Published online Aug 10, 2015. doi: 10.4253/wjge.v7.i10.928
Peer-review started: March 13, 2015
First decision: April 23, 2015
Revised: June 1, 2015
Accepted: July 8, 2015
Article in press: July 9, 2015
Published online: August 10, 2015
Processing time: 158 Days and 15.9 Hours
Gastrointestinal lymphomas represent up to 10% of gastrointestinal malignancies and about one third of non-Hodgkin lymphomas. The most prominent histologies are mucosa-associated lymphoid tissue lymphoma and diffuse large B-cell lymphoma. However, the gastrointestinal tract can be the site of rarer lymphoma subtypes as a primary or secondary localization. Due to their rarity and the multifaceted histology, an endoscopic classification has not been validated yet. This review aims to analyze the endoscopic presentation of rare gastrointestinal lymphomas from disease diagnosis to follow-up, according to the involved site and lymphoma subtype. Existing, new and emerging endoscopic technologies have been examined. In particular, we investigated the diagnostic, prognostic and follow-up endoscopic features of T-cell and natural killer lymphomas, lymphomatous polyposis and mantle cell lymphoma, follicular lymphoma, plasma cell related disease, gastrointestinal lymphomas in immunodeficiency and Hodgkin’s lymphoma of the gastrointestinal tract. Contrarily to more frequent gastrointestinal lymphomas, data about rare lymphomas are mostly extracted from case series and case reports. Due to the data paucity, a synergism between gastroenterologists and hematologists is required in order to better manage the disease. Indeed, clinical and prognostic features are different from nodal and extranodal or the bone marrow (in case of plasma cell disease) counterpart. Therefore, the approach should be based on the knowledge of the peculiar behavior and natural history of disease.
Core tip: The gastrointestinal tract can be the site of rare lymphomas as a primary or secondary localization. Their endoscopic behavior has been scantily evaluated but is emerging as a useful tool with prognostic and therapeutic implications. T-cell lymphomas present mainly with ulcerative lesions, while B-cell lymphomas (follicular or mantle cell lymphomas) present as a duodenal mass or multiple polyposis. Plasma cell-related disorders localize to the gastrointestinal tract, either as a neoplastic mass or as an amyloid deposition. Immunodeficits (primary or secondary) can lead to gastrointestinal localization of rare and seldom fatal high-grade lymphomas. More rarely, Hodgkin’s lymphoma localizes to the gastrointestinal tract with an uncertain impact on prognosis.
- Citation: Vetro C, Bonanno G, Giulietti G, Romano A, Conticello C, Chiarenza A, Spina P, Coppolino F, Cunsolo R, Raimondo FD. Rare gastrointestinal lymphomas: The endoscopic investigation. World J Gastrointest Endosc 2015; 7(10): 928-949
- URL: https://www.wjgnet.com/1948-5190/full/v7/i10/928.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i10.928
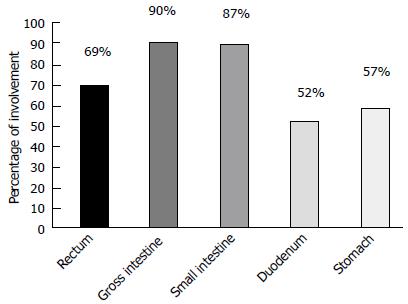
Gastrointestinal (GI) lymphomas represent 5%-10% of primary GI malignancies and almost two third of extranodal non Hodgkin’s lymphomas (NHL), that in turn account for 24%-49% of all NHL[1,2]. The most common lymphomas are mucosa-associated lymphoid tissue (MALT) and diffuse large B-cell lymphoma (DLBCL), accounting for 70%-95% of GI lymphomas[3,4]. Apart from MALT and DLBCL, the GI tract can be the site of other lymphomas, either as a primary or secondary localization[5], and these lymphomas will be the subject of this report. The knowledge of their clinical and echo-endoscopic features would help in addressing clinical questions[3,6-8], sparing inappropriate evaluations[9-13]. Nonetheless, histology, together with immunohistochemistry and molecular biology, are mandatory for diagnosis[14].
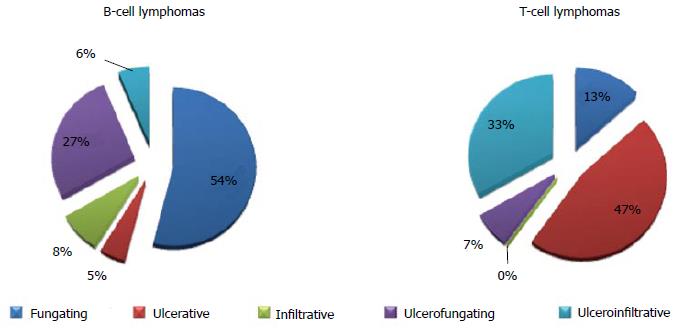
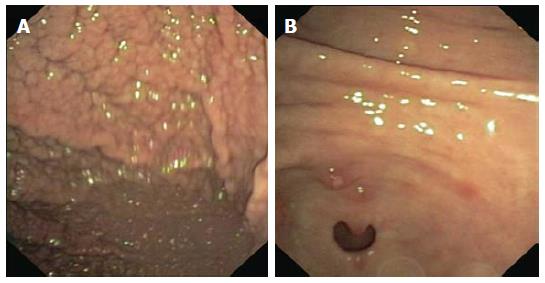
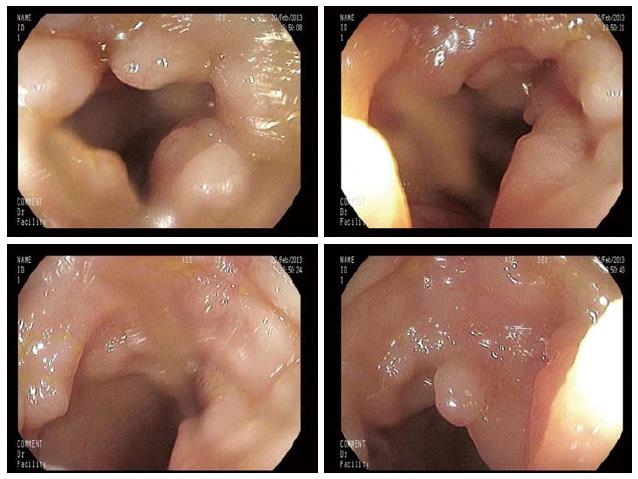
While the endoscopic classification for MALT and DLBCL has been already validated[15,16], such an analysis on rare GI lymphomas is still under debate. In 2001 and 2003, the Taiwanese[17] and the South Korean group[18] respectively published a 3/5 item classification of ileocolonic GI lymphomas. Table 1 shows patterns analyzed in both classifications. Basically, the endoscopic appearance is classified according to the presence and depth of ulcerations and of fungating lesions. To date, these were the only attempts to classify rare GI lymphomas. After that, Kim et al[19] investigated the endoscopic differences between B- and T-cell lymphomas of the colon and they observed that B-cell lymphomas occur more often as fungating or ulcerofungating lesions, while T-cell lymphomas more frequently have an ulcerative or ulceroinfiltrative pattern (Figure 1). Notwithstanding, a clear prognostic implication based on the endoscopic pattern has not been validated yet.
Newer techniques, i.e., capsule endoscopy (CE) and double-balloon enteroscopy (DBE), are emerging as useful tools in detecting small bowel tumors (15% of them represented by lymphomas)[3,20-22]. Surely both techniques can augment the endoscopic diagnostic field (especially for follicular lymphomas[21]). Moreover, spiral enteroscopy has been also evaluated as a tool for revealing GI lymphomas of the small intestine. Boudiaf et al[23] reported that 4 out of 14 patients affected by refractory celiac sprue developed a small bowel mass that was confirmed to be an enteropathy-associated T-cell lymphoma (EATL) by histological evaluation. Although less widespread, single-balloon enteroscopy has been used in the definition of small bowel lesions and recently it has been implemented with the water exchange method in order to improve the visualization of the lumen to better define and sample the lesion[24]. However, such deep diagnostic tools have not been validated for routine use in GI lymphoma staging and follow-up since they do not induce a treatment change. Thus, their application in gastric or colonic lymphomas has not been fully validated[25]. Differently, faced with T-cell lymphomas with a jejunal tropism, DBE can lead to a definitive diagnosis coupling the endoscopic investigation with the bioptic evaluation[26,27]. However, not many publications related to the usage of these techniques are available to date.
A particular consideration should be given to the role of endoscopic ultrasonography (EUS). Its role has gained more and more importance in MALT lymphomas since the locoregional staging of the disease has a great impact on the treatment approach[6]. Regarding DLBCL, the locoregional extension has significant prognostic implications, although its role in treatment definition is still under discussion[3]. In contrast, few data are available in rare GI lymphomas. In particular, they are more frequently regarded as general diseases so that the locoregional extension is not always evaluated, with some reports indicating just the EUS pattern without any clinical implication. Exceptional cases have indicated the role of EUS in defining the limited extension of the disease, thus leading to an endoscopic resection of the mass [see the paragraph “Extramedullary Plasmacytoma (EMP) and Plasma Cell-related Diseases”]. That notwithstanding, EUS information is gathered only for describing the behavior of these lesions in most cases without any significant clinical impact.
Definitively, a proper staging for GI lymphomas will include[28]: (1) physical examination: evaluation of superficial lymph nodes and Waldeyer ring inspection; abdomen palpation in order to detect liver enlargement, splenomegaly and abdominal masses; (2) endoscopic ultrasonography that is the golden standard in defining the locoregional GI involvement since it is able to distinguish the involvement of a specific layer and also of regional lymph nodes. However, as stated above, its role is under study and it is not strictly recommended in this setting; (3) computed tomography of the neck, chest and abdomen in order to detect involvement of nodes above and below the diaphragm and also other extranodal involvement not pertaining to the GI tract. In some cases, computed tomography can be of great help in defining the extension of a large bulky mass departing from the GI tract but exteriorizing outside the GI tract (see the paragraph “Plasma-cell related diseases”); (4) positron emission tomography is not generally indicated as a staging procedure, especially in MALT lymphomas, but it retains a role in defining the pre-treatment lymphomatous involvement and response to treatment; and (5) bone marrow biopsy: notwithstanding the low-grade, indolent diseases that tend to remain localized at the GI tract, bone marrow biopsy should be performed in order to exclude a marrow involvement that could influence treatment and follow-up management. However, the level of evidence on its utility is poor. A recent update of the staging recommendation in nodal lymphomas does not encourage the performance of bone marrow biopsy facing diffuse large B-cell lymphoma and Hodgkin’s lymphoma, but this strategy has not been evaluated specifically for GI lymphomas[29].
However, these are general guidelines adopted from MALT lymphoma since in more rare GI lymphomas these guidelines have not been fully validated.
The aim of the present review is to highlight macroscopic features of rare GI lymphomas using endoscopy and related techniques. In particular, we will focus on T-cell lymphomas, lymphomatous polyposis (LP) and mantle cell lymphoma (MCL), follicular lymphoma (FL), plasma cell-related diseases, gastrointestinal lymphomas in immunodeficiency and Hodgkin’s lymphoma (HL). An outline on the endoscopic presentation will be given for the diagnostic aspect and follow-up assessment. As a whole, Table 2 summarizes the clinical and molecular characteristics and prognostic features of these lymphomas.
| Lymphoma histotype | Presenting characteristics | Main GI involvement | Main endoscopic pattern | Typical immunophenotype | Typical genotype | Prognosis | ||
| T and NK lymphomas | EATL | Celiac patients with abdominal pain and small intestine obstruction/perforation | Duodenum and jejunum | Multiple erosions and ulcers | CD3+, CD4-, CD8+/-, CD7+, CD5-, CD2+, TIA+, GrBPer+, CD30-/+, CD25-/+, CD56-/+, CD16-, CD57-, BCL6-, CD10-, EBV-, EMA-/+ | TRB and TRG clonally rearranged +9q31.3 -16q12.1 +1q32.2-q41 +5q34-q35.2 +8q24 (MYC) | Poor | |
| PTCL | Poor performance status | Stomach and duodenum | Ulcerative | CD3+, CD4+, CD8-, CD7+, CD5+, CD2+, TIA-, GrBPer-, CD30-/+, CD25-, CD56-, CD16-, CD57-, BCL6-, CD10-, EBV-, EMA- | 1TCR clonally rearranged +7q/+8q/+17q/+22q/-4q -5q/-6q/-9p/-10q/-12q/-13q | 54% survival at five year Poor in case of high IPI score and stage III-IV disease | ||
| Extranodal NK/T-cell lymphoma | Gastrointestinal bleeding and B symptoms | Small intestine | Multiple erosions and ulcers | cyCD3+, CD4-, CD8-/+, CD7-, CD5-, CD2+, TIA+, GrBPer+, CD30-, CD25-, CD56+, CD16-, CD57-, BCL6-, CD10-, EBV+, EMA- | TCR in germinal configuration No specific cytogenetic studies on this specific subtype | Poor especially if perforation occurs | ||
| Adult T-cell leukemia/lymphoma | Abdominal pain, diarrhea, general fatigue, weight loss | No site preferences | Ulcers | CD3+, CD4+, CD8-, CD7-, CD5+, CD2+, TIA-, GrBPer-, CD30-/+, CD25++, CD56-, CD16-, CD57-, BCL6-, CD10-, EBV-, EMA- | TCR clonally rearranged Monoclonal integration of HTLV-1 | Poor2 Good3 | ||
| Indolent lympho-prolipherative diseases of GI tract | T-LPD | Dyspepsia and mild diarrhea | Small intestine and colon | Unremarkable/friable or erythematous mucosa | CD3+, CD4-, CD8+, CD7+/-, CD5+/-, CD2+, TIA+/-, GrBPer-/+, CD30-, CD56-, EBV- | TCR-γ monoclonal | Indolent course | |
| NK-cell enteropathy | Vague symptoms (dyspepsia) | Stomach and small intestine | Lesions exhibit superficial ulceration, flat elevations with central depression and are associated with edema and local hemorrhage | cCD3+, CD4-, CD8-, CD7+, CD5-, TIA+, GrBPer+, CD56+, EBV- | TRC polyclonal or oligoclonal | Indolent course | ||
| Mantle cell lymphoma | Vague symptoms (dyspepsia) | Colon | Multiple polyposis, seldom with ulcerations | CD19+, CD20+, CD5+, CD10-, CD43+, sIg+, BCL6-, IRF4/MUM1-, Cyclin D1+ | BCR rearranged t(11;14)(q13;q32) | Negative impact on prognosis | ||
| Follicular lymphoma | Vague symptoms (dyspepsia) | Second part of duodenum | Whitish polyps | CD19+, CD20+, CD5-, CD10+, CD43-, sIg+, BCL6+, IRF4/MUM1-/+, Cyclin D1-, α4β7+ | BCR rearranged t( 14:18)(q32:q2 1) | Good | ||
| Extramedullary plasmacytoma | Alarm symptoms and obstruction | Stomach | Infiltrating mass | Plasmacells expressing CD79a+, CD38+, CD19-, CD138+, CD56+, usually CD20- | BCR rearranged t(11;14)(q32;q13) | Poor | ||
| PTLD | Alarm symptoms | Colon | Rubbery erythematous or ulcerated | Similar to DLBCL and Burkitt’s lymphoma CD19+, CD20+, CD5-/+, CD10-/+, CD43-/+, sIg+/-, BCL6-/+, IRF4/MUM1-/+, Cyclin D1- | Monoclonal BCR | Poor median survival 6 mo | ||
| Plasmablastic lymphoma | Alarm symptoms | Stomach | Large masses and exophitic processes | CD79a+, CD138+, CD38+, IRF4/MUM1, CD45-, CD20, PAX5-, CD56- | Clonal IgH chain gene rearranqement | Poor | ||
| Hodgkin’s lymphoma | Obstruction | Colon | Protruding mass | CD30+, CD15+, CD45-, CD20-, CD79a-, PAX5+, Ig-, OCT2-, BOB1-, CD3-, CD2-, CD5-, ALK- | Clonal immunoglobulin gene rearrangeme nts | Prognostic impact not known | ||
GI T-cell lymphomas are rare, representing about 5% of GI lymphomas[14,30,31]. However, the incidence varies according to the geographical zones. European studies reported that 1.3% of primary GI lymphomas are of T-cell origin[32], while groups from eastern countries reported 7%-15%[33,34], reaching 41% in other series of intestinal lymphomas[35].
