Published online Apr 16, 2013. doi: 10.4253/wjge.v5.i4.141
Revised: February 17, 2013
Accepted: March 8, 2013
Published online: April 16, 2013
Processing time: 215 Days and 4.5 Hours
With the advent of linear echoendoscopes, endoscopic ultrasound (EUS) has become more operative and a new field of oncological application has been opened up. From tumor staging to tissue acquisition under EUS-guided fine-needle aspiration, new operative procedures have been developed on the principle of the EUS-guided puncture. A hybrid probe combining radiofrequency with cryotechnology is now available, to be passed through the operative channel of the echoendoscope into the tumor to create an area of ablation. EUS-guided fine-needle injection is emerging as a method to deliver anti-tumoral agents inside the tumor. Ethanol lavage, with or without paclitaxel, has been proposed for the treatment of cystic tumors in non-resectable cases and complete resolution has been recorded in up to 70%-80%. Many other chemical or biological agents have been investigated for the treatment of pancreatic adenocarcinoma: activated allogenic lymphocyte culture (Cytoimplant), a replication-deficient adenovirus vector carrying the tumor necrosis factor-α gene, or an oncolytic attenuated adenovirus (ONYX-015). The potential advantage of treatment under EUS control is the real-time imaging guidance into a deep target like the pancreas which is extremely difficult to reach by a percutaneous approach. To date there are no randomized controlled trials to confirm the real clinical benefits of these treatments compared to standard therapy so it seems wise to reserve them only for experimental protocols approved by ethics committees.
Core tip: New operative procedures have been developed on the principle of the endoscopic ultrasound (EUS)-guided puncture. A hybrid probe combining radiofrequency with cryotechnology is now available, to be passed through the operative channel of the echoendoscope into the tumor to create an area of ablation. The potential advantage of an ablation device employed under EUS control is the real-time imaging guidance into a deep target like the pancreas which is extremely difficult to reach by a percutaneous approach.
- Citation: Carrara S, Petrone MC, Testoni PA, Arcidiacono PG. Tumors and new endoscopic ultrasound-guided therapies. World J Gastrointest Endosc 2013; 5(4): 141-147
- URL: https://www.wjgnet.com/1948-5190/full/v5/i4/141.htm
- DOI: https://dx.doi.org/10.4253/wjge.v5.i4.141
Endoscopic ultrasound (EUS) has seen significant growth in its applications in oncology in recent years[1-9]. With the advent of linear array therapeutic probes with a large working channel EUS has become more operative. From tumor staging to tissue acquisition under EUS-guided fine-needle aspiration (FNA), new procedures have been developed on the principle of the EUS-guided puncture: if we can puncture a lesion to acquire a cytological specimen, in the same way we can puncture a tumor to carry chemical, biological, or physical therapy inside it. New accessories have been developed, and clinical research on applications in oncological patients has expanded, especially for pancreatic diseases[10-16].
Ablative therapies such as radiofrequency (RF) and cryotechnology (CT) are widely used in oncology, though not in the pancreas because of the high operative risks. Retrospective and prospective studies have, however, shown the feasibility of water-cooled monopolar RF ablation in patients with stage III pancreatic cancer in an open, percutaneous, or laparoscopic setting[17,18]. They confirmed that ablation in the pancreas is dangerous without additional cooling of adjacent tissue, real-time image control, and currently available ablation systems[19-22]. Italian surgeons applied an RF probe in locally advanced pancreatic cancer during laparotomy, demonstrating the feasibility and safety of the technique[23].
The potential advantage of an ablation device employed under EUS control is the real-time imaging guidance into a deep target like the pancreas which is extremely difficult to reach by a percutaneous approach. A minimally invasive technique to selectively ablate tumor masses could improve the efficacy of neoadjuvant treatments in patients not eligible for any other therapy. The precision of EUS in establishing the location and size of pancreatic masses could be exploited to estimate and follow up the area of ablation and help avoid damage to surrounding structures[24-26].
