Published online Apr 16, 2013. doi: 10.4253/wjge.v5.i4.191
Revised: January 16, 2013
Accepted: January 23, 2013
Published online: April 16, 2013
Processing time: 164 Days and 21.4 Hours
To evaluate the diagnostic yield of the procedure, mucosal-incision assisted biopsy (MIAB), for the histological diagnosis of gastric gastrointestinal stromal tumor (GIST), we performed a retrospective review of the 27 patients with suspected gastric GIST who underwent MIAB in our hospitals. Tissue samples obtained by MIAB were sufficient to make a histological diagnosis (diagnostic MIAB) in 23 out of the 27 patients, where the lesions had intraluminal growth patterns. Alternatively, the samples were insufficient (non-diagnostic MIAB) in remaining 4 patients, three of whom had gastric submucosal tumor with extraluminal growth patterns. Although endoscopic ultrasound and fine needle aspiration is the gold standard for obtaining tissue specimens for histological and cytological analysis of suspected gastric GISTs, MIAB can be used as an alternative method for obtaining biopsy specimens of lesions with an intraluminal growth pattern.
- Citation: Ihara E, Matsuzaka H, Honda K, Hata Y, Sumida Y, Akiho H, Misawa T, Toyoshima S, Chijiiwa Y, Nakamura K, Takayanagi R. Mucosal-incision assisted biopsy for suspected gastric gastrointestinal stromal tumors. World J Gastrointest Endosc 2013; 5(4): 191-196
- URL: https://www.wjgnet.com/1948-5190/full/v5/i4/191.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i4.191
Gastric submucosal tumors (SMTs) are a wide range of diverse conditions including neoplastic lesions such as gastrointestinal stromal tumor (GIST), leiomyoma, leiomyosarcoma, schwannoma, granular cell tumor and non-neoplastic lesions such as inflammatory fibroid polyp, gastric varices, heterotopic pancreas and heterotopic gastric mucosa[1,2]. Endoscopic ultrasonography (EUS) is one of the most useful modalities for diagnosing gastric SMTs[3,4]. However, it is usually not possible to differentiate GIST from benign conditions such as leiomyoma or schwannoma by EUS. Tissue sampling is necessary for definitive diagnosis of GIST. Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has been developed for tissue sampling of suspected GIST and is generally accepted to be a very useful for the diagnosis of this lesion[5]. When considering the diagnostic yield of EUS-FNA for suspected gastric GIST, it is important to evaluate whether the samples obtained are adequate for both cytological and histological analysis, as immunohistological analysis is indispensable for a definitive diagnosis. In general, the success rate of EUS-FNA for tissue sampling for cytology has been reported to be relatively high (83%), but the success rate for histology does not seem to be satisfactory (62%)[6]. Therefore, there has been an interest in exploring an alternative modality for tissue sampling in suspected GIST.
Endoscopic submucosal dissection (ESD) has been developed as an advanced endoscopic therapy for superficial gastric neoplasms[7] and ESD has rapidly become widely used. In this situation we have become interested in using ESD-associated techniques for tissue sampling of suspected GIST instead of using EUS-FNA. More recently, Lee et al[8] has shown the cases where the ESD-associated technique was useful for tissue sampling of suspected GISTs. It remains, however, to be determined whether the ESD-associated technique would be suitable for tissue sampling of any of suspected GISTs. Although an official term for this procedure has yet to be determined, we have named it mucosal-incision assisted biopsy (MIAB). We reviewed 27 cases with gastric SMTs in which MIAB was performed to obtain biopsy specimens. In the present study, we have shown that MIAB can be as an alternative diagnostic modality for tissue sampling of suspected GISTs when the lesions have an intraluminal growth pattern. MIAB may be contraindicated in suspected gastric GISTs with an extraluminal growth pattern.
