Published online Jun 16, 2013. doi: 10.4253/wjge.v5.i6.281
Revised: April 3, 2013
Accepted: April 18, 2013
Published online: June 16, 2013
Processing time: 115 Days and 20.5 Hours
AIM: To evaluate clinical outcomes and risk factors for endoscopic perforation during endoscopic submucosal dissection (ESD) in a prospective study.
METHODS: We investigated the clinical outcomes and risk factors for the development of perforation in 98 consecutive gastric neoplasms undergoing ESD regarding. Demographic and clinical parameters including patient-, tumor-, and treatment-related factors, clinical parameters, and duration of hospital stay were analyzed for risk factors for perforation. In subgroup analysis, we also compared the clinical outcomes between perforation and “silent” free air without endoscopically visible perforation detected only by computed tomography.
RESULTS: Perforation was identified in 8.2% of patients. All patients were managed conservatively by the administration of antibiotics. The mean procedure time was significantly longer in patients with endoscopic perforation than in those without. According to the receiver-operating characteristic analysis, the resulting cutoff value of the procedure time for perforation was 115 min (87.5% sensitivity, 56.7% specificity). Prolonged procedure time (≥ 115 min) was associated with an increased risk of perforation (odds ratio 9.15; 95%CI: 1.08-77.54; P = 0.04). Following ESD, body temperature and C-reactive protein level were significantly higher in patients with perforation than in those without (P = 0.02), whereas there was no difference between these patient groups on the starting day of oral intake or of hospitalization. In subgroup analysis, the post-ESD clinical course was not different between endoscopic perforation and silent free air.
CONCLUSION: Only prolonged procedure time (≥ 115 min) was significantly associated with perforation. The clinical outcomes of perforation are favorable and are comparable to those of patients with or without silent free air.
Core tip: There has been little prospective study on the clinical outcomes of endoscopic perforation in endoscopic submucosal dissection for gastric neoplasia. In the current study, we investigated clinical outcomes of perforation during gastric endoscopic submucosal dissection, and analyzed various demographic and clinical parameters for risk factors. The results clearly demonstrated that prolonged procedure time (≥ 115 min), but not tumor location, was significantly associated with endoscopic perforation. The clinical outcomes of perforation are favorable and comparable to those with or without silent free air without endoscopic perforation as detected only by computed tomography.
- Citation: Watari J, Tomita T, Toyoshima F, Sakurai J, Kondo T, Asano H, Yamasaki T, Okugawa T, Ikehara H, Oshima T, Fukui H, Miwa H. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: A prospective pilot study. World J Gastrointest Endosc 2013; 5(6): 281-287
- URL: https://www.wjgnet.com/1948-5190/full/v5/i6/281.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i6.281
Endoscopic submucosal dissection (ESD) is indicated for early gastric cancer in Japan, and enables en bloc resection regardless of lesion size[1,2]. Besides its positive outcomes, ESD carries controversial risks, such as perforation, bleeding, aspiration pneumonia, and technical difficulties[1-6]. According to a recent meta-analysis, although ESD had higher en bloc and curative resection rates than endoscopic mucosal resection (EMR), operation time was longer, with higher risks of complications compared to EMR[7].
Previous reports showed that large tumor size, location of the lesion in an upper region of the stomach, and long procedure time are risk factors for perforation following ESD[8-13]. Although perforation may be the most serious complication in the ESD procedure, most studies have reported recovery from perforation with conservative management such as endoscopic clipping, fasting, nasogastric aspiration, and broad-spectrum antibiotics[1,14]. However, the previous reports regarding clinical outcomes of perforation during ESD are retrospective analyses[5,8,9,13-15]. More recently, prospective studies by Onogi et al[16] and our group[17] found that “transmural air leak” or “silent” free air without endoscopically visible perforation detected only by computed tomography (CT) did not affect the post-ESD clinical course. In contrast, there has been little prospective research regarding clinical outcomes of perforation during the ESD procedure. In this study, we prospectively evaluated clinical outcomes and factors of endoscopic perforation during ESD.