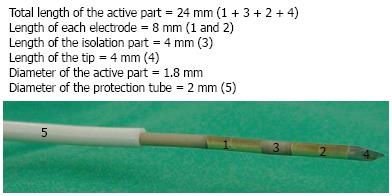
A new flexible bipolar hybrid ablation system has been developed (ERBE Elektromedizin GmbH, Tübingen, Germany) (Figures 1, 2). This hybrid cryotherm probe (CTP) combines bipolar RF ablation with CT. A bipolar system is believed to create ablations with less collateral thermal damage than monopolar systems but the trade-off is some loss of overall efficiency[27,28]. The CTP combines the advantages of the two technologies and overcomes the loss of efficiency: the more effective cooling by cryogenic gas permits more RF-induced interstitial devitalizing effects than heat alone[29]. Less power (16 W) is needed than with conventional RF ablation systems (30-60 W) to obtain the same result, so there should be less collateral damage.
The CTP has an active electrical part with a diameter of 1.8 mm. The entire probe is covered by a protection tube that can be safely passed through the operative channel of the echoendoscope without any risk for the instrument. Basically this is an internally CO2-cooled RF-ablation probe which ensures efficient cooling according to the Joule-Thomson effect. The distal tip of the probe is sharp, pointed and stiff in order to penetrate the gut wall and pancreatic parenchyma. Parameters like the power setting of the generator, the pressure of the gas through the expansion vessel, and the duration of application can be set independently.
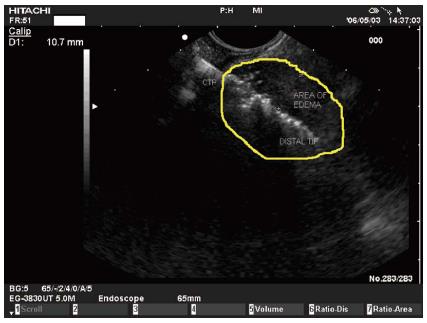
Transluminal RF ablation in the pancreas under EUS control was feasible in an animal model[30]. The power (16 W) and pressure (650 psi) settings were standardized on the basis of previous experiments. Under real-time EUS-guidance the CTP was clearly visualized as a hyperechoic line moving out of the working channel until it reached its place in the pancreatic parenchyma. During the application a hyperechoic elliptic area appeared around the distal tip of the probe, surrounded by a hypoechoic border (most likely edema) (Figure 3). There was a positive correlation between lesion size and application time: the longer the application time the more the lesion size varied, reflecting the fact that a 900-s application induces high complication rates in a healthy pancreas.
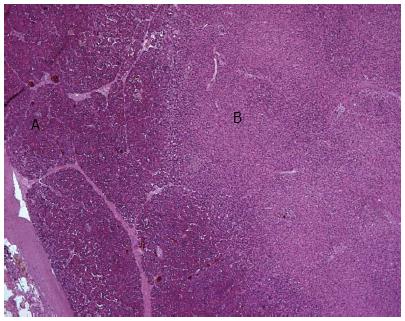
On histological examination a sharp demarcation was visible between the ablated area and the untreated pancreatic parenchyma. Coagulative necrosis was evident in the center of the lesion one week after the ablation; after two weeks the lesions showed less edema and more fibrotic transformation (Figure 4).
After the animal model experiments the efficacy of the CTP was evaluated in an ex vivo study for destroying neoplastic tissue of explanted pancreas from patients with resectable pancreatic adenocarcinoma. Again, histological examination found a positive correlation between the size of the ablated area and the application time[31].
In the animal model the complications were related to the ablation time: all but histochemical pancreatitis occurred with ablations longer than 300 s. Pancreatic tissue is very heat-sensitive and the thermal ablation of a normal pancreas usually leads to an inflammatory response with consecutive edema, fibrotic and sometimes cystic transformation. The tissue response should be different and less pronounced in a tumor mass surrounded by a capsule where a desmoplastic reaction limits the damage to the capsule to a certain extent.