Ulcerated lesions are the main endoscopic features[30,36-38]. The first definition of this disease was “ulcerative jejunitis” by Isaacson and Du, given the always present ulcerative pattern[14]. Usually, symptoms are related to malabsorption[14], although perforation[39] or intestinal bleeding[40] can occur. Incidentally, GI perforation or bleeding can occur in cases of nodal T-cell lymphomas independently from GI localization and are an infective etiology, reflecting the immune impairment that characterizes these lymphomas[41,42].
Guidelines suggest that diagnostic work-up and follow-up should be done in synergism between hematologists and gastroenterologists in order to better define the staging and the treatment needed and to ensure the best nutritional guidance (evidence level III grade B)[43].
In a study from the German group, the most frequent histotype of intestinal lymphoma was T-cell lymphomas[44]. The most commonly involved organs are the duodenum and jejunum, followed by the ileum and colon. Less frequent is the involvement of the stomach[45], also as part of composite lymphoma[46], i.e., lymphoma with B- and T-cells origin. Regarding gastric involvement, in 30% of cases there is localization in the upper part of the stomach, in 20% the localization is in the middle part and diffuse in 40% of cases[47]. Due to the fact that the prognosis and treatment strategy depends on the lymphoma histotype, bioptic evaluation is a mandatory step. In addition, each subtype presents peculiar endoscopic behaviors that can drive diagnosis and treatment. GI T-cell lymphomas typically have a mature phenotype, while acute types of T-cell neoplasms do not classically involve the GI tract[48].
According to the 2008 WHO classification of hematological malignancies, the most prevalent histotypes are[48,49]: (1) enteropathy-associated T-cell lymphomas (EATL) (distinguished in type I and II); (2) peripheral T-cell lymphomas and extranodal natural killer (NK)/T-cell lymphoma; and (3) adult T-cell leukemia/lymphoma (ATLL).
In addition, very rare cases have been reported (mostly as singular events) of colorectal T-cell prolymphocytic leukemia/lymphoma[50] or anaplastic T-cell lymphoma (ALCL) ALK+[51] or ALK-[52]. Distinct entities not described in the WHO classification are indolent T-cell/NK diseases that will also be taken into account.
Although EUS findings are not usually reported except in peculiar cases, submucosal hypoechogenic lesions destroying the involved layer would be the main pattern[53]. Another proof of the sub-mucosal origin of the tumor is given by narrow band imaging that is able to show intact gastric pits elevated from the underlying mass[51]. Very rare and unusual is the GI involvement in Sezary syndrome where, despite unremarkable gastric mucosa, EUS can show the hyperechogenic submucosa layer at giant fold level[54].
EATL can be divided into two forms[14]. The first variant is characterized by features of celiac disease with abdominal pain and small intestine obstruction/perforation. Usually there is a large mass with massive necrosis, while the neighboring mucosa shows villous atrophy and crypt hyperplasia as in typical enteropathy. Type II exhibits villous atrophy in the context of tumor mass with normal intestinal mucosa in uninvolved sites. Contrarily to type I EATL, type II EATL does not progress from undiagnosed or refractory celiac disease[14,55]. Prognosis is poor with a median overall survival of 7-10 mo[56].
The exact incidence and lymphoma risk in celiac patients is still a debated issue[57]. Some studies indicate a 200-fold increased risk of developing EATL compared to the general population[58,59]. According to other studies, the risk of developing non-Hodgkin’s lymphomas in celiac patients appears to be 6-fold higher than in the general population and this risk assumes a downward trend over years[60]. Nonetheless, it appears clear that the occurrence of complications in celiac patients, although infrequent, is an event that negatively impacts on patient survival[61]. In fact, the occurrence of intestinal perforation in a patient affected by celiac disease should lead to suspicion of lymphoma.
Usually, EATL patients tend to have a poorer performance status than B-cell lymphomas (even though tends to be localized), independent of the stage. Fever and diarrhea are the most frequent symptoms[44]. The duodenum and jejunum are the most involved sites, with secondary involvement of the gross intestine in 14% of cases[44]. The diagnosis of the disease in some cases is difficult since neoplastic lymphocytes can be present in a context of an inflammatory background.
Endoscopic features are aspecific, with multiple erosions and ulcers[31]. Nodularity and thickened folds can be seen at DBE[26,27]. Strictures and masses are less common[62]. In some cases, macroscopic findings together with the occurrence of an intense inflammatory reaction can lead to a mistaken diagnosis of Crohn’s disease (CD)[31,63]. However, although it is not a general rule, CD ulcers are transversal, while, in the presence of T-cell lymphoma, ulcers are longitudinal[63].
Peripheral T-cell lymphomas (PTCL) and NK lymphomas are more frequent in South America and Asia. These entities are distinct from other GI T-cell lymphomas by morphological and immunohistochemistry criteria[62] and should be diagnosed when other more frequent T-cell lymphomas are excluded[48]. Korean and Japanese series indicated that these are the most frequent GI T-cell lymphoma subtype, accounting for 40% of primary T-cell GI lymphomas HTLV-1 negative[64]. PTCL arises frequently in extranodal sites, especially at the skin. However, the involvement of the gastrointestinal tract is a severe prognostic factor[65,66]. The stomach and duodenum accounts for 60% of GI localizations[52]. The most frequent findings are ulcerative (46% of cases), infiltrative (9%), ulceroinfiltrative (18%), ulcerofungating (18%) and erosive (9%)[52]. Multiple polyposis can also be detected[67]. In the literature, there are two reports indicating the involvement by T-cell lymphomas in the ileocolonic anastomosis for a previously resected right colon, presenting with polypoid lesions[68] or ulcerative lesions[69].
Extranodal NK/T-cell lymphoma usually arises in nasal cavities and rarely affects the GI tract. A strict relationship exists between ENKTCL and EBV infection, with almost 70% of cases positive for Epstein–Barr virus-encoded small RNAs (EBER) detection[70]. The small intestine is the most involved organ, while the stomach is rarely involved[71]. The endoscopic pattern in the majority of cases is given by multifocal ulcers[72-75] and infiltrative lesions[52]. Sometimes the ulceration leads to intestine perforation and acute peritonitis (60% of the total complications)[52]. Additionally, perforation is more frequent in the infiltrative pattern compared to the non-infiltrative. Fungating lesions are not usually reported[76]. The most involved organ is the small intestine[77,78] and/or colon[72,76] (depending on the case series), followed by the small intestine, rectum and stomach[72]. However, the location at the GI tract does not seem to affect the prognosis[77]. Interestingly, since the perforation usually leads to the development of peritonitis, the Lugano staging system has been applied, resulting in the advanced stage of the disease being a prognostic factor at multivariate analysis[72]. Due to the high risk of perforation, many patients undergo surgery as a pre-emptive or curative strategy, rarely for diagnosis[79]. However, according to Kim et al[77], patients undergoing surgery followed by chemo/radiotherapy would show a better OS. However, as the authors themselves stated, this benefit would be ascribed to the fact that patients undergoing surgery had a better performance status and more limited disease which would have affected the outcome. Similarly, as Hong et al[78] reported in a multivariate analysis, surgery ensures a better survival compared to chemotherapy. Therefore, an appropriate locoregional staging is also useful to tailor treatment.
As for EATL, ATLL tends to present with ulcers with aggressive behavior. This is a specific variant of peripheral T-cell lymphoma that recognizes the HTLV-1 virus as an etiological agent[48]. This variant is mainly found in endemic areas of Japan[64]. In about one third of ATLL cases, GI involvement is secondary to a systemic disease[49]. According to the first data by Suzumiya, the stomach is involved in 40% of cases and the small and large intestine in 38% and 34% respectively[80]. Although four types of ATLL have been depicted (i.e., smoldering, chronic, lymphoma, acute), no endoscopic pattern has been related to a peculiar histotype. HTLV-1 infection has no role in determining the macroscopic features[47]. Noteworthy, the detection of GI involvement has a prognostic impact[49], representing the aggressiveness of the disease[43]. In fact, smoldering or chronic ATLL subtypes do not typically show GI involvement[81]. However, primary GI smoldering ATLL have been described and show long term disease-free survival after chemotherapy[82]. Gastric involvement can be enhanced by Helicobacter pylori infection that creates an inflammatory state able to lead lymphocytes (also malignant) to migrate into gastric wall through the expression of specific adhesion molecules[83].
An ulcerative pattern is present in more than half of cases of gastric involvement[47]. Single or multiple yellow-whitish polyposis of the first or second loop are more frequent in the duodenum[49] and multiple polyposis is the recurrent lesion in cases of colon involvement[84]. Although a single or multiple polyps are the most frequent lesions, flat ulcerations/erosions can also be present[84]. Red flat or elevated lesions in the rectum have been also documented[85,86]. Rarely, there is the involvement of the ileum, where polyps are the main features[87]. It should be underlined that GI lesions are not always monotone but can be variegated. For examples, case reports indicate the occurrence of protruding masses with normal or eroded mucosa at the stomach and the occurrence of flat granular, friable lesions that bled on contact with mucosa at the colon[88] or reddish irregular flat lesions at the esophagus[89].
Narrow band imaging is able to document irregular microvascular architecture, dilated and destroyed gastric pits and dense aggregations between the pits with variegated irregular nuclei without interglandular infiltration (reflecting the absence of lymphoepithelial lesions)[90].
A new category of T-cell GI lymphoproliferative disease, namely T-cell lymphoproliferative disease (T-LPD), has been recently introduced[91]. The indolent course is the main clinical hallmark while this entity has been previously treated and managed as PTCL. Noteworthy, the etiology of the disease is unknown, although many patients present with a history of inflammatory bowel disease (IBD). Basically, the clinical picture is dyspepsia and mild diarrhea, while endoscopic features can vary from unremarkable mucosa to erythema. The small intestine and colon are the most frequently involved sites, followed by the oral cavity, stomach and esophagus. Usually, the gastric mucosa is normal despite a disease localization, while the duodenum can show thickened folds and an irregular pattern. In the colon, the occurrence of friable mucosa, erythematous mucosa and small polyps can be seen. Ulcerations are not described. At immunohistochemistry, lymphoid cells have a cytotoxic phenotype (CD8+; CD4-; TIA+), clonal T-cell receptor (TCR) gene rearrangements, do not form masses, do not invade the intestinal crypts and do not cover the full thickness of the bowel[91]. Additionally, the lymphoid infiltrate is limited to the mucosa and sub-mucosa. The molecular study for TCR can show a monoclonal rearrangement of TCR-γ chain[91]. The recognition of this disease has many therapeutic implications since aggressive chemotherapy is excessive and an immunosuppressive treatment is virtually sufficient.
Indolent CD4+ T-cell lymphoma has also been described and shows a good outcome and survival despite a persistence after immunomodulatory drug-based treatment[92]. Rarely, gastric mucosa can show multiple nodularities[93]. However, a clinical and endoscopic follow-up of these lesions is always advisable[93], also for the risk of progression in the long term[92].
Similarly to T-LPD, NK cells can also give rise to an indolent form of lymphoid infiltrate in the context of the GI tract, i.e., NK-cell enteropathy[94]. Usually the symptoms are vague and the GI lesions can be present in the stomach (more frequently), duodenum, small intestine and colon. At endoscopy, these lesions exhibit superficial ulceration, flat elevations with central depression and are associated with edema and local hemorrhage. Usually these ulcers are 1 cm in diameter and the surrounding mucosa is not abnormal. This disease is distinct from ENKTL since gastric involvement in the latter is really infrequent (and if present, the localization is not limited to the stomach) and EBER is positive. In addition, in the presence of NK-enteropathy, the epithelium can be invaded, showing a lymphoepithelial-like lesion[95]. Moreover, contrarily to T-LPD, the TCR rearrangement is polyclonal or oligoclonal[94].
The pioneering study by Cornes et al[96] in 1961 first reported the term “lymphomatous polyposis (LP)”. It is defined as the presence of diffuse proliferation of monotonous small-to-intermediate sized lymphocytes presenting as multiple polypoid tumors from 2 mm to several centimeters in different GI sites. Although the preferred site is the small intestine[14], other sites can be involved alone or at the same time[97-104]. Actually, LP is present in 4%-9% of all GI lymphomas[14], more frequently in western than eastern countries[105]. B-cell lymphomas are more frequent than T-cell lymphomas and this is due to the fact that histologically these polyps originate from the mantle zone of the lymphoid follicle of the mucosa-associated lymphoid tissue[106]. Additionally, this fact justifies the augmented frequency in the small intestine (rich in lymphatic tissue) compared to other GI tract sites. Additionally, multiple tumors or different kinds of lymphomas can be simultaneously present in a context of LP[107]. Therefore, the biopsy of more than one polyp and of different types of lesions is always advisable[108,109]. Additionally, it must be underlined that although the occurrence of multiple polyposis in a patient with nodal lymphoma is not a criterion to absolutely define the involvement of the GI tract, the histological evaluation is always mandatory[110].