Between November 2010 and January 2012, 94 consecutive patients with a total of 98 gastric adenomas or cancers treated with ESD were enrolled in this study. In patients with multiple gastric neoplasms, each of the lesions was treated separately at an interval of at least 1 mo. The indications for ESD for gastric neoplasms, such as intramucosal gastric cancer and adenoma, include intramucosal differentiated tubular adenocarcinoma of any size without ulceration or signs of submucosal invasion and intramucosal differentiated-type adenocarcinoma of less than 3 cm with an ulcer scar. The histology, tumor location, and depth of invasion fulfilled the criteria of the Japanese Research Society for Gastric Cancer[18]. The histological criteria for the ESD to be considered curative were as follows: (1) margins negative for a lesion; and (2) an intramucosal lesion or minute submucosal invasion (up to 500 m invasion into the submucosal layer) without any venous or lymphatic invasion[16].
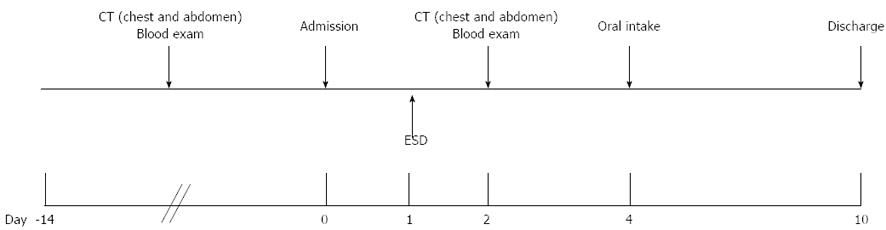
All patients were admitted on the day before ESD, and were usually discharged 9 d after the procedure. Oral intake was started 3 d after ESD. The hospital stay for patients without any clinical complications was basically 10 d, in line with the clinical protocol at our hospital (Figure 1).
Written informed consent was obtained from all patients prior to the start of the study, and all patients provided written informed consent for publication of individual clinical details. The study design was approved by the ethics committee of Hyogo College of Medicine.
The ESD procedure was performed under conscious sedation using midazolam and pethidine with or without propofol. ESD was performed using an insulation-tipped diathermic (IT-2) knife (KD-610L; Olympus Medical Systems, Tokyo, Japan) or FlushKnife BT (Fujifilm, Tokyo, Japan) for en bloc resection. We marked the normal mucosa about 5 mm outside the tumor edge with a needle knife (KD-1L-1; Olympus Medical Systems). Saline with adrenaline (1:10000 solution in saline) was injected into the submucosa, and the initial incision was made outside the marked line. Next, the diathermic knife was inserted into the initial incision, and the mucosa 5 mm outside the mark was cut circumferentially using a VIO electrosurgical generator (Erbe, Tübingen, Germany). After tumor resection, all visible vessels in the created ulcer were coagulated using coagulation forceps (Olympus Medical Systems) to reduce the risk of delayed bleeding, according to a report by Takizawa and colleagues[5]. During the ESD procedure, carbon dioxide (CO2) insufflation was used.
Endoscopic perforation was diagnosed by direct endoscopic observation of the extramural organ or fat through the muscle layer during ESD. When perforation occurred, the perforation site was immediately closed using endoclips (Olympus Medical Systems). However, endoclips sometimes make it difficult to obtain a sufficient resection margin or perform en bloc resection. In such cases, it is desirable to apply clips to perforated areas after an incision has been made or an exfoliation performed and after sufficient space for complete resection has been created. All patients with endoscopic perforation were administered antibiotics. In cases with severe pneumoperitonium such as that caused respiratory failure, decreased blood pressure or increased abdominal fullness, after which centesis was performed with an 18-gauge puncture needle to remove air from the abdominal cavity. Patients with this condition received a nasogastric tube for 1 to 2 d. In patients with perforation, oral intake was started once the white blood cell (WBC) count fell to the normal range.