We undertook a retrospective review of the 27 patients with gastric SMTs who underwent MIAB in our hospitals between May 2005 and August 2011 in order to distinguish GIST from benign causes of SMT. An extraluminal growth pattern was defined as growth in an extraluminal direction with little intraluminal growth. An intraluminal growth pattern was defined as growth in an intraluminal direction, regardless of any extraluminal growth. Informed consent was obtained from all patients before MIAB was undertaken. MIAB was performed as follows; In brief, a mucosal incision line was chosen which was usually not directly over the lesion, for easier closure with endoclips after the biopsy. Saline with 0.001% epinephrine was injected into the submucosa at the chosen incision line. A mucosal incision was made in the same way as the circumferential mucosal incision is made for ESD, using electrosurgical knives such as the flush knife or needle knife, followed by careful submucosal dissection until a portion of the SMT was exposed. When a single mucosal incision did not provide satisfactory exposure, an second incision was made perpendicular to the first incision. Several biopsy specimens were taken under direct vision using conventional biopsy forceps. The mucosal incisions were then closed with endoclips to prevent post-procedure complications including bleeding and/or perforation. The biopsy samples obtained by MIAB were fixed in formalin solution and stained with hematoxylin and eosin (HE). If applicable, specimens underwent immunohistochemical analysis. Applicable data were expressed as the mean ± SE.
Individual patient characteristics are shown in Table 1 and a summary is shown in Table 2. Fourteen females and 13 males were included in the study, with a mean age of 58.9 ± 2.4 years. Gastric SMT lesions were 10-36 mm in diameter with a mean diameter of 21.2 ± 1.0 mm. In 23 of the 27 patients, tissue samples obtained by MIAB were sufficient to make a histological diagnosis (diagnostic MIAB). We diagnosed GIST in 16 patients, leiomyoma in 4 patients, aberrant pancreas in one patient, inflammatory granuloma in one patient, and glomus tumor in one patient. In 23 patients with diagnostic MIAB, all of the lesions had intraluminal growth patterns. Fourteen of sixteen patients underwent surgical resection based on a preoperative diagnosis of GIST; the other patients (Cases 5 and 15) did not accept surgical resection and is currently under close follow-up. The post-operative pathological findings in all fourteen cases of GIST were identical to those obtained with MIAB, including findings on HE staining and immunohistochemical analysis. On the other hand, four patients (Cases 17, 25-27) resulted in non-diagnostic MIAB. In three of them, the SMT lesions had extraluminal growth patterns. In one patient with non-diagnostic MIAB (Case 17), the samples