Published online Jun 16, 2013. doi: 10.4253/wjge.v5.i6.297
Revised: April 4, 2013
Accepted: May 7, 2013
Published online: June 16, 2013
Processing time: 94 Days and 20.9 Hours
The development of pseudocysts in patients with chronic pancreatitis has been reported in 23%-60% of cases and drainage is indicated when they become symptomatic. Endoscopic ultrasound-guided drainage with the placement of plastic or metallic stents to create a cystogastric anastomosis has been shown to be a reliable and efficacious maneuver. Metallic stent use appears to be a safe and effective alternative that shortens the length of time of the procedure and maintains a greater diameter in the cystogastric communication. However, important migration rates have been reported. The use of new metallic stents that are specially designed to prevent migration represents a promising development in the treatment of these group of patients that appears to be safe and effective for pseudocyst drainage and could importantly reduce migration rates, while at the same time having the advantage of a single step procedure and a larger fistula diameter in the endoscopic cystogastric anastomosis.
Core tip: The use of novel covered self-expanding metallic stents that are specially designed to prevent migration represents a promising development in the treatment of patients with pancreatic pseudocysts that appears to be safe and effective for drainage and could importantly reduce migration rates, while at the same time having the advantage of a single step procedure and a larger fistula diameter in the endoscopic cystogastric anastomosis.
- Citation: Téllez-Ávila FI, Villalobos-Garita &, Ramírez-Luna M&. Use of a novel covered self-expandable metal stent with an anti-migration system for endoscopic ultrasound-guided drainage of a pseudocyst. World J Gastrointest Endosc 2013; 5(6): 297-299
- URL: https://www.wjgnet.com/1948-5190/full/v5/i6/297.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i6.297
Standard procedure for endoscopic ultrasound-guided drainage of peripancreatic collections includes the use of various plastic endoprostheses in the same endoscopic procedure and the need for programmed replacement to preclude their dysfunction. The use of completely covered self-expanding metallic stents (CSEMS) has recently been shown to be a safe and effective alternative that reduces the number of procedures[1]. However, there are high migration rates (up to 15%)[1,2]. The use of metallic stents designed to prevent migration are an interesting option in these patients that reduces procedure duration and provides a larger fistula diameter.
A 51-year-old man presented with chronic pancreatitis (CP) due to alcohol overuse and had a past 3-year history of obstructive jaundice with a pseudotumor at the level of the pancreatic head, along with common bile duct stricture. Cytology consistent with CP without evidence of cancer was obtained through endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNA). The patient underwent a number of endoscopic treatment sessions for the placement of multiple plastic stents and pneumatic dilatation 4 times a year for 3 years with no adequate response. During the last year of disease progression, he presented with a pseudocyst associated with early postprandial fullness and abdominal pain.
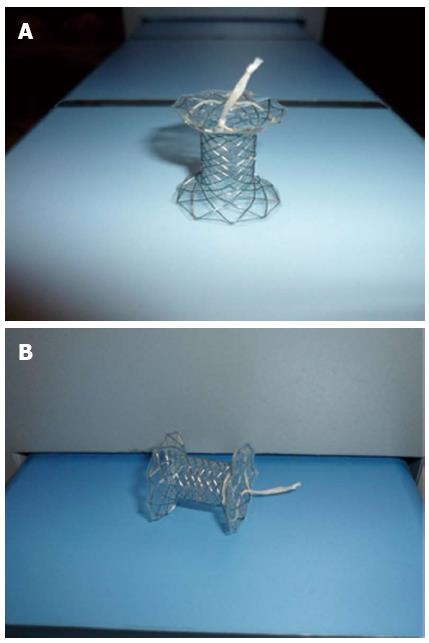
The patient rejected surgical treatment of the pseudocyst and the biliary stricture. Due to symptom persistence, the patient underwent endoscopic placement of a CSEMS in the biliary tract and endoscopic ultrasound-guided drainage of the pseudocyst with the placement of a 3 cm long “NAGI” CSEMS (Taewoong-Medical Co, Seoul, South Korea) with a 10 mm diameter in the center and 20 mm ends, for an endoscopic cystogastric anastomosis (Figure 1).
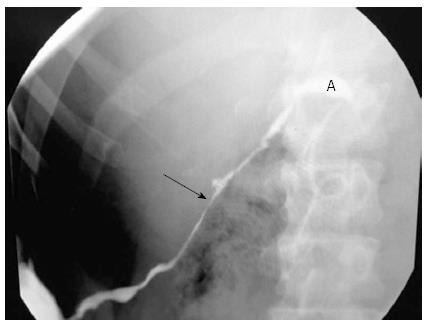
Using a duodenoscope (GIF-140, Olympus America, Melville, NY, United States), endoscopic retrograde cholangiopancreatography (ERCP) was performed. There was evidence of intrapancreatic bile duct stricture and a 6 cm long CSEMS with a 10 mm diameter (Taewoong-Medical Co, Seoul, South Korea) was placed. Pancreatography revealed an area of stricture, at the level of the neck of the pancreas, through which the passage of 0.035”, 0.025”, and 0.018” guidewires (Boston Scientific, Natick, MA, United States) was not possible. The body and tail of the pancreas were dilated and there was contrast medium leakage (Figure 2).