We evaluated the following demographic and clinical parameters: patient-related factors (age, sex, use of alcohol and tobacco, and body mass index), tumor-related factors (macroscopic type, tumor location, presence or absence of scarring in the tumor, invasion depth, and histology), treatment-related factors (operator’s skill, mean dimension (cm2) of the resected specimen, and procedure time), clinical parameters (body temperature, WBC count, and serum C-reactive protein (CRP) level at one day before and after ESD), and duration of hospital stay. The procedure time was recorded from the start of the marking around the tumor to the removal of the endoscope.
The operator’s skill is thought to affect the total procedure time and the treatment complications of ESD, according to previous reports[1-6]. Thus, differences in these outcomes between experienced and less-experienced endoscopists should be assessed. Japanese endoscopists receive board certification from the Japan Gastroenterological Endoscopy Society (JGES) after 5 years of training in a JGES-approved educational institution of endoscopy, and must also pass an examination administered by JGES. In the present study, the doctors who were defined as experienced endoscopists had board certification from the JGES and had each performed more than 30 ESD procedures for early gastric cancers[5,19,20].
The data were assessed using the Mann-Whitney U-test for comparisons between two independent groups and the χ2 test or Fisher’s exact test for comparisons between two proportions. Patient-, tumor-, and treatment-related factors were included as potential risk factors for endoscopic perforation in univariate analysis. Risk factors with a P value of < 0.05 in univariate analysis were included in the multiple logistic regression model and analyzed using the backward approach. Odds ratios (OR) and 95%CI were calculated for risk factors. The 95%CI of the OR was used to assess statistical significance at the conventional level of 0.05. Statistical analysis was performed using StatView version 5.0 (SAS Institute, Cary, NC, United States).
To identify the ESD procedure time that was associated with the highest diagnostic performance in terms of perforation development, we used receiver operating characteristic (ROC) curve analysis. The ROC curve for procedure time was plotted by using SPSS 11.0 for Windows (SPSS, Chicago, IL, United States). The area under the ROC curve (AUC) was calculated. The point with the largest AUC was defined as the point having the greatest association with perforation. Optimal cutoff points were determined on the basis of maximum values of the Youden index, calculated as [sensitivity + specificity-1], and the minimum values of the square root of [(1 - sensitivity)2 + (1 - specificity)2], which indicates the minimum distance from the upper left corner to the point on the ROC curve[21].
A total of 98 gastric lesions in 94 patients were evaluated, including 6 adenomas and 92 gastric cancers. The mean age of the patients was 70.9 ± 9.1 years (range, 48-87 years), and women accounted for 24.5% (23 of 94) of the patients. The curative en bloc resection rate was 88.8% (87 of 98), and endoscopic perforation during ESD occurred in 8.2% (8 lesions).
The mean procedure time was significantly longer in patients with perforation than in those without (controls) (P = 0.02), but the tumor location and lesion with scar were not associated with perforation (Table 1). Also, the perforation rate did not differ between experienced and less-experienced operators.