obtained by MIAB suggested a spindle cell tumor on HE staining. We could not obtain the further pathological diagnosis. In this case, since the lesion was growing rapidly and suspected to be a GIST, a surgical resection was performed. As a result, the final pathological diagnosis after surgery was a GIST (Table 1). The mean procedure time was 32.0 ± 2.4 min and no procedure-related complications (including uncontrolled bleeding or perforation) were observed. We present two representative cases below.
| Case | Age | Sex | Location of SMT | Size (mm) | Growth pattern | Diagnosis by MIAB | Post-operativediagnosis |
| 1 | 70 | M | Body, LC | 21 | Intraluminal | GIST | GIST |
| 2 | 60 | M | Body, LC | 20 | Intraluminal | GIST | GIST |
| 3 | 55 | F | Angulus, LC | 36 | Intraluminal | GIST | GIST |
| 4 | 73 | M | Body, LC | 26 | Intraluminal | GIST | GIST |
| 5 | 72 | F | Body, LC | 20 | Intraluminal | GIST | Not applicable |
| 6 | 69 | F | Fundus | 19 | Intraluminal | GIST | GIST |
| 7 | 72 | F | Body, LC | 23 | Intraluminal | GIST | GIST |
| 8 | 53 | M | Body, PW | 23 | Intraluminal | GIST | GIST |
| 9 | 79 | F | Body, GC | 24 | Intraluminal | GIST | GIST |
| 10 | 66 | F | Angulus, GC | 22 | Intraluminal | GIST | GIST |
| 11 | 66 | F | Body, PW | 25 | Intraluminal | GIST | GIST |
| 12 | 39 | M | Body, PW | 15 | Intraluminal | GIST | GIST |
| 13 | 58 | M | Body, GC | 20 | Intraluminal | GIST | GIST |
| 14 | 24 | M | Cardia, AW | 30 | Intraluminal | GIST | GIST |
| 15 | 60 | F | Body, PW | 10 | Intraluminal | GIST | Not applicable |
| 16 | 57 | M | Body, PW | 20 | Intraluminal | GIST | GIST |
| 17 | 40 | F | Body, PW | 30 | Intraluminal | IS | GIST |
| 18 | 55 | M | Cardia, LC | 23 | Intraluminal | Leiomyoma | Not applicable |
| 19 | 36 | F | Cardia, LC | 19 | Intraluminal | Leiomyoma | Not applicable |
| 20 | 62 | F | Cardia, LC | 25 | Intraluminal | Leiomyoma | Not applicable |
| 21 | 57 | F | Body, LC | 15 | Intraluminal | Leiomyoma | Not applicable |
| 22 | 50 | M | Antrum, AW | 20 | Intraluminal | Glomus tumor | Glomus tumor |
| 23 | 63 | M | Body, LC | 20 | Intraluminal | Aberrant pancreas | Not applicable |
| 24 | 57 | M | Body, GC | 20 | Intraluminal | Inflammatory change | Not applicable |
| 25 | 66 | M | Body, GC | 15 | Extraluminal | IS | Not applicable |
| 26 | 71 | F | Body, LC | 15 | Extraluminal | IS | Not applicable |
| 27 | 61 | F | Antrum, GC | 17 | Extraluminal | IS | Not applicable |
| Age | 58.9 ± 2.4 (27) |
| Sex | Female (13)/male (14) |
| Location of SMT | Fundus (1) |
| Cardia (4) | |
| Body (18) | |
| Angulus (2) | |
| Antrum (2) | |
| Size of the lesion (mm) | 21.2 ± 1.0 (27) |
| Growth pattern | Intraluminal (24) |
| Extraluminal (3) | |
| Diagnosis by MIAB | GIST (16) |
| Leiomyoma (4) | |
| Aberrant pancreas (1) | |
| Inflammatory changes (1) | |
| Glomus tumor (1) | |
| Not diagnosed (4) |
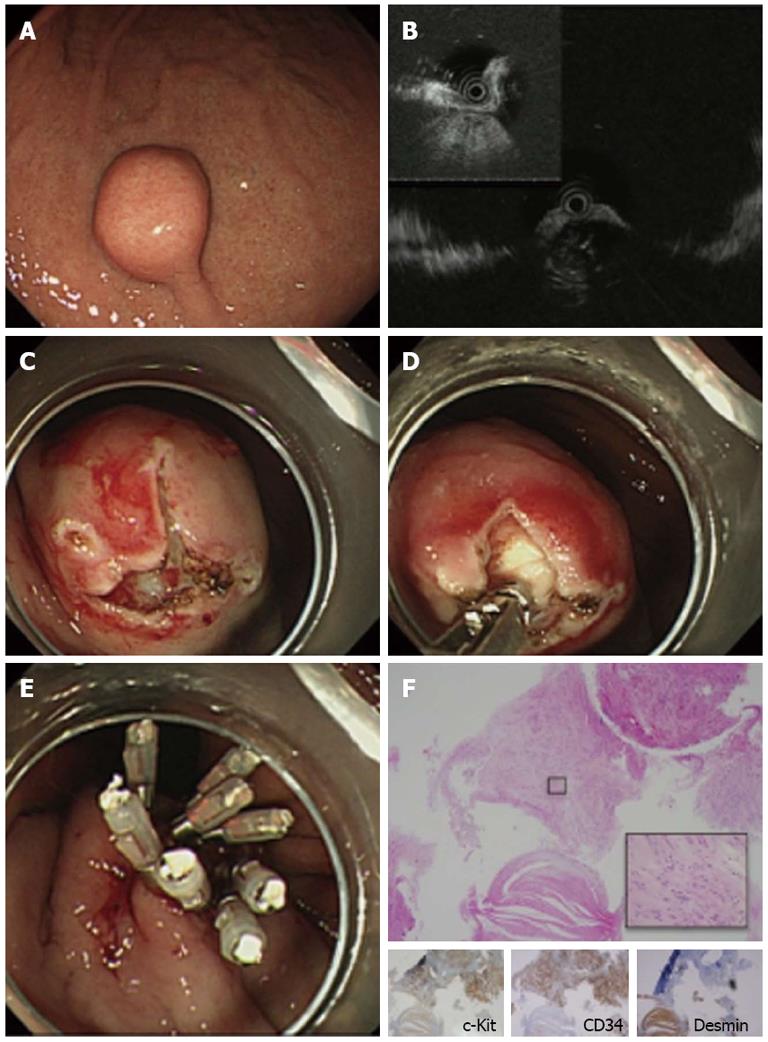
