). Particularly polyps that were “hidden” behind flexures and folds were more frequently detected with FUSE colonoscopy than with forward-viewing colonoscopy (81.9% vs 31.9%)[58]. An international, multicentre, randomised trial, the results of which were published in 2014 examined the use of FUSE further. Patients aged 18-70 years referred for colorectal cancer screening, polyp surveillance, or diagnostic assessment, were included. One hundred and eighty-five participants were assessed. The adenoma miss rate was significantly lower in patients in the FUSE group than in those in the standard forward-viewing procedure group: (7%) vs (41%) (P = 0.0001). In those who underwent standard colonoscopy first (n = 88), the FUSA system detected 39 additional polyps[59]. The authors reported a significantly longer withdrawal time (P≤ 0.01), however in real-time this was only a median time of 30 s. There certainly appears to be promise for ADR improvement with the FUSE system, more numerous and larger RCT’s will be required to confirm this.
The findings from a study examining the effectiveness of a prototype wide angled colonoscopy were recently reported. The prototype colonoscope has a extra-wide angle of view has a 144°-232°-angle lateral-backward viewing lens in addition to a standard 140°-angle forward-viewing lens. Views from both lenses are simultaneously constructed and displayed on a video monitor as a single image. The ADR reported from this study was 57.1%, achieved whilst maintaining appropriate caecal intubation rate, completion times and no adverse event[60].
Balloon assisted colonoscopy
The NaviAid G-EYE colonoscope (SMART Medical Systems) is one such system. With this there is an integrated balloon on the flexible tip of the scope. The balloon can be reprocessed and reinflated by the endoscopist upon withdrawal of the scope. The mechanical flattening and straightening of haustral folds with the inflated balloon permit visualization of hidden anatomic areas, thus increasing the ADR[28]. Only simulated studies on anatomical models exist for this device. One such study showed a significantly greater ADR in the balloon assisted group (P≤ 0.0001)[61]. Clearly larger scale human studies are required to more about the utility of this device.
Real-time histology and confocal microscopy
Confocal laser endomicroscopy (CLE) is an emerging technology, which allows in vivo imaging of cellular and subcellular details of the gut mucosa and vessels during ongoing endoscopy. The most commonly used contrast agents are acriflavine hydrochloride and fluorescein sodium. For colon pathology assessment, the administration of fluorescein intravenously produces a strong staining of both surface epithelium and deeper layers of lamina propria[62,63]. Mounted into the end of a regular colonoscope is a miniature confocal microscope. When the tip of the scope is placed in direct contact with the mucosa and an argon ion laser excites the tissue a grayscale image can be produced, with a 7 μm thickness and a lateral resolution of 0.7 μm, the field of view being 475 μm × 475 μm[63] (Figure 6).
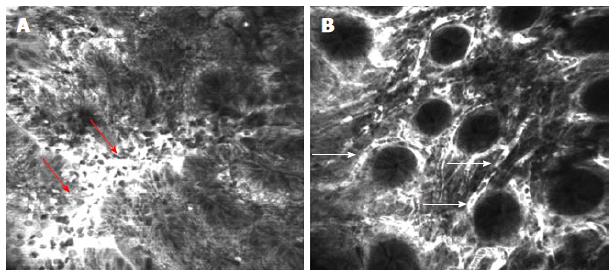
Figure 6 Confocal laser endomicroscopy of the colon using intravenous fluorescein.
A: Colon carcinoma with total disorganization of cell architecture, invasion and destruction of the vessels with leakage of fluorescein (arrows); B: Severe inflammatory changes in ulcerative colitis with cellular infiltrate causing an increase in the distance between crypts and excessive vascularity (arrows)[63].
A number of studies have demonstrated the ability of confocal microendosocopy to perform real time histological analysis. Showing its ability to separate hyperplastic and adenomatous polyps, whilst identifying malignant features also[62-64]. The application of confocal is somewhat in its infancy, however as things develop real-time microendoscopy may become mainstream for endoscopist. There is little evidence to suggest that confocal can improve ADR, but it can improve decision making once the adenoma is detected.
DEVELOPMENTS IN ACCESSORIES
Third eye retroscope®
Third eye retroscope® (Avantis Medical Systems, Inc) consists of a video processor, a single-use polarizing filter cap for the colonoscope light source, and a 3.5 mm flexible single-use catheter with a camera and diode light source at the tip. The retroscope is retroflexed 180 degrees after being advanced through the working channel of the colonoscope and provides a 135 degrees retrograde view of the colon[27]. The system has been quoted to increase mucosal visualisation from 87% to 99%[65]. Like the FUSE system, initial studies of the third-eye system used anatomical models with simulated polyps. Standard colonoscopy detected 12% of the polyps located on the proximal aspects of folds, while 81% of these polyps were detected with the third eye retroscope[66]. A study demonstrated a 14.8% increase in polyp detection and a 16.0% increase in adenoma detection in their study that included 298 patients[67]. A further study reported similar result with a 13.2% increase in polyp detection and a 11.0% increase in adenoma detection[68]. The largest study for Third eye was the TERRACE study. TERRACE was a multi-centred study that included 349 patients. A net additional detection rate with the third eye retroscope of 29.8% for polyps and 23.2% for adenomas was reported. The study was criticised as the withdrawal time for the Third eye scopes were on average 2 min longer, however post-hoc analysis found withdrawal time to be independent of ADR[69] (Figure 7).