| Control (n = 90) | Perforation (n = 8) | P value | |
| Patient-related factors | |||
| Age (yr) | 70.8 ± 9.2 | 72.4 ± 7.5 | NS |
| Sex, male/female | 69/21 | 6/2 | NS |
| Active alcohol drinking Positive/negative | 40/50 | 4/4 | NS |
| Active smokingPositive/negative | 16/74 | 2/6 | NS |
| Body mass index (kg/m2) | 23.2 ± 2.9 | 23.0 ± 3.3 | NS |
| Tumor-related factors | |||
| Macroscopic type: I/IIa/II b/IIc | 9/43/2/36 | 0/5/0/3 | NS |
| Location: Upper/middle/ lower | 12/48/30 | 2/6/0 | NS |
| Scar: Positive/negative | 9/81 | 0/8 | NS |
| Depth of invasion: M/SM and beyond | 77/13 | 5/3 | NS |
| Histology: DAC/poorly DAC/adenoma | 5/6/1979 | 7/1/0 | NS |
| Treatment-related factors | |||
| Operator: Experienced/ less-experienced | 32/58 | 2/6 | NS |
| Resected dimensions (cm2) | 9.7 ± 6.0 | 24.0 ± 24.9 | NS |
| Procedure time (min) | 122.5 ± 75.6 | 203.1 ± 114.3 | 0.02 |
| Clinical parameters | |||
| Body temperature | 36.9 ± 0.5 | 37.3 ± 0.6 | NS |
| White blood cell (/mL) | 10566.9 ± 2903.6 | 9898.8 ± 3149.4 | NS |
| C-reactive protein (mg/dL) | 1.5 ± 1.4 | 2.4 ± 1.3 | 0.04 |
| Hospital stay (d) | 10.5 ± 2.4 | 10.9 ± 1.5 | NS |
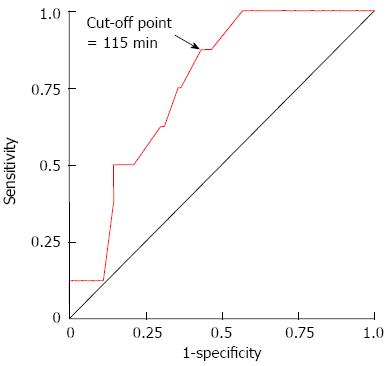
The association between endoscopic perforation and procedure time was evaluated using ROC curve analysis (Figure 2). According to this analysis, cutoff points showing optimal performance were chosen by the distance to the ROC curve and the Youden index for the procedure time. The resulting cutoff value of the procedure time for perforation was 115 min (sensitivity, 87.5%; specificity, 56.7%) for patients who underwent gastric ESD.
Based on the ROC curve analysis and optimal cutoff points of the procedure time of gastric ESD determined above, a procedure time of ≥ 115 min was used in the analyses. We analyzed the strength of the association between perforation development and procedure time (≥ 115 min). As a result, procedure time (≥ 115 min) was significantly associated with increased endoscopic perforation (OR = 9.15, 95%CI: 1.08-77.54; P = 0.04).
Following ESD, only the CRP level was significantly higher in patients with perforation than in those without (P = 0.04) (Table 1). The clinical courses of patients with perforation are summarized in Table 2. Four patients with endoscopic perforation received a nasogastric tube for a mean of 1.3 d. None of the patients with this condition required surgery, and there was no perforation-related mortality. Oral intake was started from a mean of 4.0 d after ESD (range, 3-7 d). Patients with perforation were discharged after a mean stay of 10.9 d (9.9 d after ESD); this did not differ significantly from the average stays of patients without perforation (Table 1).
| Age (yr) | Sex | Macroscopic type | Location | Depth of invasion | Scar | Resected dimensions (cm2) | Procedure time (min) | Nasogastric tube (d) | Beginning of oral intake after ESD (d) | Hospitalization (d) |
| 62 | Male | IIa | Upper | M | - | 69.1 | 460 | 1 | 4 | 10 |
| 63 | Male | IIc | Middle | M | - | 5.5 | 130 | - | 3 | 10 |
| 77 | Male | IIb +IIa | Middle | SM | - | 18.8 | 220 | 1 | 3 | 11 |
| 71 | Male | IIa | Middle | M | - | 8.2 | 160 | 2 | 3 | 10 |
| 83 | Female | IIc | Lower | SM | - | 56.1 | 220 | - | 5 | 12 |
| 72 | Female | IIa | Middle | M | - | 22.0 | 215 | - | 3 | 10 |
| 80 | Male | IIa | Upper | M | - | 3.1 | 100 | - | 3 | 10 |
| 71 | Male | IIc | Lower | SM | - | 9.4 | 120 | 1 | 7 | 14 |
All patients underwent plain abdominal CT on the day after ESD. If free air close to the stomach was detected by CT on the day after ESD even though no evidence of endoscopic perforation was seen during ESD and peritonitis, the case was defined as silent free air as reported previously[17]. We compared the clinical outcomes between patients with perforation and silent free air.