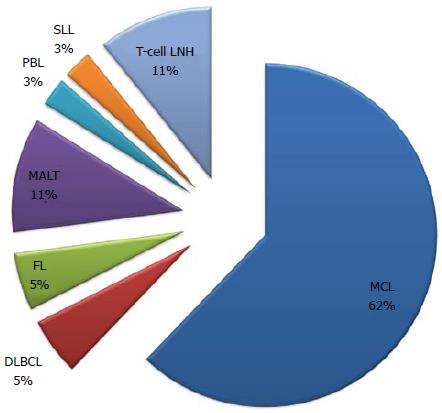
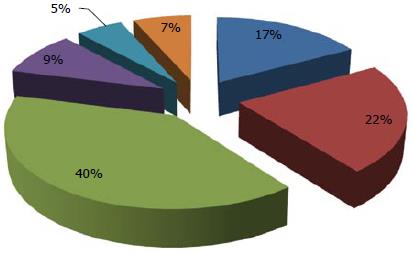
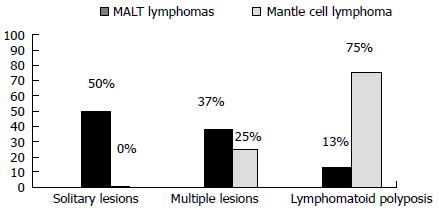
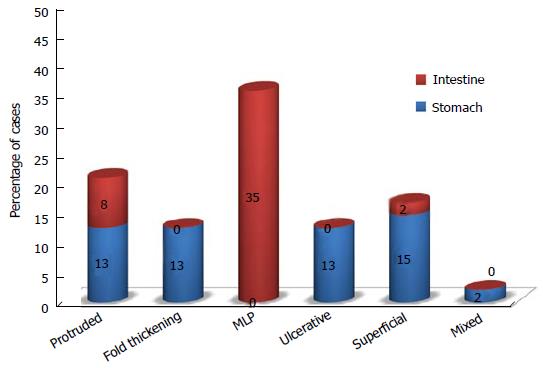
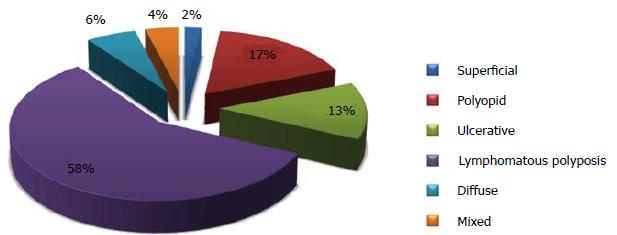
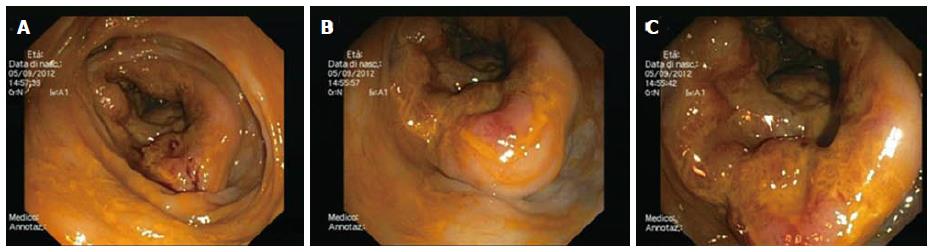
Typical lymphoma presenting with LP is MCL[14,111], although other tumors can show this feature[98-100,112-114]. Among 37 case reports of LP since 2000[67,84,98-100,103,112-142], MCL was indeed the most frequent disease (more than 50% of cases) (Figure 2). The most involved site was the colon (Figure 3). In the case series by Saito et al[143], regarding patients affected by MALT lymphomas or MCL at the ileal site, it was underlined that LP was the most frequent presentation of MCL and the least common lesion in MALT lymphomas (Figure 4).
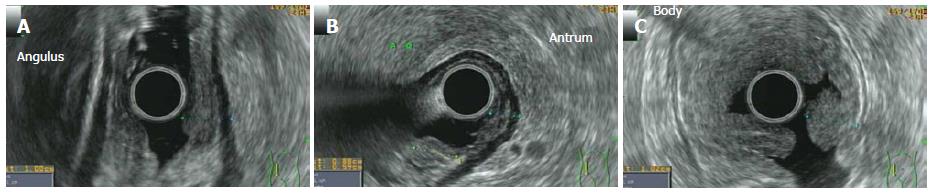
MCL can locate at the GI tract secondary to the generalized disease[102] and, although only 25% of patients with nodal mantle cell lymphoma suffer GI symptoms, 77%-88% have a localization at the gross intestine and 43%-77% in the upper GI tract, also in the absence of macroscopic lesions[14] (Figure 5). LP is the most frequent endoscopic pattern although other endoscopic features can be present[144], for example, a granular pattern associated with polyps (Figure 6) or ulcerated polyps[145] or masses[146]. In addition, the endoscopic pattern varies according to the part of the GI tract involved (Figure 7). EUS has been applied in this setting, giving the possibility of identifying submucosal lesions[115]. MCL appears echo-poor, usually departing from the second layer and remaining confined to the GI wall (Figure 8)[115,147,148]. In some cases, the diagnosis of MCL could be incidental during the endoscopic definition of gastric bleeding caused by gastric ulcers[149].
Contrarily to GI follicular lymphoma (discussed below), the GI tract involvement by MCL assumes a great prognostic implication and is useful to monitor patients after the treatment[14,101]. Indeed, the occurrence of LP designates a median survival of 3-4 years[14,101]. Due to the fact that the small intestine can be also involved by the tumor, the performance of CE or DBE would be advisable in order to correctly stage the patient and assess the follow-up evaluations[116,117].
Although the disease presentation has been well studied, there are no data about the management of LP during follow-up assessment. Our opinion is that endoscopic evaluation with mapping biopsies should be performed in these patients since in some cases the presence of aspecific abnormalities during follow-up can lead to the finding of lymphoma reappearance[146], sometimes many years after complete remission[103,119].
GI FL is a rare entity, representing up to 3.6% of all GI NHL[150,151]. Primary GI FL was recognized as an histological variant of FL in the 2008 WHO classification of hematopoietic tumors[152]. Sites most frequently involved are the duodenum (55.6% of cases)[101], in particular the second part[152], and the terminal ileum (33.3% of cases)[151,153]. Since positron emission tomography and computed tomography have low sensitivity and specificity[154] in catching small intestine involvement, CE and DBE have acquired more and more importance[155,156]. Indeed, these techniques have shown that the small bowel can be involved in 70%-83% of cases[157,158], even in cases of duodenal lymphoma[152].
To date, a clear endoscopic classification of GI-FL has not been done, as for GI MALT lymphomas. However, Yamamoto et al[151], reviewing 249 GI-FL cases, reported a reliable endoscopic classification of the disease. Whitish polyps usually up to 2 mm[151,153] and/or white granules-nodular aggregates, with or without ulceration of the mucosa layer (Figure 9), are the typical endoscopic pattern[150,159,160]. This can be unifocal or multifocal and is mainly present in intestinal FL. A large mass with or without ulceration is less frequent and in half of cases can be associated with multifocal whitish polyps. The latter is the most frequent endoscopic pattern of primary gastric FL. Multiple lymphomatous polyposis can also be found[30,101,150,158,161,162]. Interestingly, in the series of 48 patients with GI FL reported by Yanai et al[163], it was found that the LP was the most frequent endoscopic feature (more than 50% of cases), followed by polypoid or ulcerative lesions (Figure 10).
Recently, high-definition endoscopy, as well as magnifying endoscopy (ME), has been used to describe the surface microstructures of GI FL, such as enlarged whitish villi and tiny whitish depositions and an irregular microvascular pattern[164,165]. This fact indicates that the tumor is of non-epithelial origin and usually reflects the formation of lymphoid follicles[164,166-169]. EUS has not been widely applied. A few reports have indicated that the echoendoscopic pattern is given by second and third layer thickening, dotted by hypoechogenic nodules[170].
Capsule endoscopy and double-balloon enteroscopy are useful in the definition of small intestine involvement in a non-invasive way. The typical picture is a whitish submucosal elevation presenting as nodules or polyps[21], usually multifocal[171,172]. However, the limitation is the inability to perform a biopsy that is postponed until the enteroscopy and the risk of retention in cases of stenosis (unusual in cases of GI-FL).
Nodal spread is rare and GI FL tends to be localized in the gastrointestinal tract (stage IE according to Ann Arbor staging system)[173] and to have an indolent course[152,174]. However, transformation to aggressive lymphoma has been documented[175]. Different from other form of lymphomas, the GI involvement is not an adverse prognostic factor[176]. Lymphoma grading is low in the majority of cases, while in the nodal counterpart grade I-II FL is documented in 1 case out of 10[173]. Furthermore, in contrast to nodal FL, these cells do not acquire additional mutations and this justifies the absence of grade 3 GI FL and the very low rate of transformation[173,175].
Treatment strategies are not uniform, although GI FL are treated more frequently compared to the nodal counterpart[177]. Different case series have demonstrated that a watch and wait approach is as useful as the pharmacological approach, except for relieving clinical symptoms[163,178-180]. However, case series differ greatly in identifying the correct treatment approach to be applied. Surgical resection is not recommended and chemoimmunotherapy is preferred[151,171]. It must also be considered that the introduction of anti CD20 antibodies has augmented the survival rate and in some series localized/low-grade GI FL have been treated with anti CD20 monoclonal antibody alone, without chemotherapy[151].
It is debatable whether CE and/or DBE are truly useful. Indeed, no studies have demonstrated that the detection of small bowel involvement (especially if duodenal lymphoma is present) would have changed the treatment needed. Surely, these procedures would change the treatment strategy in cases of radiation or surgical treatment and are needed in cases of obscure gastrointestinal bleeding[172,181]. Apart from these occurrences, the effectiveness of chemoimmunotherapy or immunotherapy alone would render these procedures less practical in patient management. However, since no clear data exists regarding survival and quality of life in dependence of small bowel involvement, clinician choice is the only way to proceed.
That notwithstanding, the diagnostic suspicion based on the endoscopic features, together with the patient history, is fundamental in addressing the pathological diagnosis. Indeed, in almost 20% of cases, FL can be misdiagnosed by endoscopic biopsy evaluation[182]. Therefore, multiple biopsies would be necessary. In particular, biopsies of the peripheral mucosa would be more informative than biopsies from the erosion/ulceration since the probability of catching necrotic tissue decreases significantly.
EMP belongs to a precise type of lymphoid malignancies, i.e., plasma cell neoplasms, representing 3%-4% of cases[183]. It is important to distinguish this subtype from lymphomas with plasmacytic differentiation, particularly MALT lymphomas[48]. The upper respiratory tract is the most involved organ (almost 80% of cases), while GI localization is rare[48]. Among these cases, the stomach is the most involved site, followed by the liver, colon and the small intestine (duodenum, jejunum and ileum)[184].
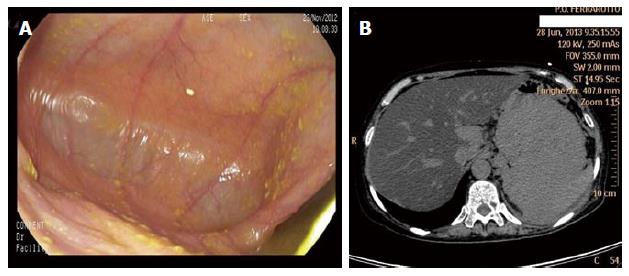
Usually, gastric localization is secondary to a plasma cell myeloma (PCM) and often emerges through a clone selection process. Indeed, multiple myeloma treatment itself can select a particular chemoresistant PC clone able to migrate at extra-nodal organs. In these cases, an accurate endoscopic investigation is critical for the diagnostic assessment and disease monitoring[185]. Due to the strict relationship with plasma cell myeloma, the clinical course is poor. The most frequent endoscopic finding consists of an infiltrating mass or masses in the stomach and/or the duodenum[186,187] or well-demarcated, flat, yellow-whitish mucosal changes[188] or nodular lesion with central umbilication[189]. Endoscopic appearance as diffusely thickened mucosal folds simulating linitis plastica is rare[190]. Sometimes, large ulcerations can be seen[191,192]. However, the gastric mucosa can appear normal, while the extramural growth is incredibly vast (Figure 11). EUS could be of great help in defining the disease extension that appears as a large echo-poor mass infiltrating surrounding organs[186]. However, sometimes EUS can be useful to detect limited gastric wall involvement and in these cases, an endoscopic resection of the mass can be performed, resulting in safety for the patient and effective in the treatment of the disease[188,193,194]. Alternatively, patients with localized disease can be treated with radiation treatment[190,195].
Small intestine involvement is generally primary with a benign course. These lesions can be explored by enteroscopy and/or capsule endoscopy[196], paying attention to the cases in which obstructions or retention are expected. Differential diagnosis is other cases of sub-mucosal masses in the small intestine, as reported by Lopes da Silva[196]. Colon involvement appears more frequently as stricture[197,198], in some cases difficult to differentiate from colon adenocarcinoma[199], returning to the differential diagnosis of sub-mucosal tumors[193]. Rarely it can determine rectal bleeding[200]. The localization at the rectum appears as a mild granularity as well as a reddish, protruded lesion[201]. Usually these lesions disappear after treatment and this is a confirmation of treatment efficacy[186], although mucosal atrophy and non-specific inflammation can be reinstated[195].
Apart from EMP, other plasma cell-related disorders can involve the GI tract. This is due to the production of amyloid protein in AL amyloidosis (light chain amyloidosis)[187]. The most involved organ is the small intestine. In some cases the amyloid deposition is synchronous with EMP[187,193,195] or other GI lymphomas[202]. Usually, the amyloid protein in AL amyloidosis involves the submucosa and the muscularis mucosae, resulting in thickened folds and valvulae conniventes and polypoid lesions in the GI tract. The typical deposition of AL amyloid proteins result in pseudo-obstruction, constipation and mechanical obstruction as the main symptoms[203]. Intestinal bleeding can also occur[204] and if this event occurs in a patient with multiple myeloma, the occurrence of aspecific elevated lesions at the endoscopic evaluations should lead to suspicion of systemic amyloidosis. More rarely, submucosal hematoma, ulcers and hemorrhagic bullous colitis can be seen[205]. On the other hand, nodularity, fine granular appearance and mucosal friability are more frequent in other types of amyloidosis, i.e., AA amyloidosis (amyloidosis secondary to systemic disorders). This is due to the deposit of amyloid proteins into the lamina propria with impaired absorption and subsequent diarrhea[203].