Patients with unresectable, locally advanced pancreatic adenocarcinoma were recently enrolled in a prospective case study to investigate the feasibility of EUS-guided CTP application in vivo and to assess to what extent progression of the disease was slowed[32]. The inclusion and exclusion criteria are listed in Table 1. From September 2009 to May 2011, 22 patients (11 males and 11 females, mean age 61.9 years) with unresectable stage III pancreatic adenocarcinoma were enrolled. The cryotherm ablation was feasible in 16 patients (72.8%). The probe was clearly visible throughout the procedure. No severe complications arose during or immediately after the ablation. Three patients reported post-interventional abdominal pain, which responded well to analgesic drugs. Only one patient experienced a minor bleed in the duodenal lumen after the procedure, which was treated by endoscopic placement of hemostatic clips and did not require blood transfusion. Late complications arose in four cases: three were related mainly to tumor progression. A computed tomography scan was done in all patients but only in 6/16 was it possible to clearly define the tumor margins after ablation. In these patients the tumor seemed smaller than the initial mass (P = 0.07).
| Inclusion criteria | Exclusion criteria |
| Age > 18 yr | Severe alteration of hemostasis |
| Able to give consent for the procedure | Unwilling or unable to give consent |
| Pregnancy | |
| PLT > 100 000/μL | Infection and/or severe leucopenia |
| INR < 1.5 | Acute pancreatitis |
| Unresectable locally advanced pancreatic adenocarcinoma already treated with neoadjuvant chemotherapy | Distant metastasis |
For experts familiar with the EUS-FNA procedure, the EUS-guided placement of the CTP and the ablation itself should not present any technical challenge.
A hepatocellular carcinoma of the caudate lobe unsuitable for surgery was treated with EUS-guided neodymium: yttrium-aluminium-garnet (Nd:YAG) laser ablation. A 300-μm optical fiber was passed through a 22-G needle which was then positioned in the tumor under EUS guidance. After two months computed tomography scan showed uniform hypo-attenuation without enhancement in the ablated zone[33].
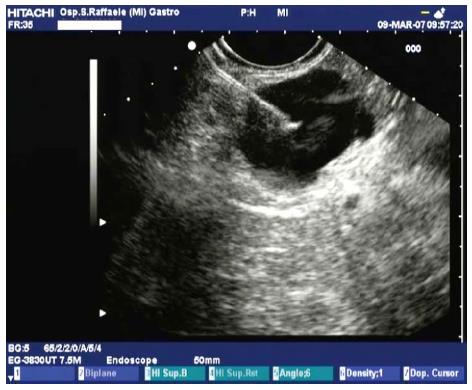
Only few studies have examined the role of ethanol injection in ablation of the lining epithelium of cystic tumors. Pancreatic cystic tumors encompass a wide spectrum of histopathologies and biological behaviors (from benign to borderline to malignant) and can be differentiated essentially as mucinous or non-mucinous. They are often detected by chance in asymptomatic patients during radiological examinations for non-specific gastrointestinal complaints. For the treatment of mucinous cystic tumors, surgical resection is usually the first choice, but EUS-guided ethanol lavage has been proposed as an alternative for patients not suitable for surgery. The rationale for the use of ethanol is that it can sclerose the lining epithelium and reduce the influx of fluid. The cyst is punctured with a 22-G fine needle under EUS-guidance, the fluid is aspirated, then ethanol is injected into the cyst and re-aspirated after 3-5 min (Figure 5). In the initial pilot study the Boston group showed the feasibility and safety of EUS-guided ethanol lavage for pancreatic cystic tumors in 25 patients[34]. They obtained complete resolution of the cysts in eight (33%), with variable degrees of epithelial ablation observed at histological examination of resected specimens in patients who subsequently underwent surgery.