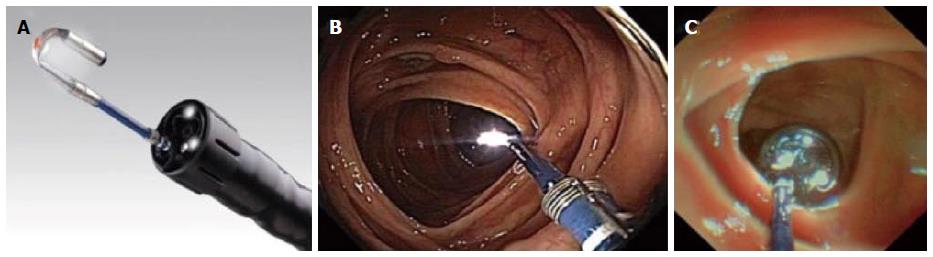
Figure 7 Third eye retroscope®.
A: Image of the third eye retroscope® protruding from the working channel of the colonoscope; B: Forward view of third-eye retroscope®; C: View from lens of third-eye retroscope®[27] .
Despite the apparent improved ADR and reduced miss rate, third eye endoscopy has some significant flaws. It results in a 50% reduction in suction capacity; it needs to be removed from the working channel as another device is required and is very expensive[27].
Cap assistance
Transparent caps attached to the distal tip of the colonoscope were first designed to assist during endoscopic mucosal resection but they have also been suggested to be of help in depressing colonic folds to improve visualization of their proximal aspects[27]. Particularly in the hands of trainees and less-experienced colonoscopist they have been shown to improve caecal intubation times and rates[70]. Most recently, a study, assessed ADR using cap-assisted colonoscopy vs normal colonoscopy. A total of 1380 patients were randomly allocated cap-assisted or normal, these consisted of asymptomatic participants (aged 50-75 years) in a primary colonoscopy screening programme. There was no significant difference in the type, location, size or number of polyps detected between the two groups. Caecal intubation time and Gloucester Comfort Scores were lower in the cap-assisted group[71]. A further study had similar finding, only demonstrated a superior ADR for polyps < 5 mm in the cap-assisted group[72]. Such finding have been persistent in other studies over the last decade with one of the original cap-assisted studies that examined 684 patients failing to demonstrate a significant difference in ADR[70]. This has been supported further by a meta-analysis performed in 2012 that concluded cap-assisted colonoscopy does not significantly improve ADR[73]. It would appear that cap-assisted colonoscopy may be of benefit in reducing caecal intubation time, but has limited or no benefit on polyp detection.
A similar device is Endocuff (Arc Medical, United States). Endocuff has been introduced as a means of enhancing visualization and scope stability during endoscopic mucosal resection of large or flat polyps of the sigmoid colon[74]. The Endocuff is a 2-cm long, flexible cuff with 2 rows of small flexible, hinged wings that help flatten large mucosal folds during withdrawal of the instrument. A prospective randomized trial in 498 patients undergoing screening colonoscopy showed Endocuff-assisted colonoscopy increased the absolute rate of polyp detection by 14% over unassisted colonoscopy from 42% to 56% (P = 0.001). The increase was particularly marked for polyps in the sigmoid colon 32% vs 15% (P = 0.0001) and caecum 4% vs 7% (P = 0.019)[75].
CONCLUSION
Novel and refinement of existing techniques, together with advancements in technologies can improve ADRs, and thus, potentially reduce cancer mortality. The use of chromoendoscopy in high risk groups such as colitis or HNPCC is becoming standard practice, but remains unsubstantiated for general use and is impractical. However, the development oral preparation given with the bowel preparation is a promising development. Increased ADR is yet to be proven with NBI, FICE and AFI beyond the use of high quality colonoscopes, and the marginal gains of using water exchange endoscopy are negated by time constraints, expense and further technical points for widespread application. Extra-wide angle colonoscopes such has FUSE has additional cost but its significant ADR may in the long-term make this economically viable, but more studies investigating the diagnostic before this device can be recommended for routine practice. The third-eye retroscope may be prohibited by cost, despite the apparent benefit, In contrast, cap-assistance is relatively inexpensive and further studies may show such devices as the Endo-cuff to be cost effective in improving ADR. However, at present, it seems that education, team work and optimising current practice will provide the biggest gains in ADR whilst maintaining financial acceptability.