Silent free air was observed in 35.7% (35 lesions) in this period. Body temperature and CRP levels following ESD were significantly higher in patients with endoscopic perforation than in those with silent free air (P = 0.04 and P = 0.03, respectively) (Table 3). Oral intake was started from 3 d after ESD in all patients with silent free air, as scheduled based on the clinical protocol (Figure 1), but no significant difference in the starting day of oral intake was found between these conditions.
| Perforation (n = 8) | Silent free air on CT (n = 35) | P value | |
| Tumor-related factors | |||
| Location: Upper/middle/ lower | 2/6/0 | 9/21/5 | NS |
| Scar: Positive/negative | 0/6 | 5/30 | NS |
| Depth of invasion: M/SM and beyond | 5/3 | 5/30 | NS |
| Treatment-related factors | |||
| Operator: Experienced/ less-experienced | 2/6 | 16/19 | NS |
| Resected dimensions (cm2) | 24.0 ± 24.9 | 10.4 ± 7.2 | NS |
| Procedure time (min) | 203.1 ± 114.3 | 145.1 ± 76.5 | NS |
| Clinical parameters | |||
| Body temperature | 37.3 ± 0.6 | 36.8 ± 0.6 | 0.04 |
| White blood cell (/mm3) | 9898.8 ± 3149.4 | 10658.0 ± 3119.3 | NS |
| C-reactive protein (mg/dL) | 2.4 ± 1.3 | 1.4 ± 1.0 | 0.03 |
| Oral intake (d) | 3.0 | 4.0 ± 1.5 | NS |
| Hospital stay (d) | 10.9 ± 1.5 | 10.7 ± 2.1 | NS |
Even though ESD is widely accepted and performed worldwide in patients with gastric cancer, perforation is a common and serious complication. In contrast, many retrospective studies show that conservative management by immediate endoscopic closure with endoclips is effective in most patients with perforation[1,14]. Recently in prospective studies, Onogi et al[16] and we reported that an “air leak” after gastric ESD, detected only by CT in patients without endoscopically visible perforation, was observed frequently, and this asymptomatic (silent) free air does not affect the post-ESD clinical course. Likewise, the current work, which is based on our recent study[17], clearly demonstrated that perforation was not associated with clinically significant complications, and showed clinical outcomes similar to those of cases without perforation. Therefore, perforations might be considered part of the procedure and not as a complication[22].
In the current study, a procedure time exceeding 115 min was considered to be a reliable marker associated with perforation development by ROC curve analysis. Thus, prolonged procedure time was a highly significant factor for endoscopic perforation; this finding is consistent with those of other studies[9,11-13,16]. However, tumor location was not related to perforation. In our previous study[17], tumor location was also not an independent risk factor for silent free air. Previous studies showed that tumor location (the upper portion of the stomach) was a significant and independent predictor of perforation by multivariate analysis[8-13,16,17]. A possible explanation for the discrepancy may be the difference in the number of patients with perforation investigated between ours and other studies. Indeed, only 8 of the patients in our study had perforation. In reports from Japan and South Korea, perforation was observed in 1.2% to 6.1% of patients[8-15]. Our perforation rate (8.2%) was slightly higher than in the other studies. Of the 8 cases with endoscopic perforation, 6 were treated by less-experienced operators. However, operator skill was not associated with either perforation or silent free air (Tables 1 and 2). This was attributed to the fact that more experienced endoscopists were more likely to perform ESD in patients with larger tumors or tumors with scars than were less-experienced endoscopists. Actually, the features of the lesions, i.e., ulcer scarring, tumor size, and tumor location, in addition to technical skill, may be significant risk factors for perforation, as many reports have pointed out.
Silent free air was detected in 35.7% of the cases in this study. Jeon et al[14] recently reported a similar study, which compared the clinical outcomes of treatment for macro- and micro-perforations with ESD and determined the short-term prognosis after ESD. Those authors defined micro-perforation as a perforation identified by a pneumoperitoneum seen on plain radiographs after ESD. According to their report, a micro-perforation, resembling the silent free air in our study, was observed in only 0.76% (13 of 1711) of the patients undergoing gastric ESD, an extremely lower incidence than we found in our study. The difference may be attributable to different sensitivities between plain radiograph and CT.