Immunodeficiency is defined as a state of impaired function of the immune system that can be congenital, acquired or iatrogenic. The reduced immune-surveillance can determine an augmented rate of lymphomas. Two conditions mainly determine the arising of lymphomas: human immunodeficiency virus (HIV) infection with the correlated acquired immunodeficiency syndrome (AIDS) and post-transplant immunosuppression. In both conditions, the GI tract is the most involved site[206]. Apart from HIV and PTLD, common variable immunodeficiency (CVID) has been associated with the development of gastrointestinal NHL, although this is a very rare finding[207,208].
In HIV patients, the rate of GI lymphomas was higher in the pre-HAART era before 1996[209] and the risk of gastric NHL was 353-fold compared with normal subjects, with aggressive lymphomas the most prevalent feature[59]. In cases of AIDS-related lymphoma, the GI tract is involved in 20% to 50% of cases[206,210]. However, the decrease of GI lymphoma incidence has not been as high as in central nervous system lymphomas[209]. A recent analysis of 243 HIV patients performed at the University of Sao Paolo revealed an incidence of gastric NHL of 2.5%[211]. Co-infection with EBV and/or CMV would complicate the prognosis[212], although the occurrence of viral infection is less pathogenetically important compared to PTLD[206]. The main histologies are B-cell lymphomas (67%) (DLBCL, Burkitt lymphoma, MALT lymphoma)[213], while T-cell lymphomas are less frequent (33%)[209] and other types of hematological malignancies are anecdotal[214,215]. In 5%-10% of cases, cMyc rearrangement is present and confers a poor prognosis[212]. Additionally, the prompt recognition of this lymphoma subtype has a great impact in patient management since the presenting symptoms are usually alarm symptoms in about half of patients. However, in the majority of patients, the lymphoma is diagnosed at Ann Arbor stage III-IV[206]. The most frequent endoscopic features are multifocal ulcerations, followed by polypoid or a bulky mass together with bloody spots[206,212]. The most involved sites are the stomach and duodenum[216], followed by the small bowel and colon-rectum (Figure 12)[211]. However, unusual presentations can be seen more commonly than in immunocompetent patients[206]. At narrow-band, a honeycomb-like pattern is present without irregularity in the microvasculature[212]. The localization can also be perirectal and in these cases, EUS-guided fine needle biopsy would be a valid tool for diagnosis given the high grade nature of this kind of lymphomas[217]. Noteworthy, EUS appearance is of a hypoechoic poorly defined mass[217] and is important for the locoregional staging[206]. Prognosis is poor with a median survival of 6 mo and a rate of complete remission less than 40%[211]. Prognosis is also impaired by the occurrence of opportunistic infections[210]. Extremely suggestive is the development of EBV-related DLBCL in patients suffering other types of lymphomas that induce a state of immunosuppression, such as AITL[218]. In these peculiar cases, the outcome is really poor and alarm symptoms and perforation can occur with fatal implications[218].
GI lymphomas are also more frequent in solid organ transplant recipients, particularly after renal, heart and small bowel transplantation, encompassing the spectrum of PTLD (Table 3). The pathogenetic events seem to be different compared to HIV-related lymphomas since in this kind of lymphomas, Epstein-Barr virus re-activation due to immunosuppressants plays a pivotal role[219]. Apart from negative EBV serology prior to transplantation, length of immunosuppression is an overt risk factor[220,221]. EBV-positive lymphomas arise earlier than EBV-negative lymphomas[221]. In adults, the majority of cases arises over 12 mo from transplantation[222], at a median of 36 mo[223]. A second peak is after 5-10 years[206]. Median overall survival is 8 years and the principal histotype is B-cell lymphoma, although lymphomas of T-cell origin can also be present. Noteworthy, the GI tract is involved in one third of cases. Endoscopy is of great help in establishing the diagnosis. Especially in small bowel transplantation, endoscopic follow-up has gained a pivotal role in defining the transplant-related complications, including the onset of PTLD[224]. Typically, lesions are raised, rubbery, erythematous or ulcerated[222,225,226]. The most involved organ is the colon, followed by the small intestine and stomach[223]. However, the recognition of symptoms together with the patient history is of great help in driving the diagnosis. Additionally, endoscopic procedures are essential in order to follow the course of disease[225], also valid in the long-term[226]. Interestingly, early stage PTLD can be safely removed endoscopically and this would be a valid approach in the treatment of localized PTLD[224].
| Transplant | Prevalence |
| Bone marrow | 0.50% |
| Liver | 1%-2% |
| Kidney | 0.7%-4% |
| Heart | 2%-10% |
| Small bowel | up to 30% |
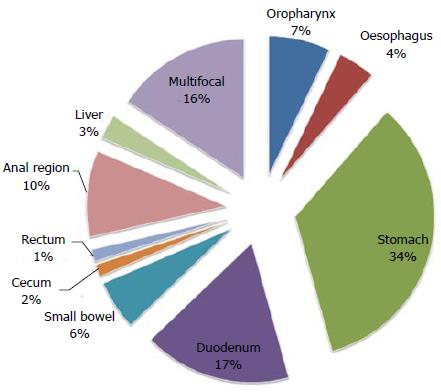
Plasmablastic lymphoma (PBL) is a rare and aggressive type of lymphoma characterized at histological evaluation by the presence of large immunoblasts with plasmacytic differentiation with an high replication index[227]. Usually, this lymphoma arises in the oral cavity in HIV-infected patients and in the literature there are few cases of GI localization (Table 4)[228-237]. The stomach is the most involved site (about 50% of cases), followed by the small intestine, anal region, cecum, colon and esophagus[237]. Large masses and exophytic processes are the main endoscopic appearance in the stomach and anal region. Intestinal localization is extremely rare and when present, the endoscopic appearance is of multiple nodularity[227]. Moreover, PBL can also arise in immunocompetent patients with ulcerated lesions at the stomach[236]. These patients are normally older than HIV+ patients, tend to present with GI localizations more that HIV+ patients and have a worse overall survival[236,237].
| Manuscript | Year | localization | Endoscopic appearance | HIV |
| Pruneri et al[230] | 1998 | Stomach | Large polypoid mass | - |
| Colomo et al[231] | 2004 | Anal region | Mass | + |
| Dong et al[232] | 2005 | GI tract | Not reported | + |
| Small Intestine | Not reported | + | ||
| Tavora et al[228] | 2006 | Anal region | Not reported | + |
| Anal region | Exophytic mass | + | ||
| Taddesse-Heath et al[233] | 2010 | Small intestine/colon (2 cases) | Not reported | + |
| Brahmania et al[234] | 2011 | Ano-rectal junction | Hypervascular cauliflower-like mass | - |
| Mihaljevic et al[235] | 2012 | Stomach | Not reported | - |
| Hashimoto et al[236] | 2012 | Stomach | Not reported | - |
| Chapman-Fredricks et al[229] | 2012 | Stomach | Not reported | + |
| Luria et al[237] | 2014 | Anal region | Mass | + |
| Sigma | Mass | - | ||
| Small bowel | Not reported | - | ||
| Ileum | Not reported | - |
Additionally, CD has also been linked to the development of lymphomas of the gastrointestinal tract. Most of them are of B origin, comprising DLBCL and HL, although T-cell lymphomas can also arise. In the recent report by Kappelman et al[238], patients with CD showed a greater risk of developing hematological malignancies compared to the general population. This study confirmed the previous report by Askling et al[239], also showing an augmented rate of hematological malignancies compared to the general population and 10% of developed lymphomas were T-type. Probably, it would be related to the state of immunosuppression leading to infection of lymphotropic and oncogenic viruses, but the specific mechanism is still to be clarified. This predisposition seems to be unrelated to immunosuppressive treatment. In this setting, anti-TNFα treatment has been related to development of hepatosplenic T-cell lymphoma[240]. However, two years later, a meta-analysis by Siegel et al[241] indicated that immunosuppressive treatment is not a risk factor for the development of NHL in CD patients. However, it is still a matter of discussion since the augmented incidence of GI lymphomas in these patients is related to the more intensive examinations. Moreover, the histological evaluation is a crucial point since the inflammatory background can lead to a false positive result. That notwithstanding, anti-TNFα treatment seems to be safe regarding the incidence of NHL and should not be regarded as a risk factor. Therefore, more epidemiological studies will be needed in order to better define the link between CD and GI lymphomas.
Lymphomatous GI involvement in HL appears as a stricture (Figure 13) or ulceration[242-245]. The abundant lymphoid tissue present at this site renders it one of the most involved regions[246]. HL rarely presents as a colonic localization (almost 1%-3% of extra-nodal HL cases[247] and less than 5% of gastrointestinal lymphomas[243]) and the prognostic impact is still obscure. Mixed-cellularity subtype is the most common feature[248]. As for the nodal counterpart, the inflammatory background is a key feature of HL[249]. In some cases, the endoscopic and histological presentation can resemble IBD, that in turn is seldom associated with colonic HL[244,250]. Additionally, immunodeficiency is a risk factor[251], although this type of lymphoma can also arise in immunocompetent patients[247].
Recently, a new entity has been proposed, i.e., ‘‘EBV-associated mucocutaneous ulcer” (EBVMCU)[252]. This disease subtype resembles HL but there are peculiar clinical and histological differences. Indeed, the presence of ‘‘plasmacytoid’’ apoptotic cells and the confinement to mucosa and sub-mucosal layers are the histological hallmark that can lead to a differential diagnosis from cHL. However, EBV infection is always present, as in GI-HL.
Endoscopic features of GI lymphomas are variegated encompassing ulcers, erosions, polyps and so on. It is a fascinating matter of study for both hematologists and gastroenterologists. As stated in guidelines, a synergism between these two figures is fundamental. This is due to the lack of data and the fact that information regarding rare GI lymphomas are extrapolated from case series or case reports. Actually, the scientific community is gaining more and more knowledge about the recognition and management of these lymphomas, with the creation of proper guidelines for specific lymphoma subtypes. In this setting, the collection of different case series and their analysis will assume a pivotal role in drawing general guidance on disease characterization. Certainly, as has emerged in the manuscript, the management of these lymphomas is different from the nodal or medullary counterparts and a proper understanding of the endoscopic features together with clinical and histological characteristics is crucial for better management of patients, with the ultimate goal of improving clinical outcome and quality of life for patients.
| 1. | Zullo A, Hassan C, Cristofari F, Perri F, Morini S. Gastric low-grade mucosal-associated lymphoid tissue-lymphoma: Helicobacter pylori and beyond. World J Gastrointest Oncol. 2010;2:181-186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Vetro C, Romano A, Palumbo GA, Bonanno G, Di Raimondo F. Role of the endoscopic ultrasonography in the management of gastric lymphomas: our experience and review of literature. Russian Federation: Novosibirsk State technical University: InTech 2011; 235-258. |
| 3. | Vetro C, Romano A, Amico I, Conticello C, Motta G, Figuera A, Chiarenza A, Di Raimondo C, Giulietti G, Bonanno G. Endoscopic features of gastro-intestinal lymphomas: from diagnosis to follow-up. World J Gastroenterol. 2014;20:12993-13005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Vetro C, Romano A, Donnarumma D, La Fauci A, Fiumara P, Chiarenza A, Figuera A, Palumbo G, Bonanno G, Di Raimondo F. Role of the endoscopic ultrasonography in the management of gastric lymphomas: metanalysis of literature. Haematologica. 2011;96:567. |
| 5. | Ferry JA. Extranodal Lymphomas. Publisher: Elsevier Health Sciences 2011; . |
| 6. | Vetro C, Chiarenza A, Romano A, Amico I, Calafiore V, Di Raimondo C, Coppolino F, Di Raimondo F. Prognostic assessment and treatment of primary gastric lymphomas: how endoscopic ultrasonography can help in tailoring patient management. Clin Lymphoma Myeloma Leuk. 2014;14:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Vetro C, Romano A, Chiarenza A, Conticello C, Donnarumma D, Gorgone A, Coppolino F, Palumbo GA, Bonanno G, Di Raimondo F. Endoscopic ultrasonography in gastric lymphomas: appraisal on reliability in long-term follow-up. Hematol Oncol. 2012;30:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | O’Malley DP, Goldstein NS, Banks PM. The recognition and classification of lymphoproliferative disorders of the gut. Hum Pathol. 2014;45:899-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Zullo A, Esposito G, Ridola L, Hassan C, Lahner E, Perri F, Bianco MA, De Francesco V, Buscarini E, Di Giulio E. Prevalence of lesions detected at upper endoscopy: an Italian survey. Eur J Intern Med. 2014;25:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Di Giulio E, Hassan C, Marmo R, Zullo A, Annibale B. Appropriateness of the indication for upper endoscopy: a meta-analysis. Dig Liver Dis. 2010;42:122-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Faintuch JJ, Silva FM, Navarro-Rodriguez T, Barbuti RC, Hashimoto CL, Rossini AR, Diniz MA, Eisig JN. Endoscopic findings in uninvestigated dyspepsia. BMC Gastroenterol. 2014;14:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Hassan C, Di Giulio E, Marmo R, Zullo A, Annibale B. Appropriateness of the indication for colonoscopy: systematic review and meta-analysis. J Gastrointestin Liver Dis. 2011;20:279-286. [PubMed] |