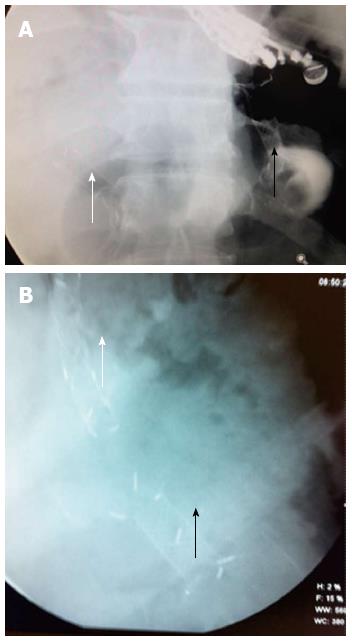
A pseudocyst with a 6 cm × 5 cm diameter was then seen with a GF-UCT140AL5 echo endoscope (Olympus America, Melville, NY, United States). Under endosonographic vision, and after using the Doppler mode to detect blood vessels in the tract, the pseudocyst was punctured through the gastric wall with a 19G-caliber Echotip® needle (Cook Endoscopy, Winston-Salem, NC, United States) followed by the introduction of a 0.035” Hydra Jagwire® (Boston Scientific, Natick, MA, United States). The needle-knife (Boston Scientific, Natick, MA, United States), 6, 7, 8.5, and 10 F Soehendra® catheters (Cook Endoscopy, Winston-Salem, NC, United States), and lastly, a Max Force® balloon dilator (Boston Scientific, Galway, Ireland) were progressively advanced along the guidewire to dilate the puncture tract up to 8 mm. A “NAGI” CSEMS was put in place under fluoroscopic vision to provide support to the cystogastrostomy (Figure 3).
At 6 mo of outpatient evaluation, the patient is asymptomatic and his liver function tests are normal (Table 1).
| Parameter | Before procedure | 12-wk after procedure |
| Total bilirubin | 5.66 | 0.45 |
| Direct bilirubin | 4.09 | 0.08 |
| ALT | 351 | 49 |
| AST | 271 | 25 |
| ALP | 391 | 112 |
High success rates have been reported for ultrasound-guided pseudocyst drainage since 2001 and this procedure has shown advantages over the surgical option in relation to hospital stay and costs[3,4].
The placement of multiple plastic stents is technically difficult and so the use of a single CSEMS has been proposed[1]. Procedure duration and resolution time is lower with CSEMSs and this is probably related to the larger fistula diameter, while the technical success, clinical outcome, and complications are similar[5]. Nevertheless, the probability of stent migration in 15% of the patients is a concern[1,2]. In the present case, a stent with a specially designed feature to reduce the high migration rate was used. The design of the “NAGI” stent, with 20 mm large and acute angled flare ends, implies a decrease in the migration rates due to better anchoring in the gastric and pseudocyst extremes. Besides this is fully covered with silicone that prevents leakage and tissue ingrowth and with retrieval string allows for easy removal. With a reduced migration rate, severe complications such as gastrointestinal tract obstruction, impaction, and/or perforation of the gastrointestinal tract wall could be prevented[6-11].
In conclusion, the use of CSEMSs that are designed with an anti-migration system is an alternative that appears to be safe and effective for pseudocyst drainage and could importantly reduce migration rates, while at the same time having the advantage of a single step procedure and a larger fistula diameter in the endoscopic cystogastric anastomosis.
| 1. | Penn DE, Draganov PV, Wagh MS, Forsmark CE, Gupte AR, Chauhan SS. Prospective evaluation of the use of fully covered self-expanding metal stents for EUS-guided transmural drainage of pancreatic pseudocysts. Gastrointest Endosc. 2012;76:679-684. |
| 2. | Varadarajulu S, Wilcox CM. Endoscopic placement of permanent indwelling transmural stents in disconnected pancreatic duct syndrome: does benefit outweigh the risks? Gastrointest Endosc. 2011;74:1408-1412. |
| 3. | Giovannini M, Pesenti C, Rolland AL, Moutardier V, Delpero JR. Endoscopic ultrasound-guided drainage of pancreatic pseudocysts or pancreatic abscesses using a therapeutic echo endoscope. Endoscopy. 2001;33:473-477. |
| 4. | Varadarajulu S, Lopes TL, Wilcox CM, Drelichman ER, Kilgore ML, Christein JD. EUS versus surgical cyst-gastrostomy for management of pancreatic pseudocysts. Gastrointest Endosc. 2008;68:649-655. |
| 5. | Lee S, Park D, Seo D, Lee SK, Kim MH. Comparing Plastic versus Covered Self Expandable Metallic Stents in EUS guided transmural drainage for peripancreatic flui collections, Which is better? A pilot study. Gastrointest Endosc. 2012;75:AB434. |
| 6. | Ho H, Mahajan A, Gosain S, Jain A, Brock A, Rehan ME, Ellen K, Shami VM, Kahaleh M. Management of complications associated with partially covered biliary metal stents. Dig Dis Sci. 2010;55:516-522. |
| 7. | Baron TH. Minimizing endoscopic complications: endoluminal stents. Gastrointest Endosc Clin N Am. 2007;17:83-104, vii. |
| 8. | Kundu R, Pleskow D. Biliary and Pancreatic Stents: Complications and Management. Tech in Gastrointest Endosc. 2007;9:125-134. |
| 9. | Baron TH. Expandable gastrointestinal stents. Gastroenterology. 2007;133:1407-1411. |
| 10. | Navarta-Herrera GJ, Blumtritt G, Telayna F, Trouboul F. Migración de stent biliar con perforación de sigmoides. Reporte de un caso. Rev Arg Res Cir. 2011;16:40-43. |
| 11. | Zerrweck-López C, de la Peña Rodríguez J, Orozco Obregón P. Oclusión intestinal causada por migración de endoprótesis de vía biliar. An Med (Mex). 2007;52:86-89. |
P- Reviewer Guan YS S- Editor Song XX L- Editor A E- Editor Zhang DN