Other studies used taxol for lavage after the ethanol. Paclitaxel is a viscous, hydrophobic chemotherapeutic agent that is believed to have prolonged action in the cyst. In a preliminary study 11 out of 14 patients showed complete cyst resolution after ethanol lavage and paclitaxel injection[35,36].
A more recent cohort study determined the duration of successful cyst resolution after EUS-guided ethanol lavage. Computed tomography scans at a median of 26 mo suggested resolution lasted well[37]. In the Editorial commenting this study, Goodman et al[38] suggest that until we have better randomized controlled trials EUS-guided ethanol ablation of pancreatic cysts is best reserved for experimental protocols and for patients who cannot undergo surgery.
EUS-guided fine-needle injection is emerging as a method to deliver anti-tumoral agents inside pancreatic tumors. Many chemical or biological agents have been investigated for the treatment of pancreatic adenocarcinoma: activated allogenic lymphocyte culture (Cytoimplant)[39], a replication-deficient adenovirus vector carrying the tumor necrosis factor-α gene[40,41], and an oncolytic attenuated adenovirus (ONYX-015)[42]. The procedure was developed on the principle of EUS-guided FNA: the needle is passed through the operative channel of the echoendoscope and is followed in real time while it punctures the tumor and the agent is delivered inside the mass. A Doppler signal helps avoid interposing vessels and makes the procedure safer.
Allogenic mixed lymphocyte culture (Cytoimplant): The first study, by Chang et al[40], assessed the technical feasibility and safety of EUS-guided injection of allogenic mixed lymphocyte culture (Cytoimplant) in locally advanced pancreatic adenocarcinoma. Eight patients with unresectable pancreatic cancer were given a single EUS-guided injection of Cytoimplant. The first two received three billion cells, the next three six billion cells and the last three nine billion cells. The procedures were safe and there were no severe complications. The only side effect reported was low-grade fever. Median survival was 13.2 mo. No other studies have followed this first phase I trial.
Replication-deficient adenovirus vector carrying the tumor necrosis factor-αgene: Chang et al[40] also tested EUS-guided TNFerade injection in patients with locally advanced pancreatic cancer. TNFerade is a replication-deficient adenovector that contains the human tumor necrosis factor (TNF)-α gene. Patients received five weekly EUS-guided intratumoral injections of TNFerade (4 × 109, 4 × 1010, and 4 × 1011 particle units in 2 mL). This was combined with iv chemotherapy (fluorouracil, 5-FU) and radiation. The rationale for this triple strategy lies in the synergism between the three therapies. 5-FU is directly toxic to malignant cells and is also a radiosensitizer; radiation therapy destroys tumor cells and up-regulates TNF production; and TNFerade, which is also a radiosensitizer, kills the tumor cells. The procedure was well tolerated. Patients who received the higher doses had better locoregional control of the disease, better median survival rates, and a higher percentage of resective surgery after the treatment[41,42].
Adenovirus ONYX-015: Another anti-tumoral viral therapy schedule is ONYX-015, a replication selective adenovirus with a deletion in the E1B-55 kDa gene, which preferentially replicates in tumoral cells and kills them. Twenty-one patients were given EUS-guided injections of ONYX-015 over an eight-week period. Complications were more severe than in the previous studies described: two patients had sepsis and two had duodenal perforation. None showed tumor regression with the ONYX-015 injection alone after five weeks, but two patients had a partial response after the combination with gemcitabine[42].
Although EUS-guided antitumoral injection seems feasible and safe, and the results of these studies seem promising, the efficacy in phase III randomized controlled trials has still to be demonstrated and published.
EUS guidance can also be used to place fiducial markers or radioactive seeds inside a tumor. Fiducial markers are radiopaque spheres, coils, or seeds that are implanted in or near the tumor in order to demarcate the borders of the tumor to facilitate image-guided radiation therapy. Many studies have been published on EUS-guided placement of these markers in different tumors[43-47].