| 13. | Buri L, Bersani G, Hassan C, Anti M, Bianco MA, Cipolletta L, Di Giulio E, Di Matteo G, Familiari L, Ficano L. How to predict a high rate of inappropriateness for upper endoscopy in an endoscopic centre? Dig Liver Dis. 2010;42:624-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Burke JS. Lymphoproliferative disorders of the gastrointestinal tract: a review and pragmatic guide to diagnosis. Arch Pathol Lab Med. 2011;135:1283-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Ahmad A, Govil Y, Frank BB. Gastric mucosa-associated lymphoid tissue lymphoma. Am J Gastroenterol. 2003;98:975-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Zullo A, Hassan C, Andriani A, Cristofari F, Cardinale V, Spinelli GP, Tomao S, Morini S. Primary low-grade and high-grade gastric MALT-lymphoma presentation. J Clin Gastroenterol. 2010;44:340-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Myung SJ, Joo KR, Yang SK, Jung HY, Chang HS, Lee HJ, Hong WS, Kim JH, Min YI, Kim HC. Clinicopathologic features of ileocolonic malignant lymphoma: analysis according to colonoscopic classification. Gastrointest Endosc. 2003;57:343-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Wang MH, Wong JM, Lien HC, Lin CW, Wang CY. Colonoscopic manifestations of primary colorectal lymphoma. Endoscopy. 2001;33:605-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Kim YH, Lee JH, Yang SK, Kim TI, Kim JS, Kim HJ, Kim JI, Kim SW, Kim JO, Jung IK. Primary colon lymphoma in Korea: a KASID (Korean Association for the Study of Intestinal Diseases) Study. Dig Dis Sci. 2005;50:2243-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Chen WG, Shan GD, Zhang H, Li L, Yue M, Xiang Z, Cheng Y, Wu CJ, Fang Y, Chen LH. Double-balloon enteroscopy in small bowel tumors: a Chinese single-center study. World J Gastroenterol. 2013;19:3665-3671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Egea-Valenzuela J, Castillo-Espinosa JM, Sánchez-Torres A, Alberca-de-las-Parras F, Carballo-Álvarez F. Diagnosis of follicular lymphoma of the small bowel by video capsule endoscopy. Rev Esp Enferm Dig. 2014;106:51-52. [PubMed] |
| 22. | Nakamura M, Ohmiya N, Hirooka Y, Miyahara R, Ando T, Watanabe O, Itoh A, Kawashima H, Ohno E, Kinoshita T. Endoscopic diagnosis of follicular lymphoma with small-bowel involvement using video capsule endoscopy and double-balloon endoscopy: a case series. Endoscopy. 2013;45:67-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Boudiaf M, Jaff A, Soyer P, Bouhnik Y, Hamzi L, Rymer R. Small-bowel diseases: prospective evaluation of multi-detector row helical CT enteroclysis in 107 consecutive patients. Radiology. 2004;233:338-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 156] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Liang S, Pan Y, Wang B, Luo H, Xue X, Qin J, Guo X, Wu K. Complete small-bowel examination by oral single-balloon enteroscopy using the water-exchange method. Endoscopy. 2013;45 Suppl 2 UCTN:E415-E417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Al-Taie O, Dietrich CG, Flieger D, Katzenberger T, Fischbach W. Is there a role for capsule endoscopy in the staging work-up of patients with gastric marginal zone B-cell lymphoma of MALT? Z Gastroenterol. 2013;51:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 26. | Yanai S, Matsumoto T, Nakamura S, Fujisawa K, Ueki T, Hirahashi M, Yao T, Iida M. Endoscopic findings of enteropathy-type T-cell lymphoma. Endoscopy. 2007;39 Suppl 1:E339-E340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Matsumoto T, Nakamura S, Esaki M, Yada S, Moriyama T, Yanai S, Hirahashi M, Yao T, Iida M. Double-balloon endoscopy depicts diminutive small bowel lesions in gastrointestinal lymphoma. Dig Dis Sci. 2010;55:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Zucca E, Copie-Bergman C, Ricardi U, Thieblemont C, Raderer M, Ladetto M , Ladetto M; ESMO Guidelines Working Group. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi144-vi148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 29. | Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059-3068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2850] [Cited by in RCA: 4195] [Article Influence: 349.6] [Reference Citation Analysis (8)] |
| 30. | Terada T. Gastrointestinal malignant lymphoma: a pathologic study of 37 cases in a single Japanese institution. Am J Blood Res. 2012;2:194-200. [PubMed] |
| 31. | Freeman HJ. Malignancy in adult celiac disease. World J Gastroenterol. 2009;15:1581-1583. [PubMed] |
| 32. | Koch P, Probst A, Berdel WE, Willich NA, Reinartz G, Brockmann J, Liersch R, del Valle F, Clasen H, Hirt C. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96). J Clin Oncol. 2005;23:7050-7059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 171] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Park YH, Kim WS, Bang SM, Lee SI, Uhm JE, Kang HJ, Na II, Yang SH, Lee SS, Kim K. Prognostic factor analysis and proposed prognostic model for conventional treatment of high-grade primary gastric lymphoma. Eur J Haematol. 2006;77:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Kohno S, Ohshima K, Yoneda S, Kodama T, Shirakusa T, Kikuchi M. Clinicopathological analysis of 143 primary malignant lymphomas in the small and large intestines based on the new WHO classification. Histopathology. 2003;43:135-143. [PubMed] |
| 35. | Gou HF, Zang J, Jiang M, Yang Y, Cao D, Chen XC. Clinical prognostic analysis of 116 patients with primary intestinal non-Hodgkin lymphoma. Med Oncol. 2012;29:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 36. | Ho HC, Nagar AB, Hass DJ. Obscure gastrointestinal bleeding and video capsule retention due to enteropathy-associated T-cell lymphoma. Gastroenterol Hepatol (N Y). 2013;9:536-538. [PubMed] |
| 37. | Kawamoto K, Nakamura S, Iwashita A, Watanabe J, Oshiro Y, Nakayama Y, Nimura S, Kimura N, Aoyagi K, Yao T. Clinicopathological characteristics of primary gastric T-cell lymphoma. Histopathology. 2009;55:641-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Radersma M, Mulder CJ, Tushuizen ME. A rare cause of weight loss and night sweats. Gastroenterology. 2014;147:e11-e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Dughayli MS, Baidoun F, Lupovitch A. Synchronous perforation of non-Hodgkin’s lymphoma of the small intestine and colon: a case report. J Med Case Rep. 2011;5:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Kinaci E, Gunes ME, Huq GE. An unusual presentation of EATL type 1: Emergency surgery due to life-threatening gastrointestinal bleeding. Int J Surg Case Rep. 2013;4:961-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Saitoh T, Matsushima T, Matsuo A, Yokohama A, Irisawa H, Handa H, Tsukamoto N, Karasawa M, Nojima Y, Murakami H. Small-bowel perforation accompanied by Aspergillus endocarditis in a patient with angioimmunoblastic T-cell lymphoma. Ann Hematol. 2007;86:71-73. [PubMed] |
| 42. | Keiser PB, Nutman TB. Strongyloides stercoralis in the Immunocompromised Population. Clin Microbiol Rev. 2004;17:208-217. [PubMed] |
| 43. | Dearden CE, Johnson R, Pettengell R, Devereux S, Cwynarski K, Whittaker S, McMillan A. Guidelines for the management of mature T-cell and NK-cell neoplasms (excluding cutaneous T-cell lymphoma). Br J Haematol. 2011;153:451-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, Zeitz M, Riecken EO. Intestinal non-Hodgkin’s lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin’s Lymphoma. J Clin Oncol. 2003;21:2740-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 165] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 45. | Sugita S, Iijima T, Furuya S, Kano J, Yanaka A, Ohta K, Kojima H, Noguchi M. Gastric T-cell lymphoma with cytotoxic phenotype. Pathol Int. 2007;57:108-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Tachikawa Y, Shiratsuchi M, Sada E, Idutsu K, Kiyasu J, Karube K, Ohshima K, Nishimura J, Takayanagi R, Abe Y. Composite gastrointestinal lymphoma consisting of diffuse large B-cell lymphoma and peripheral T-cell lymphoma. Int J Hematol. 2009;90:275-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 47. | Hatano B, Ohshima K, Katoh A, Kanda M, Kawasaki C, Tsuchiya T, Shimazaki K, Haraoka S, Sugihara M, Suzumiya J. Non-HTLV-1-associated primary gastric T-cell lymphomas show cytotoxic activity: clinicopathological, immunohistochemical characteristics and TIA-1 expression in 31 cases. Histopathology. 2002;41:421-436. [PubMed] |
| 48. | Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardimar JW; WHO classification of tumours of haematopoietic and lymphoid tissues: International Agency for Research on Cancer, 2008. . |
| 49. | Isomoto H, Ohnita K, Mizuta Y, Maeda T, Onizuka Y, Miyazaki M, Omagari K, Takeshima F, Murase K, Haraguchi M. Clinical and endoscopic features of adult T-cell leukemia/lymphoma with duodenal involvement. J Clin Gastroenterol. 2001;33:241-246. [PubMed] |
| 50. | Toyota S, Nakamura N, Dan K. T-cell prolymphocytic leukemia with hemorrhagic gastrointestinal involvement and a new chromosomal abnormality. Int J Hematol. 2002;75:314-317. [PubMed] |
| 51. | Ishii H, Isomoto H, Taniguchi H, Kinoshita N, Matsushima K, Taguchi J, Miyazaki Y, Nakao K. Education and Imaging: Gastrointestinal: gastroduodenal involvement of ALK-positive anaplastic large cell lymphoma. J Gastroenterol Hepatol. 2011;26:933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Kim do H, Lee D, Kim JW, Huh J, Park SH, Ha HK, Suh C, Yoon SM, Kim KJ, Choi KD. Endoscopic and clinical analysis of primary T-cell lymphoma of the gastrointestinal tract according to pathological subtype. J Gastroenterol Hepatol. 2014;29:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Okada M, Maeda K, Suzumiya J, Hagimoto T, Wakamatsu S, Ohshima K, Kanda M, Sonoda T, Sakamoto A, Tamura K. Primary colorectal T-cell lymphoma. J Gastroenterol. 2003;38:376-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Schuler MK, Kroschinsky F, Schaich M, Kalauch A, Stroszczynski C, Kellermann S, Ehninger G, Benter T. Sézary syndrome: infiltration of the gastric wall--does it matter? Ann Hematol. 2012;91:1507-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Okumura K, Ikebe M, Shimokama T, Takeshita M, Kinjo N, Sugimachi K, Higashi H. An unusual enteropathy-associated T-cell lymphoma with MYC translocation arising in a Japanese patient: a case report. World J Gastroenterol. 2012;18:2434-2437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Sieniawski MK, Lennard AL. Enteropathy-associated T-cell lymphoma: epidemiology, clinical features, and current treatment strategies. Curr Hematol Malig Rep. 2011;6:231-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Rostami Nejad M, Aldulaimi D, Ishaq S, Ehsani-Ardakani MJ, Zali MR, Malekzadeh R, Rostami K. Geographic trends and risk of gastrointestinal cancer among patients with celiac disease in Europe and Asian-Pacific region. Gastroenterol Hepatol Bed Bench. 2013;6:170-177. [PubMed] |
| 58. | d’Amore F, Brincker H, Grønbaek K, Thorling K, Pedersen M, Jensen MK, Andersen E, Pedersen NT, Mortensen LS. Non-Hodgkin’s lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol. 1994;12:1673-1684. [PubMed] |
| 59. | Chang ST, Menias CO. Imaging of primary gastrointestinal lymphoma. Semin Ultrasound CT MR. 2013;34:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 60. | Askling J, Linet M, Gridley G, Halstensen TS, Ekström K, Ekbom A. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology. 2002;123:1428-1435. [PubMed] |
| 61. | Biagi F, Gobbi P, Marchese A, Borsotti E, Zingone F, Ciacci C, Volta U, Caio G, Carroccio A, Ambrosiano G. Low incidence but poor prognosis of complicated coeliac disease: a retrospective multicentre study. Dig Liver Dis. 2014;46:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 62. | Arps DP, Smith LB. Classic versus type II enteropathy-associated T-cell lymphoma: diagnostic considerations. Arch Pathol Lab Med. 2013;137:1227-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Jiao G, Zheng Z, Jiang K, Zhang J, Wang B. Enteropathy-associated T-cell lymphoma presenting with gastrointestinal tract symptoms: A report of two cases and review of diagnostic challenges and clinicopathological correlation. Oncol Lett. 2014;8:91-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Park YH, Kim WS, Bang SM, Lee SI, Kang HJ, Na II, Yang SH, Lee SS, Uhm JE, Kwon JM. Primary gastric lymphoma of T-cell origin: clinicopathologic features and treatment outcome. Leuk Res. 2006;30:1253-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 65. | Bellei M, Chiattone CS, Luminari S, Pesce EA, Cabrera ME, de Souza CA, Gabús R, Zoppegno L, Zoppegno L, Milone J. T-cell lymphomas in South america and europe. Rev Bras Hematol Hemoter. 2012;34:42-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 66. | Velagapudi P, Turagam M, Uzoaru I, Graham D. Small bowel obstruction due to mycosis fungoides: an unusual presentation. Am J Med Sci. 2011;341:508-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Isomoto H, Maeda T, Akashi T, Tsuchiya T, Kawaguchi Y, Sawayama Y, Koida S, Ohnita K, Kohno S, Tomonaga M. Multiple lymphomatous polyposis of the colon originating from T-cells: a case report. Dig Liver Dis. 2004;36:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 68. | Cooperberg MR, Fiedler PN. Ki-1 anaplastic large-cell lymphoma occurring at the site of ileocolonic anastomosis in a patient treated surgically for colonic adenocarcinoma: case report and review of the literature. Ann Diagn Pathol. 2001;5:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Ikeda J, Yamauchi A, Hoshida Y, Okamura S, Hashimoto K, Aozasa K, Morii E. Peripheral T-cell lymphoma developing at ileocolonic anastomosis site after colectomy for adenocarcinoma. Pathol Res Pract. 2010;206:376-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 70. | Jung CK, Lee KY, Kim Y, Han K, Shim SI, Kim BK, Kang CS. Epstein-Barr virus infection, drug resistance and prognosis in Korean T- and NK-cell lymphomas. Pathol Int. 2001;51:355-363. [PubMed] |