A 70-year-old man was referred to our hospital for evaluation of a suspected gastric SMT. EGD revealed a solid, round, protruding lesion covered with normal mucosa, measuring about 20 mm in diameter, at the middle of the lesser curvature of the body of the stomach (Figure 1A). EUS with a miniature probe showed a hypoechoic mass was observed, which originated from the 4th layer (muscularis propria) (Figure 1B), confirming that the lesion was an SMT. The lesion was thought to be a gastrointestinal mesenchymal tumor (GIMT) such as a GIST, leiomyoma or schwannoma. EUS findings showed an intraluminal growth pattern. MIAB was performed to obtain biopsy samples for histological diagnosis. Two mucosal incision lines were made perpendicular to each other to expose the surface of the SMT (Figure 1C) and tissue samples were successfully obtained (Figure 1D), followed by closure of the mucosal incisions with endoclips (Figure 1E). Pathological examination of the biopsy specimens showed a spindle cell mesenchymal tumor with abundant hyalinized fibrous stroma on HE staining. Immunohistochemical analysis was positive for c-Kit and CD34 and negative for desmin, which enabled us to make a diagnosis of GIST. The patient underwent surgical resection of the lesion. The final pathological diagnosis after surgery was GIST with a 21 mm diameter and mitotic index less than 5/50 HPFs, indicating a very low risk GIST according to Miettinen et al[9] (Figure 1F).
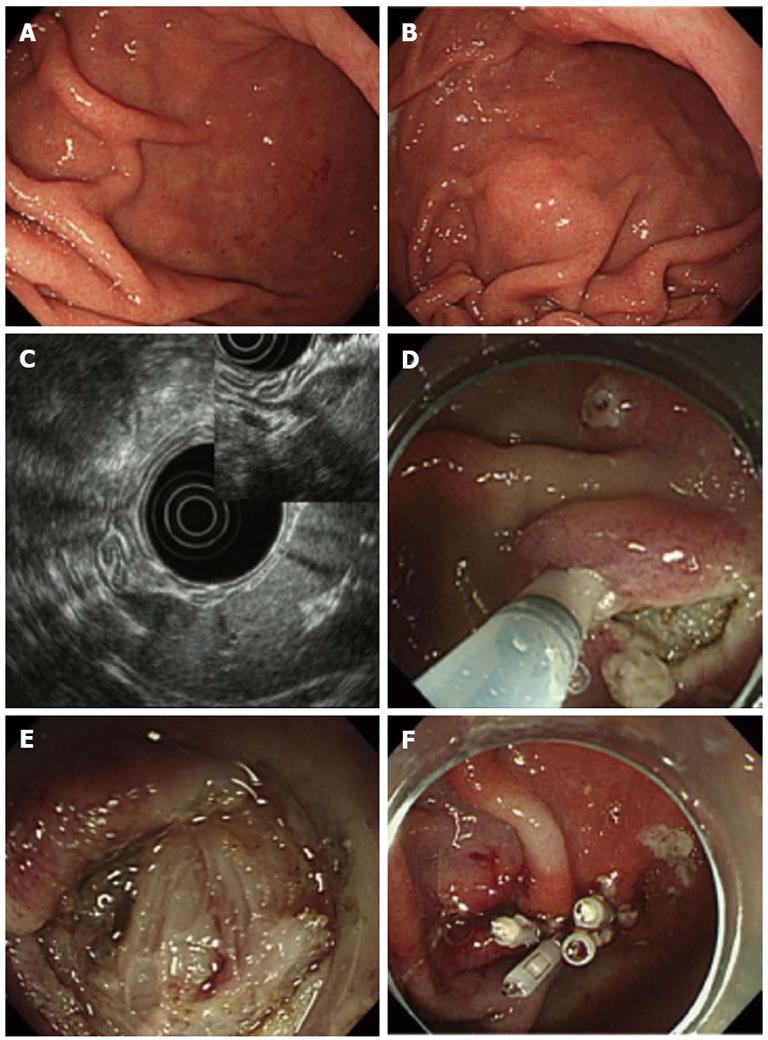
A 66-year-old man was referred to our hospital for evaluation of a suspected gastric SMT at the greater curvature of the lower body. EGD did not initially reveal any lesion (Figure 2A), but an SMT-like lesion was detected later during the examination (Figure 2B). As we were unable to detect the lesion by EUS with a miniature prove, conventional EUS was undertaken, revealing a hypoechoic, oval mass originating from the 4th layer (Figure 2C) which was suggestive of a GIMT such as a GIST, leiomyoma or schwannoma. The lesion had an extraluminal growth pattern. MIAB was undertaken to obtain biopsy specimens for a histological diagnosis. In this case we were unable to expose the lesion clearly due to risk of perforation (Figure 2D and E). The lesion appeared to be covered with normal smooth muscle of the muscularis propria. Some tissue samples were obtained, followed by closure of the incision with endoclips (Figure 2F). Pathological examination of the biopsy specimens with HE staining showed fascicles of smooth muscle cells accompanied by small fragments of spindle-shaped cells. Immunohistochemical analysis showed that the spindle-shaped cells were probably positive for c-Kit and CD34. These findings were suggestive of GIST, but not conclusive. In this case, MIAB was considered a non-diagnostic procedure.