With regards to inflammatory markers after ESD, such as body temperature, WBC level, and CRP level, only CRP level was significantly higher in perforation patients than in controls (P = 0.04). All the patients with endoscopic perforation were exposed to antibiotics, and 4 patients received a nasogastric tube. By conservative treatments, these patients with perforation were able to start oral intake from a mean of 4 d following ESD; this time to resume oral intake was not significantly different from that in patients with or without silent free air. Furthermore, the hospital stay did not differ according to the presence or absence of perforation or silent free air. These results indicate that immediate closure of the perforation site, intravenous antibiotic therapy, or brief nasogastric tube replacement may be important for favorable outcomes. In our clinical protocol of ESD, the hospital stay was 10 d, and oral intake was started 3 d after ESD; these may be slightly longer than in other hospitals. It remains possible, therefore, that this longer hospitalization in our protocol affected the present results.
In our series, we used CO2 insufflation during the ESD procedure. It has been reported that ESD with CO2 insufflation is safe and reduces both abdominal discomfort and the risk of perforation after ESD[9,23,24]. Hereafter, ESD with CO2 insufflation should be performed during lengthy endoscopic treatment procedures to avoid complications during and after ESD.
In the present study, there has been no evidence of peritoneal seeding after endoscopic perforation with short follow-up periods by CT or ultrasonography, and this was consistent with previous results[10,14]. Similarly, Ikehara et al[25] reported that perforation associated with EMR and ESD does not lead to peritoneal dissemination even in the long term (median 53.6 mo, range 7.0-136.6 mo). Further studies are needed before definitive conclusions can be drawn about the risk of peritoneal seeding after perforation or silent free air[10].
The limitation of this study is the small number of patients with perforation in a single center, limiting our ability to draw conclusions, as mentioned previously[8,9,13,14]. Our results do not necessarily mean, therefore, that perforation during ESD can be managed conservatively. Seewald et al[22] previously showed an algorithm for endoscopic management of gastrointestinal perforation. Therefore further studies with larger numbers of patients will be needed to clarify the long-term outcomes of patients with endoscopic perforation.
In conclusion, the current prospective pilot study showed that prolonged procedure time (≥ 115 min) was associated with an increased risk of perforation. However, conservative management of perforation was successful and did not affect the post-ESD clinical course. Therefore, clinical outcomes of endoscopic perforation are favorable and comparable to those with or without silent free air.
The authors thank the late Takayuki Matsumoto (Professor, Hyogo College of Medicine) for his helpful supports and encouragement, and Dr. Takashi Daimon and Ms. Kazuko Nagase for their valuable help with the statistical analyses.
Endoscopic submucosal dissection (ESD) is indicated for early gastric cancer in Japan, and enables en bloc resection regardless of lesion size. Besides its positive outcomes, ESD carries controversial risks, such as perforation, bleeding, aspiration pneumonia, and technical difficulties.
Even though ESD is widely accepted and performed worldwide in patients with gastric cancer, perforation is a common and serious complication. In contrast, many retrospective studies show that conservative management by immediate endoscopic closure with endoclips is effective in most patients with perforation.
There has been little prospective study on the clinical outcomes of endoscopic perforation in endoscopic submucosal dissection for gastric neoplasia. In the current study, authors investigated clinical outcomes of perforation during gastric endoscopic submucosal dissection, and analyzed various demographic and clinical parameters for risk factors.
The clinical outcomes of perforation are favorable and comparable to those with or without silent free air without endoscopic perforation as detected only by computed tomography.
Generally, this is an interesting and well written prospective study about clinical outcomes and risk factors for perforation in gastric ESD. Authors prospectively investigated 98 consecutive gastric neoplasms undergoing ESD regarding the clinical outcomes and risk factors for development of perforation. They clearly showed that prolonged procedure time was associated with an increased risk of perforation.