| 71. | Zhang YC, Sha Zhao JB, Lei Shi MX, Zhang HY, Liu WP. Gastric involvement of extranodal NK/T-cell lymphoma, nasal type: a report of 3 cases with literature review. Int J Surg Pathol. 2008;16:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Jiang M, Chen X, Yi Z, Zhang X, Zhang B, Luo F, Jiang Y, Zou L. Prognostic characteristics of gastrointestinal tract NK/T-cell lymphoma: an analysis of 47 patients in China. J Clin Gastroenterol. 2013;47:e74-e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Wakabayashi S, Arai A, Oshikawa G, Araki A, Watanabe M, Uchida N, Taniguchi S, Miura O. Extranodal NK/T cell lymphoma, nasal type, of the small intestine diagnosed by double-balloon endoscopy. Int J Hematol. 2009;90:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Li JZ, Tao J, Ruan DY, Yang YD, Zhan YS, Wang X, Chen Y, Kuang SC, Shao CK, Wu B. Primary duodenal NK/T-cell lymphoma with massive bleeding: A case report. World J Clin Oncol. 2012;3:92-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Kobold S, Merz H, Tiemann M, Mahuad C, Bokemeyer C, Koop I, Fiedler W. Primary NK/T cell lymphoma nasal type of the stomach with skin involvement: a case report. Rare Tumors. 2009;1:e58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 76. | Kim JH, Lee JH, Lee J, Oh SO, Chang DK, Rhee PL, Kim JJ, Rhee JC, Lee J, Kim WS. Primary NK-/T-cell lymphoma of the gastrointestinal tract: clinical characteristics and endoscopic findings. Endoscopy. 2007;39:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Kim SJ, Jung HA, Chuang SS, Hong H, Guo CC, Cao J, Hong XN, Suzuki R, Kang HJ, Won JH. Extranodal natural killer/T-cell lymphoma involving the gastrointestinal tract: analysis of clinical features and outcomes from the Asia Lymphoma Study Group. J Hematol Oncol. 2013;6:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 78. | Hong H, Liang C, Huang H, Guo C, Tian Y, Liu T, Zhang M, Li X, Wang Z, Fang X. Surgical resection followed by chemotherapy may be an effective treatment strategy for primary gastrointestinal natural killer/T-cell lymphoma: a single center experience. Leuk Lymphoma. 2014;55:2649-2651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 79. | Yang H, Wu M, Yin W, Sun W, Zhang G. Diagnosis and treatment of a patient with primary gastric extranodal natural killer/T-cell lymphoma, nasal type. Leuk Lymphoma. 2010;51:2137-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Suzumiya J, Marutsuka K, Nabeshima K, Nawa Y, Koono M, Tamura K, Kimura N, Hisano S, Tachibana N, Inoue S. Autopsy findings in 47 cases of adult T-cell leukemia/lymphoma in Miyazaki prefecture, Japan. Leuk Lymphoma. 1993;11:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Bazarbachi A, Suarez F, Fields P, Hermine O. How I treat adult T-cell leukemia/lymphoma. Blood. 2011;118:1736-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 82. | Tanaka K, Nakamura S, Matsumoto T, Hirakawa K, Yanaru-Fujisawa R, Onoyama K, Sakata H, Ohshima K, Yao T, Iida M. Long-term remission of primary gastric T cell lymphoma associated with human T lymphotropic virus type 1: a report of two cases and review of the literature. Intern Med. 2007;46:1783-1787. [PubMed] |
| 83. | Ohnita K, Isomoto H, Mizuta Y, Maeda T, Haraguchi M, Miyazaki M, Murase K, Murata I, Tomonaga M, Kohno S. Helicobacter pylori infection in patients with gastric involvement by adult T-cell leukemia/lymphoma. Cancer. 2002;94:1507-1516. [PubMed] |
| 84. | Hokama A, Tomoyose T, Yamamoto Y, Watanabe T, Hirata T, Kinjo F, Kato S, Ohshima K, Uezato H, Takasu N. Adult T-cell leukemia/lymphoma presenting multiple lymphomatous polyposis. World J Gastroenterol. 2008;14:6584-6588. [PubMed] |
| 85. | Asada Y, Isomoto H, Shikuwa S, Ito M, Momita S, Matsumura N, Ohba K, Ohnita K, Nakamura T, Mizuta Y. Adult T-cell leukemia with colorectal involvement. Gastrointest Endosc. 2004;60:983-984. [PubMed] |
| 86. | Onishi T, Tamura S, Onishi S. A case of adult T-cell leukemia with colon involvement. Gastrointest Endosc. 2007;65:712-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 87. | Ohnita K, Isomoto H, Maeda T, Jubashi T, Nakamura H, Misuta Y, Murase K, Tomonaga M, Kohno S. Two cases of adult T-cell leukemia/lymphoma involving the terminal ileum. Leuk Lymphoma. 2003;44:973-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 88. | Isomoto H, Furusu H, Onizuka Y, Kawaguchi Y, Mizuta Y, Maeda T, Kohno S. Colonic involvement by adult T-cell leukemia/lymphoma mimicking ulcerative colitis. Gastrointest Endosc. 2003;58:805-808. [PubMed] |
| 89. | Isomoto H, Ohnita K, Mizuta Y, Maeda T, Omagari K, Miyazaki M, Murase K, Hasui K, Murata I, Kohno S. Adult T-cell leukemia with an unusual esophageal lesion. Gastrointest Endosc. 2001;53:673-675. [PubMed] |
| 90. | Isomoto H, Matsushima K, Hayashi T, Imaizumi Y, Shiota J, Ishii H, Minami H, Ohnita K, Takeshima F, Shikuwa S. Endocytoscopic findings of lymphomas of the stomach. BMC Gastroenterol. 2013;13:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 91. | Perry AM, Warnke RA, Hu Q, Gaulard P, Copie-Bergman C, Alkan S, Wang HY, Cheng JX, Bacon CM, Delabie J. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122:3599-3606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 92. | Margolskee E, Jobanputra V, Lewis SK, Alobeid B, Green PH, Bhagat G. Indolent small intestinal CD4+ T-cell lymphoma is a distinct entity with unique biologic and clinical features. PLoS One. 2013;8:e68343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 93. | Leventaki V, Manning JT, Luthra R, Mehta P, Oki Y, Romaguera JE, Medeiros LJ, Vega F. Indolent peripheral T-cell lymphoma involving the gastrointestinal tract. Hum Pathol. 2014;45:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Mansoor A, Pittaluga S, Beck PL, Wilson WH, Ferry JA, Jaffe ES. NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood. 2011;117:1447-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 95. | Takeuchi K, Yokoyama M, Ishizawa S, Terui Y, Nomura K, Marutsuka K, Nunomura M, Fukushima N, Yagyuu T, Nakamine H. Lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood. 2010;116:5631-5637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 96. | Cornes JS. Multiple lymphomatous polyposis of the gastrointestinal tract. Cancer. 1961;14:249-257. [PubMed] |
| 97. | Shen KH, Chen CJ, Yen HH. Multiple polyposis of the esophagus: mantle cell lymphoma. Clin Gastroenterol Hepatol. 2012;10:e65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Esteban JM, Gutiérrez del Olmo A, Baki W, Fernández S, Soria MT, Díaz Mediavilla J, Ramírez Armengol JA. Colonic mucosa-associated lymphoid tissue lymphoma presenting as multiple polyposis. Gastrointest Endosc. 2005;61:928-930. [PubMed] |
| 99. | Isomoto H, Maeda T, Ohnita K, Nakayama T, Kohno S. Colonic lymphomatous polyposis. Gastrointest Endosc. 2004;59:261. [PubMed] |
| 100. | Peters JH, Rondonotti E, Weijmer MC, Mulder CJ, Jacobs MA. Lymphomatous polyposis of the small intestine. Gastrointest Endosc. 2008;67:763-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 101. | Ruskoné-Fourmestraux A, Audouin J. Primary gastrointestinal tract mantle cell lymphoma as multiple lymphomatous polyposis. Best Pract Res Clin Gastroenterol. 2010;24:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Moynihan MJ, Bast MA, Chan WC, Delabie Jan RS, Wu G, Weisenburger DD. Lymphomatous polyposis. A neoplasm of either follicular mantle or germinal center cell origin. Am J Surg Pathol. 1996;20:442-452. [PubMed] |
| 103. | Saito M, Mori A, Irie T, Tanaka M, Morioka M, Ozasa M, Kobayashi T, Saga A, Miwa K, Tanaka S. Endoscopic follow-up of 3 cases with gastrointestinal tract involvement of mantle cell lymphoma. Intern Med. 2010;49:231-235. [PubMed] |
| 104. | Chung R, Peters AC, Armanious H, Anand M, Gelebart P, Lai R. Biological and clinical significance of GSK-3beta in mantle cell lymphoma--an immunohistochemical study. Int J Clin Exp Pathol. 2010;3:244-253. [PubMed] |
| 105. | Chuang SS, Chang ST, Liu H, Ye H, Sheu MJ, Chen MJ, Tsao CJ. Intramucosal follicular lymphoma in the terminal ileum with nodal involvement. Pathol Res Pract. 2007;203:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 106. | Isaacson PG, MacLennan KA, Subbuswamy SG. Multiple lymphomatous polyposis of the gastrointestinal tract. Histopathology. 1984;8:641-656. [PubMed] |
| 107. | Kanehira K, Braylan RC, Lauwers GY. Early phase of intestinal mantle cell lymphoma: a report of two cases associated with advanced colonic adenocarcinoma. Mod Pathol. 2001;14:811-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (1)] |
| 108. | Chong Y, Shin JJ, Cho MY, Cui Y, Kim HY, Park KH. Synchronous primary gastric mantle cell lymphoma and early gastric carcinoma: a case report. Pathol Res Pract. 2008;204:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Okazaki K. Multiple lymphomatous polyposis form is common but not specific for mantle cell lymphoma in the gastrointestinal tract. J Gastroenterol. 2004;39:1023-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 110. | Au WY, Lo SH, Law WL, Khong PL, Shek TW, Lau WH, Chau EM. Concomitant Eber-positive lymphomatoid papulosis and malignant transformation of colonic polyposis in a heart transplant recipient. J Heart Lung Transplant. 2008;27:575-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 111. | Lee JA, Taxier MS, Nicely CJ. Electronic clinical challenges and images in GI. Lymphomatous polyposis. Gastroenterology. 2008;135:e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 112. | Hirata N, Tominaga K, Ohta K, Kadouchi K, Okazaki H, Tanigawa T, Shiba M, Watanabe T, Fujiwara Y, Nakamura S. A case of mucosa-associated lymphoid tissue lymphoma forming multiple lymphomatous polyposis in the small intestine. World J Gastroenterol. 2007;13:1453-1457. [PubMed] |
| 113. | Bahari A, Jahantigh M, Mashhadi A, Bari Z, Bari A. Plasmablastic Lymphoma presenting as small intestinal polyposis: A case-report. Iran Red Crescent Med J. 2012;14:669-675. [PubMed] |
| 114. | Khaled S, Gotlieb V, Schuster IP, Saif MW. Multiple lymphomatous polyposis associated with small lymphocytic lymphoma: a unique presentation. J Gastrointestin Liver Dis. 2008;17:461-463. [PubMed] |
| 115. | Songür Y, Tezel A, Ensari A, Sahin B. EUS diagnosis of lymphomatous polyposis. Gastrointest Endosc. 2000;52:685-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 116. | Rodrigues S, Cardoso H, Macedo G. Mantle cell lymphoma presenting as multiple lymphomatous polyposis of the small bowel. Clin Gastroenterol Hepatol. 2013;11:e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 117. | Hotta K, Oyama T, Kitamura Y, Tomori A, Miyata Y, Mitsuishi T. Mantle cell lymphoma presenting as multiple lymphomatous polyposis spreading widely to the small intestine and diagnosed by double-balloon endoscopy. Endoscopy. 2007;39 Suppl 1:E347-E348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 118. | Han EJ, Yang SA, Sohn HS, Kim JI, Kang CS, Cho SG. Successful treatment with tandem consolidation using 90yttrium-ibritumomab tiuxetan (Zevalin) and high-dose therapy with autologous PBSCT in a patient with relapsed mantle cell lymphoma presenting as multiple lymphomatous polyposis. Bone Marrow Transplant. 2012;47:877-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 119. | Tanţău M, Tanţău A, Zaharia T, Cucuianu A. Gastrointestinal lymphomatous polyposis--clinical, endoscopical and evolution features. A case report. Rom J Gastroenterol. 2005;14:273-278. [PubMed] |
| 120. | Chung HH, Kim YH, Kim JH, Cha SH, Kim BH, Kim TK, Kim AR, Cho SJ. Imaging findings of mantle cell lymphoma involving gastrointestinal tract. Yonsei Med J. 2003;44:49-57. [PubMed] |
| 121. | Yu SC, Liu KL, Chang LC. Lymphomatous polyposis as primary manifestation of mantle cell lymphoma. Eur J Haematol. 2012;89:185-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 122. | Christopoulos C, Stathakis S, Theodoropoulos I, Papavassiliou E, Savva S. Multiple lymphomatous polyposis. Br J Haematol. 2004;125:544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 123. | Chim CS, Shek TW, Chung LP, Liang R. Unusual abdominal tumors: case 3. Multiple lymphomatous polyposis in lymphoma of colon. J Clin Oncol. 2003;21:953-955. [PubMed] |
| 124. | Watanabe T, Homma N, Ogata N, Saito H, Kanefuji T, Hasegawa K, Soga K, Shibasaki K, Endo T, Ajioka Y. Complete response in a patient with colonic mantle cell lymphoma with multiple lymphomatous polyposis treated with combination chemotherapy using anti-CD20 antibody and cladribine. Gut. 2007;56:449-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 125. | Meral M, Demirpençe M, Gönen C, Akarsu M, Kayahan H, Demirkan F, Kargi A, Akpinar H. Diffuse gastrointestinal involvement of mantle cell lymphoma. Turk J Gastroenterol. 2008;19:117-120. [PubMed] |
| 126. | Alvarez-Martínez P, Pipa-Muñiz M, Ordieres-Díaz C, Fernández-Vega I, Palacio-Galán MA, Granero-Castro P, Suárez-González A. Multiple lymphomatous polyposis of intestine as presentation of mantle cell lymphoma. Rev Esp Enferm Dig. 2012;104:491-492. [PubMed] |
| 127. | Kyung Shin B, Lee H, Choi J, Kim A, Kim H, Park J, Kim I. Primary marginal zone B-cell lymphoma of gastrointestinal tract presenting as multiple lymphomatoid polyposis. Int J Colorectal Dis. 2007;22:991-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 128. | Wolfsen HC, Achem SR. Diffuse colonic polyposis detected at screening colonoscopy: mantle-cell lymphoma. Endoscopy. 2004;36:471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 129. | Lam KC, Yeo W, Lee JF, Chow J, Mok TS. Unusual abdominal tumors: case 4. Multiple lymphomatous polyposis in mantle cell lymphoma. J Clin Oncol. 2003;21:955-956. [PubMed] |
| 130. | Michopoulos S, Petraki K, Matsouka C, Kastritis E, Chrysanthopoulou H, Dimopoulos MA. Mantle-cell lymphoma (multiple lymphomatous polyposis) of the entire GI tract. J Clin Oncol. 2008;26:1555-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 131. | Xu XJ, Wu SM. Multiple lymphomatous polyposis of the gastrointestinal tract: report of three cases and literature review. J Dig Dis. 2012;13:649-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 132. | Barut F, Kandemır NO, Karakaya K, Kökten N, Ozdamar SO. Primary intestinal diffuse large B-cell lymphoma forming multiple lymphomatous polyposis. Turk J Gastroenterol. 2011;22:324-328. [PubMed] |
| 133. | Cestaro G, De Rosa M, Vitiello C, Galloro G, Gentile M. Multiple lymphomatous polyposis with diffuse involvement of the gastrointestinal tract. Case report. G Chir. 2013;34:173-175. [PubMed] |
| 134. | Murase K, Matsunaga T, Sato T, Kuribayashi K, Kogawa K, Kawano Y, Okamoto T, Takayama T, Watanabe H, Niitsu Y. Allogeneic bone marrow transplantation in a patient with T-prolymphocytic leukemia with small-intestinal involvement. Int J Clin Oncol. 2003;8:391-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 135. | Chen CY, Wu CC, Hsiao CW, Lee TY, Jao SW. Colonic manifestation of mantle cell lymphoma with multiple lymphomatous polyposis. Int J Colorectal Dis. 2009;24:729-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 136. | Franco MI, Waisberg J, Lopes LS. Multiple lymphomatous polyposis of the gastrointestinal tract. Sao Paulo Med J. 2004;122:131-133. [PubMed] |