In the present study, we retrospectively reviewed 27 cases with suspected GIST, in which MIAB was undertaken to obtain tissue samples for histological diagnosis. A definitive histological diagnosis was obtained in 23 of the 27 patients (85.2 %) who had gastric SMTs with intraluminal growth pattern. MIAB resulted in insufficient tissue sampling in the other four patients. In three of them, the SMT lesions had extraluminal growth patterns. We have shown that MIAB can be as an alternative diagnostic modality for tissue sampling of suspected GISTs when the lesions have an intraluminal growth pattern. MIAB may be contraindicated in suspected gastric GISTs with an extraluminal growth pattern[10,11].
EUS-FNA has been developed for tissue sampling and analysis of suspected GIST and plays an important role in making a histological diagnosis of this lesion[5]. Even though EUS-FNA has become the gold standard for obtaining biopsy samples for cytological and histological analysis of suspected gastric GIST, the procedure does not seem satisfactory. Mekky et al[6] recently reported the diagnostic yield from EUS-FNA for a total of 141 patients with gastric SMTs. They reported adequate samples in 117 of 141 cases (83%). In 29 cases of the 117 cases, however, the samples were sufficient for suggestion of a diagnosis based on cytological examination, but were inadequate for immunohistochemical analysis. Adequate samples for histological diagnosis were therefore obtained in only 88 of 141 cases (62%). Since immunohistochemical analysis is indispensable for a definitive diagnosis of GIST, the diagnostic yield of EUS-FNA for suspected GIST was not satisfactory. Therefore, there has been an interest in developing an alternative modality for tissue sampling of suspected GIST. Reasonably, we have become interested in using ESD-associated techniques for tissue sampling of suspected GIST instead of using EUS-FNA as recently shown by Lee et al[8].
MIAB has the following advantages over EUS-FNA. First, MIAB would be less costly than EUS-FNA. Although both ESD and EUS-FNA require a high skill level, ESD only needs an electrosurgical generator and electrosurgical knives (such as the flush knife, insulation-tipped electrosurgical knife, or grasping-type scissors forceps[12], and does not need expensive equipment such as the linear echoendoscopy used for EUS-FNA. Second, on-site cytologists are not required for MIAB, whereas they need to be scheduled for successful EUS-FNA. Third, when the gastric SMT proves to be a GIST, tissue samples obtained by MIAB are large enough for pathologists to calculate or estimate the Ki-67 labeling index, which gives information about the relative risk of malignant behavior. Calculation of the Ki-67 labeling index is not possible with EUS-FNA biopsy samples. It is very advantageous to have an indication of the risk of malignant behavior of a GIST before surgical resection.