P- Reviewer Komatsu K S- Editor Gou SX L- Editor A E- Editor Zhang DN
| 1. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. |
| 2. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. |
| 3. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. |
| 4. | Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776-782. |
| 5. | Takizawa K, Oda I, Gotoda T, Yokoi C, Matsuda T, Saito Y, Saito D, Ono H. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection--an analysis of risk factors. Endoscopy. 2008;40:179-183. |
| 6. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. |
| 7. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. |
| 8. | Imagawa A, Okada H, Kawahara Y, Takenaka R, Kato J, Kawamoto H, Fujiki S, Takata R, Yoshino T, Shiratori Y. Endoscopic submucosal dissection for early gastric cancer: results and degrees of technical difficulty as well as success. Endoscopy. 2006;38:987-990. |
| 9. | Mannen K, Tsunada S, Hara M, Yamaguchi K, Sakata Y, Fujise T, Noda T, Shimoda R, Sakata H, Ogata S. Risk factors for complications of endoscopic submucosal dissection in gastric tumors: analysis of 478 lesions. J Gastroenterol. 2010;45:30-36. |
| 10. | Ohta T, Ishihara R, Uedo N, Takeuchi Y, Nagai K, Matsui F, Kawada N, Yamashina T, Kanzaki H, Hanafusa M. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest Endosc. 2012;75:1159-1165. |
| 11. | Akasaka T, Nishida T, Tsutsui S, Michida T, Yamada T, Ogiyama H, Kitamura S, Ichiba M, Komori M, Nishiyama O. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc. 2011;23:73-77. |
| 12. | Ahn JY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim do H, Song HJ, Lee GH, Jung HY. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: analysis of 916 dissections performed by 4 experts. Gastrointest Endosc. 2011;73:911-916. |
| 13. | Abe Y, Inamori M, Iida H, Endo H, Akiyama T, Yoneda K, Fujita K, Takahashi H, Yoneda M, Hirokawa S. Clinical characteristics of patients with gastric perforation following endoscopic submucosal resection for gastric cancer. Hepatogastroenterology. 2009;56:921-924. |
| 14. | Jeon SW, Jung MK, Kim SK, Cho KB, Park KS, Park CK, Kwon JG, Jung JT, Kim EY, Kim TN. Clinical outcomes for perforations during endoscopic submucosal dissection in patients with gastric lesions. Surg Endosc. 2010;24:911-916. |
| 15. | Oda I, Gotoda T, Hamanaka H, Eguchi T, Saito Y, Matsuda T, Bhandari P, Emura F, Saito D, Ono H. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54-58. |
| 16. | Onogi F, Araki H, Ibuka T, Manabe Y, Yamazaki K, Nishiwaki S, Moriwaki H. “Transmural air leak”: a computed tomographic finding following endoscopic submucosal dissection of gastric tumors. Endoscopy. 2010;42:441-447. |
| 17. | Watari J, Tomita T, Toyoshima F, Sakurai J, Kondo T, Asano H, Yamasaki T, Okugawa T, Tanaka J, Daimon T. The incidence of “silent” free air and aspiration pneumonia detected by CT after gastric endoscopic submucosal dissection. Gastrointest Endosc. 2012;76:1116-1123. |
| 18. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. |
| 19. | Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning curve for advanced endoscopic resection. Gastrointest Endosc. 2005;62:866-867. |
| 20. | Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923-928. |
| 21. | Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644-647. |
| 22. | Seewald S, Soehendra N. Perforation: part and parcel of endoscopic resection? Gastrointest Endosc. 2006;63:602-605. |
| 23. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. |
| 24. | Nonaka S, Saito Y, Takisawa H, Kim Y, Kikuchi T, Oda I. Safety of carbon dioxide insufflation for upper gastrointestinal tract endoscopic treatment of patients under deep sedation. Surg Endosc. 2010;24:1638-1645. |