| 137. | Kella VK, Constantine R, Parikh NS, Reed M, Cosgrove JM, Abo SM, King S. Mantle cell lymphoma of the gastrointestinal tract presenting with multiple intussusceptions--case report and review of literature. World J Surg Oncol. 2009;7:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 138. | Remes-Troche JM, De-Anda J, Ochoa V, Barreto-Zuñiga R, Arista-Nasr J, Valdovinos MA. A rare case of multiple lymphomatous polyposis with widespread involvement of the gastrointestinal tract. Arch Pathol Lab Med. 2003;127:1028-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 139. | Mar N, Khaled S, Kencana F, Gergis U, Khattak F, Brodsky N, Gotlieb V. Multiple lymphomatous polyposis as a sole presentation of mantle cell lymphoma. J Clin Gastroenterol. 2006;40:653-654. [PubMed] |
| 140. | Tomita S, Kojima M, Imura J, Hori H, Ueda Y, Koitabashi A, Suzuki Y, Nakamura T, Nakamura Y, Mitani K. Extranodal diffuse follicular center lymphoma mimicking mantle cell lymphoma of the intestine. Am J Hematol. 2003;74:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 141. | Sciaudone G, Pellino G, Selvaggi F. Diagnostic pitfalls: cancerization in IBD versus mantle cell lymphoma presenting with multiple lymphomatous polyposis. Inflamm Bowel Dis. 2011;17:E28-E30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 142. | Yang SF, Liao YL, Kuo SY, Ye H, Lin SF, Chen FM, Chen CY, Chuang SS. Primary intestinal diffuse large B-cell lymphoma presenting as multiple lymphomatous polyposis. Leuk Lymphoma. 2009;50:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 143. | Saito T, Toyoda H, Yamaguchi M, Nakamura T, Nakamura S, Mukai K, Fuke H, Wakita Y, Iwata M, Adachi Y. Ileocolonic lymphomas: a series of 16 cases. Endoscopy. 2005;37:466-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 144. | Iwamuro M, Okada H, Kawahara Y, Shinagawa K, Morito T, Yoshino T, Yamamoto K. Endoscopic features and prognoses of mantle cell lymphoma with gastrointestinal involvement. World J Gastroenterol. 2010;16:4661-4669. [PubMed] |
| 145. | Yilmaz B, Posul E, Col C, Uyeturk U, Boran C, Aktas G, Kurt M. Diffuse gastric mantle cell lymphoma resembling gastrointestinal stromal tumors. Gastrointest Endosc. 2014;79:375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 146. | Kelkitli E, Atay H, Yıldız L, Bektaş A, Turgut M. Mantle cell lymphoma mimicking rectal carcinoma. Case Rep Hematol. 2014;2014:621017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 147. | Raderer M, Püspök A, Birkner T, Streubel B, Chott A. Primary gastric mantle cell lymphoma in a patient with long standing history of Crohn’s disease. Leuk Lymphoma. 2004;45:1459-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 148. | Viana HL, Henrique RM, Ferreira ES, Correia AM, Silva RA, Dias LM, Viana RL. Endoscopic ultrasonography in multiple lymphomatous polyposis. J Clin Gastroenterol. 2002;34:150-154. [PubMed] |
| 149. | Zheng W, Song Y, Lin N, Tu M, Liu W, Zhu J. Primary gastrointestinal mantle lymphoma with massive bleeding: a case report and literature review. Chin J Cancer Res. 2013;25:250-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 150. | Yoshino T, Miyake K, Ichimura K, Mannami T, Ohara N, Hamazaki S, Akagi T. Increased incidence of follicular lymphoma in the duodenum. Am J Surg Pathol. 2000;24:688-693. [PubMed] |
| 151. | Yamamoto S, Nakase H, Yamashita K, Matsuura M, Takada M, Kawanami C, Chiba T. Gastrointestinal follicular lymphoma: review of the literature. J Gastroenterol. 2010;45:370-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 152. | Takata K, Miyata-Takata T, Sato Y, Yoshino T. Pathology of follicular lymphoma. J Clin Exp Hematop. 2014;54:3-9. [PubMed] |
| 153. | Shia J, Teruya-Feldstein J, Pan D, Hegde A, Klimstra DS, Chaganti RS, Qin J, Portlock CS, Filippa DA. Primary follicular lymphoma of the gastrointestinal tract: a clinical and pathologic study of 26 cases. Am J Surg Pathol. 2002;26:216-224. [PubMed] |
| 154. | Iwamuro M, Okada H, Takata K, Shinagawa K, Fujiki S, Shiode J, Imagawa A, Araki M, Morito T, Nishimura M. Diagnostic role of 18F-fluorodeoxyglucose positron emission tomography for follicular lymphoma with gastrointestinal involvement. World J Gastroenterol. 2012;18:6427-6436; discussion p.6434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 155. | Higuchi N, Sumida Y, Nakamura K, Itaba S, Yoshinaga S, Mizutani T, Honda K, Taki K, Murao H, Ogino H. Impact of double-balloon endoscopy on the diagnosis of jejunoileal involvement in primary intestinal follicular lymphomas: a case series. Endoscopy. 2009;41:175-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 156. | Hoffmann M, Chott A, Püspök A, Jäger U, Kletter K, Raderer M. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) does not visualize follicular lymphoma of the duodenum. Ann Hematol. 2004;83:276-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 157. | Chowdhury M, Endo M, Chiba T, Kudara N, Oana S, Sato K, Akasaka R, Tomita K, Fujiwara S, Mizutani T. Characterization of follicular lymphoma in the small intestine using double-balloon endoscopy. Gastroenterol Res Pract. 2009;2009:835258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 158. | Wright DH. Pathology of extra-nodal non Hodgkin lymphomas. Clin Oncol (R Coll Radiol). 2012;24:319-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 159. | Matsumoto H, Haruma K, Akiyama T. An unusual case of multiple small ulcerations throughout the GI tract. Gastroenterology. 2011;141:e11-e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 160. | Hayashi H, Onishi Y, Mitsuoka H, Ogura T, Maeda M, Nishigami T, Harada M. Regression of follicular lymphoma of the duodenum following eradication of H. pylori infection. Intern Med. 2013;52:2611-2614. [PubMed] |
| 161. | Ruskoné-Fourmestraux A, Delmer A, Lavergne A, Molina T, Brousse N, Audouin J, Rambaud JC. Multiple lymphomatous polyposis of the gastrointestinal tract: prospective clinicopathologic study of 31 cases. Groupe D’étude des Lymphomes Digestifs. Gastroenterology. 1997;112:7-16. [PubMed] |
| 162. | Matsuura M, Nakase H, Mochizuki N. Ileal polyposis caused by follicular lymphoma. Clin Gastroenterol Hepatol. 2007;5:e30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 163. | Yanai S, Nakamura S, Takeshita M, Fujita K, Hirahashi M, Kawasaki K, Kurahara K, Sakai Y, Matsumoto T. Translocation t(14; 18)/IGH-BCL2 in gastrointestinal follicular lymphoma: correlation with clinicopathologic features in 48 patients. Cancer. 2011;117:2467-2477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 164. | Nakase H, Matsuura M, Mikami S, Chiba T. Magnified endoscopic view of primary follicular lymphoma at the duodenal papilla. Intern Med. 2007;46:141-142. [PubMed] |
| 165. | Nakazuru S, Yoshio T, Suemura S, Iwasaki R, Hasegawa H, Sakakibara Y, Mita E, Ikeda H, Mori K, Mano M. Education and Imaging. Gastrointestinal: Unusual duodenal follicular lymphoma observed by magnifying endoscopy with narrow-band imaging. J Gastroenterol Hepatol. 2013;28:1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 166. | Iwamuro M, Kawai Y, Takata K, Kawano S, Yoshino T, Okada H, Yamamoto K. Primary intestinal follicular lymphoma: How to identify follicular lymphoma by routine endoscopy. World J Gastrointest Endosc. 2013;5:34-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 167. | Iwamuro M, Okuda M, Yumoto E, Suzuki S, Shirakawa A, Takata K, Yoshino T, Okada H, Yamamoto K. Magnifying endoscopy for intestinal follicular lymphoma is helpful for prompt diagnosis. Gut Liver. 2013;7:258-261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 168. | Inoue N, Isomoto H, Shikuwa S, Mizuta Y, Hayashi T, Kohno S. Magnifying endoscopic observation of primary follicular lymphoma of the duodenum by using the narrow-band imaging system. Gastrointest Endosc. 2009;69:158-159; discussion 159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 169. | Norimura D, Isomoto H, Imaizumi Y, Akazawa Y, Matsushima K, Inoue N, Yamaguchi N, Ohnita K, Shikuwa S, Arima T. Case series of duodenal follicular lymphoma, observed by magnified endoscopy with narrow-band imaging. Gastrointest Endosc. 2011;74:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 170. | Higuchi K, Komatsu K, Wakamatsu H, Kawasaki H, Murata M, Miyazaki K, Oikawa K, Ohwada M, Nanjo H, Otaka M. Small intestinal follicular lymphoma with multiple tumor formations diagnosed by double-balloon enteroscopy. Intern Med. 2007;46:705-709. [PubMed] |
| 171. | Akamatsu T, Kaneko Y, Ota H, Miyabayashi H, Arakura N, Tanaka E. Usefulness of double balloon enteroscopy and video capsule endoscopy for the diagnosis and management of primary follicular lymphoma of the gastrointestinal tract in its early stages. Dig Endosc. 2010;22:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 172. | Nakamura M, Ohmiya N, Hirooka Y, Miyahara R, Ando T, Watanabe O, Itoh A, Kawashima H, Ohno E, Kinoshita T. Endoscopic diagnosis of follicular lymphoma with small-bowel involvement using video capsule endoscopy and double-balloon endoscopy: a case series. Endoscopy. 2013;45:67-70. |
| 173. | Schmatz AI, Streubel B, Kretschmer-Chott E, Püspök A, Jäger U, Mannhalter C, Tiemann M, Ott G, Fischbach W, Herzog P. Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol. 2011;29:1445-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 174. | Sagaert X, Tousseyn T, Yantiss RK. Gastrointestinal B-cell lymphomas: From understanding B-cell physiology to classification and molecular pathology. World J Gastrointest Oncol. 2012;4:238-249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 175. | Miyata-Takata T, Takata K, Sato Y, Taniguchi K, Takahashi Y, Ohara N, Yoshino T. A case of diffuse large B-cell lymphoma transformed from primary duodenal follicular lymphoma. Pathol Int. 2014;64:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 176. | Hawkes EA, Wotherspoon A, Cunningham D. Diagnosis and management of rare gastrointestinal lymphomas. Leuk Lymphoma. 2012;53:2341-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 177. | Watanabe T. Treatment strategies for nodal and gastrointestinal follicular lymphoma: current status and future development. World J Gastroenterol. 2010;16:5543-5554. [PubMed] |
| 178. | Damaj G, Verkarre V, Delmer A, Solal-Celigny P, Yakoub-Agha I, Cellier C, Maurschhauser F, Bouabdallah R, Leblond V, Lefrère F. Primary follicular lymphoma of the gastrointestinal tract: a study of 25 cases and a literature review. Ann Oncol. 2003;14:623-629. [PubMed] |
| 179. | Huang WT, Hsu YH, Yang SF, Chuang SS. Primary gastrointestinal follicular lymphoma: a clinicopathologic study of 13 cases from Taiwan. J Clin Gastroenterol. 2008;42:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 180. | Mori M, Kobayashi Y, Maeshima AM, Gotoda T, Oda I, Kagami Y, Bennett S, Nomoto J, Azuma T, Yokoyama H. The indolent course and high incidence of t(14; 18) in primary duodenal follicular lymphoma. Ann Oncol. 2010;21:1500-1505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 181. | Rondonotti E, Pennazio M, Toth E, Menchen P, Riccioni ME, De Palma GD, Scotto F, De Looze D, Pachofsky T, Tacheci I. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40:488-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 182. | Iwamuro M, Okada H, Takata K, Nose S, Miyatani K, Yoshino T, Yamamoto K. Diagnostic accuracy of endoscopic biopsies for the diagnosis of gastrointestinal follicular lymphoma: a clinicopathologic study of 48 patients. Ann Diagn Pathol. 2014;18:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 183. | Nolan KD, Mone MC, Nelson EW. Plasma cell neoplasms. Review of disease progression and report of a new variant. Surg Oncol. 2005;14:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 184. | Hu K, Yahalom J. Radiotherapy in the management of plasma cell tumors. Oncology (Williston Park). 2000;14:101-108, 111; discussion 101-108, 115. [PubMed] |
| 185. | Terpos E, Rezvani K, Basu S, Milne AE, Rose PE, Scott GL, Rahemtulla A, Samson D, Apperley JF. Plasmacytoma relapses in the absence of systemic progression post-high-dose therapy for multiple myeloma. Eur J Haematol. 2005;75:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 186. | Katodritou E, Kartsios C, Gastari V, Verrou E, Mihou D, Banti A, Lazaraki G, Lazaridou A, Kaloutsi V, Zervas K. Successful treatment of extramedullary gastric plasmacytoma with the combination of bortezomib and dexamethasone: first reported case. Leuk Res. 2008;32:339-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 187. | Carneiro FP, Sobreira MN, Maia LB, Sartorelli AC, Franceschi LE, Brandão MB, Calaça BW, Lustosa FS, Lopes JV. Extramedullary plasmocytoma associated with a massive deposit of amyloid in the duodenum. World J Gastroenterol. 2009;15:3565-3568. [PubMed] |
| 188. | Park CH, Lee SM, Kim TO, Kim DU, Jung WJ, Kim GH, Song GA. Treatment of solitary extramedullary plasmacytoma of the stomach with endoscopic submucosal dissection. Gut Liver. 2009;3:334-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 189. | Krishnamoorthy N, Bal MM, Ramadwar M, Deodhar K, Mohandas KM. A rare case of primary gastric plasmacytoma: an unforeseen surprise. J Cancer Res Ther. 2010;6:549-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 190. | Chim CS, Wong WM, Nicholls J, Chung LP, Liang R. Extramedullary sites of involvement in hematologic malignancies: case 3. Hemorrhagic gastric plasmacytoma as the primary presentation in multiple myeloma. J Clin Oncol. 2002;20:344-347. [PubMed] |
| 191. | Matsumoto Y, Matsumoto T, Nakamura S, Kawasaki A, Aso M, Aoyagi K, Sadoshima S, Onoyama K, Fujishima M. Primary ileal plasmacytoma arising in mixed low- and high-grade B-cell lymphoma of mucosa-associated lymphoid tissue type. Abdom Imaging. 2000;25:139-141. [PubMed] |