There are, however, some disadvantages and limitations to MIAB. First, MIAB does not seem to be appropriate for tissue sampling of gastric SMTs with an extraluminal growth pattern. In our study, MIAB was non-diagnostic in cases 25-27 in which the gastric SMTs had an extraluminal growth pattern. In contrast, EUS-FNA is generally considered to be useful for obtaining tissue samples of gastric SMTs regardless of growth patterns. Other possible disadvantages are procedure-related complications including bleeding and perforation. MIAB may have a higher rate of procedure-related bleeding than EUS-FNA, but all bleeding was easily controlled by endoscopic hemostatic procedures in our cases. No perforation occurred in our cases, but extra care should be taken to prevent perforation in cases with an extraluminal growth pattern. It is not known whether procedure-related dissemination will be a possible late complication, but this has not been reported to date. It is important to close the mucosal incisions appropriately with endoclips after tissue sampling to prevent post-procedure complications.
In conclusion, although it is generally accepted that EUS-FNA is the gold standard for obtaining biopsies for histological and cytological analysis of suspected gastric GIST, MIAB may be chosen as an alternative diagnostic modality only when the lesion has an intraluminal growth pattern. Further studies will be required to further assess MIAB, including randomized controlled trials to compare MIAB with EUS-FNA.
| 1. | Papanikolaou IS, Triantafyllou K, Kourikou A, Rösch T. Endoscopic ultrasonography for gastric submucosal lesions. World J Gastrointest Endosc. 2011;3:86-94. [PubMed] [DOI] [Full Text] |
| 2. | Ponsaing LG, Kiss K, Loft A, Jensen LI, Hansen MB. Diagnostic procedures for submucosal tumors in the gastrointestinal tract. World J Gastroenterol. 2007;13:3301-3310. [PubMed] |
| 3. | Buscarini E, Stasi MD, Rossi S, Silva M, Giangregorio F, Adriano Z, Buscarini L. Endosonographic diagnosis of submucosal upper gastrointestinal tract lesions and large fold gastropathies by catheter ultrasound probe. Gastrointest Endosc. 1999;49:184-191. [PubMed] |
| 4. | Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, Ulm K. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: a prospective multicenter study. Scand J Gastroenterol. 2002;37:856-862. [PubMed] |
| 5. | Ando N, Goto H, Niwa Y, Hirooka Y, Ohmiya N, Nagasaka T, Hayakawa T. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 233] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 6. | Mekky MA, Yamao K, Sawaki A, Mizuno N, Hara K, Nafeh MA, Osman AM, Koshikawa T, Yatabe Y, Bhatia V. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 7. | Kakushima N, Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J Gastroenterol. 2008;14:2962-2967. [PubMed] |
| 8. | Lee HL, Kwon OW, Lee KN, Jun DW, Eun CS, Lee OY, Jeon YC, Han DS, Yoon BC, Choi HS. Endoscopic histologic diagnosis of gastric GI submucosal tumors via the endoscopic submucosal dissection technique. Gastrointest Endosc. 2011;74:693-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006;23:70-83. [PubMed] |
| 10. | Lee CK, Chung IK, Lee SH, Lee SH, Lee TH, Park SH, Kim HS, Kim SJ, Cho HD. Endoscopic partial resection with the unroofing technique for reliable tissue diagnosis of upper GI subepithelial tumors originating from the muscularis propria on EUS (with video). Gastrointest Endosc. 2010;71:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | de la Serna-Higuera C, Pérez-Miranda M, Díez-Redondo P, Gil-Simón P, Herranz T, Pérez-Martín E, Ochoa C, Caro-Patón A. EUS-guided single-incision needle-knife biopsy: description and results of a new method for tissue sampling of subepithelial GI tumors (with video). Gastrointest Endosc. 2011;74:672-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Akahoshi K, Akahane H. A new breakthrough: ESD using a newly developed grasping type scissor forceps for early gastrointestinal tract neoplasms. World J Gastrointest Endosc. 2010;2:90-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
P- Reviewers Armstrong D, Cho YS S- Editor Gou SX L- Editor A E- Editor Zhang DN