| 192. | Ammar T, Kreisel F, Ciorba MA. Primary antral duodenal extramedullary plasmacytoma presenting with melena. Clin Gastroenterol Hepatol. 2010;8:A32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 193. | Kodani T, Osada T, Terai T, Ohkusa T, Shibuya T, Sakamoto N, Beppu K, Kato J, Nagahara A, Watanabe H. Successful endoscopic mucosal resection of a solitary extramedullary plasmacytoma in the sigmoid colon. Endoscopy. 2011;43 Suppl 2 UCTN:E298-E299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 194. | Nakagawa Y, Nagai T, Okawara H, Nakashima H, Hisamatsu A, Syutou M, Yamauchi M, Kai S, Nakayama T, Yokoyama S. Minute primary extramedullary plasmacytomas of the large intestine. Endoscopy. 2011;43 Suppl 2 UCTN:E105-E106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 195. | Tan J, Lade S, Harrison S, Opat S, Mac Manus MP. Complete remission of localised gastric plasmacytomas following definitive radiotherapy. J Med Imaging Radiat Oncol. 2012;56:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 196. | Lopes da Silva R. Extramedullary plasmacytoma of the small intestine: clinical features, diagnosis and treatment. J Dig Dis. 2012;13:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 197. | Lattuneddu A, Farneti F, Lucci E, Garcea D, Ronconi S, Saragoni L. A case of primary extramedullary plasmacytoma of the colon. Int J Colorectal Dis. 2004;19:289-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 198. | Doki T, Takeuchi O, Kaiho T, Tsuchiya S, Matsuzaki O, Miyazaki M. Primary isolated extramedullary plasmacytoma of the colon. Int J Colorectal Dis. 2008;23:719-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 199. | Makis W, Ciarallo A, Hickeson M, Lisbona R. Gastric recurrence of a primary colon plasmacytoma: staging and evaluating response to therapy with 18F-FDG PET/CT. Br J Radiol. 2012;85:e4-e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 200. | Pacheco DC, de Solórzano MM, Lázaro EQ, Alvarez CI, Torre LR. Extramedullary plasmacytoma of the colon: a rare cause of gastrointestinal bleeding. Endoscopy. 2009;41 Suppl 2:E306-E307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 201. | Hashiguchi K, Iwai A, Inoue T, Okudaira K, Miyazaki J, Matsuzaki K, Tsuzuki Y, Kawaguchi A, Nagao S, Itoh K. Extramedullary plasmacytoma of the rectum arising in ulcerative colitis: case report and review. Gastrointest Endosc. 2004;59:304-307. [PubMed] |
| 202. | Yaita H, Nakamura S, Kurahara K, Nagasue T, Kochi S, Oshiro Y, Ohshima K, Ikeda Y, Fuchigami T. Primary small-bowel adult T-cell leukemia/lymphoma with gastric AL amyloidosis. Endoscopy. 2014;46 Suppl 1 UCTN:E613-E614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 203. | Hokama A, Kishimoto K, Nakamoto M, Kobashigawa C, Hirata T, Kinjo N, Kinjo F, Kato S, Fujita J. Endoscopic and histopathological features of gastrointestinal amyloidosis. World J Gastrointest Endosc. 2011;3:157-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (4)] |
| 204. | Chang SS, Lu CL, Tsay SH, Chang FY, Lee SD. Amyloidosis-induced gastrointestinal bleeding in a patient with multiple myeloma. J Clin Gastroenterol. 2001;32:161-163. [PubMed] |
| 205. | Sattianayagam PT, Hawkins PN, Gillmore JD. Systemic amyloidosis and the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2009;6:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 206. | Heise W. GI-lymphomas in immunosuppressed patients (organ transplantation; HIV). Best Pract Res Clin Gastroenterol. 2010;24:57-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 207. | Delia M, Liso V, Capalbo S, Napoli A, Ricco R, Semenzato G, Liso A. Common variable immunodeficiency patient with large granular lymphocytosis developing extranodal diffuse large B-cell lymphoma: a case report. Haematologica. 2006;91:ECR61. [PubMed] |
| 208. | Castellano G, Moreno D, Galvao O, Ballestín C, Colina F, Mollejo M, Morillas JD, Solís Herruzo JA. Malignant lymphoma of jejunum with common variable hypogammaglobulinemia and diffuse nodular hyperplasia of the small intestine. A case study and literature review. J Clin Gastroenterol. 1992;15:128-135. [PubMed] |
| 209. | Persson EC, Shiels MS, Dawsey SM, Bhatia K, Anderson LA, Engels EA. Increased risk of stomach and esophageal malignancies in people with AIDS. Gastroenterology. 2012;143:943-950.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 210. | Gao GJ, Yang D, Lin KK, Xiao J, Li X, Liang HY, Liu L, Han N, Zhao HX. Clinical analysis of 10 AIDS patients with malignant lymphoma. Cancer Biol Med. 2012;9:115-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (5)] |
| 211. | Rezende RE, Mantelmacher M, Ferreira Sda C, Hyppólito EB, Machado AA, Ardengh JC, Módena JL. Clinical, endoscopic and prognostic aspects of primary gastric non-hodgkin’s lymphoma associated with acquired immunodeficiency syndrome. Braz J Infect Dis. 2009;13:2-4. [PubMed] |
| 212. | Tanaka S, Nagata N, Mine S, Igari T, Kobayashi T, Sugihara J, Honda H, Teruya K, Kikuchi Y, Oka S. Endoscopic appearance of AIDS-related gastrointestinal lymphoma with c-MYC rearrangements: case report and literature review. World J Gastroenterol. 2013;19:4827-4831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 213. | Kim Y, Leventaki V, Bhaijee F, Jackson CC, Medeiros LJ, Vega F. Extracavitary/solid variant of primary effusion lymphoma. Ann Diagn Pathol. 2012;16:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 214. | Flanagan PK, Coupland SE, Arumainathan A, Probert CS. A rare cause of weight loss. Gut. 2014;63:1024, 1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 215. | Manley K, Dunning J, Nelson M, Bower M. HIV-associated gastric natural killer/T-cell lymphoma. Int J STD AIDS. 2012;23:66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 216. | Smith G, Crocker J, Nar P, Chesner I, Leyland MJ. Immunogold-silver technique applied to showing malignant B cell infiltration of gastrointestinal tract in patients with chronic lymphocytic leukaemia and non-Hodgkin’s lymphoma. J Clin Pathol. 1987;40:756-759. [PubMed] |
| 217. | Palakodeti S, Hoda K, Munroe CA. Perirectal Burkitt lymphoma presenting as an uncommon cause of lower gastrointestinal bleeding. Clin Gastroenterol Hepatol. 2014;12:A23-A24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 218. | Takahashi T, Maruyama R, Mishima S, Inoue M, Kawakami K, Onishi C, Miyake T, Tanaka J, Nabika T, Ishikura H. Small bowel perforation caused by Epstein-Barr virus-associated B cell lymphoma in a patient with angioimmunoblastic T-cell lymphoma. J Clin Exp Hematop. 2010;50:59-63. [PubMed] |
| 219. | Ghobrial IM, Habermann TM, Maurer MJ, Geyer SM, Ristow KM, Larson TS, Walker RC, Ansell SM, Macon WR, Gores GG. Prognostic analysis for survival in adult solid organ transplant recipients with post-transplantation lymphoproliferative disorders. J Clin Oncol. 2005;23:7574-7582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 220. | Shroff R, Rees L. The post-transplant lymphoproliferative disorder-a literature review. Pediatr Nephrol. 2004;19:369-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 221. | Knight JS, Tsodikov A, Cibrik DM, Ross CW, Kaminski MS, Blayney DW. Lymphoma after solid organ transplantation: risk, response to therapy, and survival at a transplantation center. J Clin Oncol. 2009;27:3354-3362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 222. | Haverkos BM, Oza VM, Johnson A, Walker J, Shana’ah A. Rare presentation of post-transplant lymphoproliferative disorder isolated to gastroesophageal junction. World J Gastrointest Oncol. 2013;5:230-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 223. | Shitrit D, Shitrit AB, Dickman R, Sahar G, Saute M, Kramer MR. Gastrointestinal involvement of posttransplant lymphoproliferative disorder in lung transplant recipients: report of a case. Dis Colon Rectum. 2005;48:2144-2147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 224. | Lauro A, Di Simone M, Zanfi C, Pironi L, D’Errico A, Pinna AD. Operative endoscopy for treatment of native rectal post-transplantation lymphoproliferative disease after adult small bowel transplantation: a case report. Transplant Proc. 2013;45:3442-3443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 225. | Sharma S, Gurakar A, Camci C, Jabbour N. Avoiding pitfalls: what an endoscopist should know in liver transplantation--part II. Dig Dis Sci. 2009;54:1386-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 226. | Lauro A, Pinna AD, Pellegrini S, Bagni A, Zanfi C, Dazzi A, Pironi L, Di Simone MP. Long-term endoscopic follow-up in small bowel transplant recipients: single-center series. Transplant Proc. 2014;46:2325-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 227. | Wang HW, Yang W, Sun JZ, Lu JY, Li M, Sun L. Plasmablastic lymphoma of the small intestine: case report and literature review. World J Gastroenterol. 2012;18:6677-6681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 228. | Tavora F, Gonzalez-Cuyar LF, Sun CC, Burke A, Zhao XF. Extra-oral plasmablastic lymphoma: report of a case and review of literature. Hum Pathol. 2006;37:1233-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 229. | Chapman-Fredricks J, Montague N, Akunyili I, Ikpatt O. Extraoral plasmablastic lymphoma with intravascular component and MYC translocation. Ann Diagn Pathol. 2012;16:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 230. | Pruneri G, Graziadei G, Ermellino L, Baldini L, Neri A, Buffa R. Plasmablastic lymphoma of the stomach. A case report. Haematologica. 1998;83:87-89. [PubMed] |
| 231. | Colomo L, Loong F, Rives S, Pittaluga S, Martínez A, López-Guillermo A, Ojanguren J, Romagosa V, Jaffe ES, Campo E. Diffuse large B-cell lymphomas with plasmablastic differentiation represent a heterogeneous group of disease entities. Am J Surg Pathol. 2004;28:736-747. [PubMed] |
| 232. | Dong HY, Scadden DT, de Leval L, Tang Z, Isaacson PG, Harris NL. Plasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. Am J Surg Pathol. 2005;29:1633-1641. [PubMed] |
| 233. | Taddesse-Heath L, Meloni-Ehrig A, Scheerle J, Kelly JC, Jaffe ES. Plasmablastic lymphoma with MYC translocation: evidence for a common pathway in the generation of plasmablastic features. Mod Pathol. 2010;23:991-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 234. | Brahmania M, Sylwesterowic T, Leitch H. Plasmablastic lymphoma in the ano-rectal junction presenting in an immunocompetent man: a case report. J Med Case Rep. 2011;5:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 235. | Mihaljevic BS, Todorovic MR, Andjelic BM, Antic DA, Perunicic Jovanovic MD. Unusual presentation of gastric plasmablastic lymphoma in HIV-negative patient. Med Oncol. 2012;29:1186-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 236. | Hashimoto M, Inaguma S, Kasai K, Kuwabara K, Noda N, Hayakawa M, Fujino M, Ito M, Ikeda H. Plasmablastic lymphoma of the stomach in an HIV-negative patient. Pathol Int. 2012;62:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 237. | Luria L, Nguyen J, Zhou J, Jaglal M, Sokol L, Messina JL, Coppola D, Zhang L. Manifestations of gastrointestinal plasmablastic lymphoma: a case series with literature review. World J Gastroenterol. 2014;20:11894-11903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 238. | Kappelman MD, Farkas DK, Long MD, Erichsen R, Sandler RS, Sørensen HT, Baron JA. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12:265-73.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 239. | Askling J, Brandt L, Lapidus A, Karlén P, Björkholm M, Löfberg R, Ekbom A. Risk of haematopoietic cancer in patients with inflammatory bowel disease. Gut. 2005;54:617-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 240. | Mackey AC, Green L, Liang LC, Dinndorf P, Avigan M. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2007;44:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 241. | Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: a meta-analysis. Clin Gastroenterol Hepatol. 2009;7:874-881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 386] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 242. | Venizelos I, Tamiolakis D, Bolioti S, Nikolaidou S, Lambropoulou M, Alexiadis G, Manavis J, Papadopoulos N. Primary gastric Hodgkin’s lymphoma: a case report and review of the literature. Leuk Lymphoma. 2005;46:147-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 243. | Sharma S, Rana S, Kapur S, Jairajpuri ZS. Primary Intestinal Hodgkin’s Lymphoma: An Uncommon Presentation. J Lab Physicians. 2013;5:124-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 244. | Kashi MR, Belayev L, Parker A. Primary extranodal Hodgkin lymphoma of the colon masquerading as new diagnosis of Crohn’s disease. Clin Gastroenterol Hepatol. 2010;8:A20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 245. | Hossain FS, Koak Y, Khan FH. Primary gastric Hodgkin’s lymphoma. World J Surg Oncol. 2007;5:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 246. | Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17:697-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 245] [Cited by in RCA: 281] [Article Influence: 18.7] [Reference Citation Analysis (3)] |
| 247. | da Costa AA, Flora AC, Stroher M, Soares FA, de Lima VC. Primary Hodgkin’s lymphoma of the rectum: an unusual presentation. J Clin Oncol. 2011;29:e268-e270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 248. | Gupta D, Sharma A, Dwary A, Kark AK. Hodgkin lymphoma involving ascending colon and mesenteric lymphnodes: A rare entity. Indian J Cancer. 2009;46:175-176. [PubMed] |
| 249. | Romano A, Vetro C, Caocci G, Greco M, Parrinello NL, Di Raimondo F, La Nasa G. Immunological deregulation in classic hodgkin lymphoma. Mediterr J Hematol Infect Dis. 2014;6:e2014039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 250. | Bautista-Quach MA, Ake CD, Chen M, Wang J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest Oncol. 2012;3:209-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 251. | Vadmal MS, LaValle GP, DeYoung BR, Frankel WL, Marsh WL. Primary localized extranodal hodgkin disease of the transverse colon. Arch Pathol Lab Med. 2000;124:1824-1827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 252. | Dojcinov SD, Venkataraman G, Raffeld M, Pittaluga S, Jaffe ES. EBV positive mucocutaneous ulcer--a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 409] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Boulay B, Braden B, Polistina F S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wu HL