The fiducials are passed through a 19-G or 22-G needle and deployed with different techniques into the mass, using the stylet, or by injecting sterile water into the needle[46]. The fact that the 19-G needle is stiffer can make it harder to position the fiducials in pancreatic head tumors with the echoendoscope placed in the second portion of the duodenum, while with the smaller-caliber 22-G needle it may be easier to place the fiducials in the deepest portions of the pancreas[48].
Few trials have evaluated EUS-guided implantation of radioactive seeds (iodine-125) in patients with unresectable pancreatic cancer[49,50]. Patients treated with a combination of radioactive seeds and chemotherapy showed tumor regression and reported some relief of pain[50].
EUS, born as an extremely accurate imaging technique, is emerging as a tool to guide interventional endoscopy in oncological patients, from EUS-guided FNA, to EUS-guided injection of anti-tumoral agents, to EUS-guided ablation devices. Table 2 summarizes the potential oncological applications of therapeutic EUS.
| Ref. | Year of publication | Type of cancer | n | Materials | Results | Complications |
| Arcidiacono et al[32] | 2012 | Adeno-carcinoma | 22 | Cryotherm probe | Feasible (72%), and safe | Pain (3 pts); minor bleeding (1 pt) |
| Gan et al[34] | 2005 | Cystic tumors | 25 | Ethanol lavage | Complete resolution (35%) | No complications |
| Oh et al[36] | 2008 | Cystic tumors | 52 | Ethanol lavage + paclitaxel | Complete resolution (62%) | Mild pancreatitis and splenic vein obliteration (1 pt) |
| Chang et al[39] | 2000 | Adeno-carcinoma | 8 | Cytoimplant | 2 partial responses and 1 minor response | Low-grade fever (86%); GI toxicities (37%) |
| Hecht | 2012 | Adeno-carcinoma | 50 | TNFerade | 1 complete response; 3 partial responses; 12 stable diseases | Pancreatitis and cholangitis (3 pts) |
| Hecht et al[42] | 2003 | Adeno-carcinoma | 21 | ONYX-015 + iv gemcitabine | Partial response (2 pts) | Sepsis (2 pts); duodenal perforation (2 pts) |
| Jin et al[50] | 2008 | Adeno-carcinoma | 22 | iodine 125-seeds | Successful implantation in all pts; partial remission (13%); stable disease (45%) | No complications |
Many case series and reports have confirmed the feasibility and safety of EUS-guided operative procedures, but there are still no randomized controlled trials to confirm the real clinical benefits of these treatments compared to standard therapy. At the moment it seems wise to reserve them only for experimental protocols approved by ethics committees.
| 1. | DeWitt J, Devereaux B, Chriswell M, McGreevy K, Howard T, Imperiale TF, Ciaccia D, Lane KA, Maglinte D, Kopecky K. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Intern Med. 2004;141:753-763. [PubMed] |
| 2. | Săftoiu A. State-of-the-art imaging techniques in endoscopic ultrasound. World J Gastroenterol. 2011;17:691-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Barresi L, Tarantino I, Granata A, Curcio G, Traina M. Pancreatic cystic lesions: How endoscopic ultrasound morphology and endoscopic ultrasound fine needle aspiration help unlock the diagnostic puzzle. World J Gastrointest Endosc. 2012;4:247-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Krishna SG, Lee JH. Endosonography in solid and cystic pancreatic tumors. J Interv Gastroenterol. 2011;1:193-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Chong AK, Caddy GR, Desmond PV, Chen RY. Prospective study of the clinical impact of EUS. Gastrointest Endosc. 2005;62:399-405. [PubMed] |
| 6. | Bhutani MS. Interventional endoscopic ultrasonography: state of the art at the new millenium. Endoscopy. 2000;32:62-71. [PubMed] |
| 7. | Fusaroli P, Kypraios D, Caletti G, Eloubeidi MA. Pancreatico-biliary endoscopic ultrasound: a systematic review of the levels of evidence, performance and outcomes. World J Gastroenterol. 2012;18:4243-4256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Tharian B, Tsiopoulos F, George N, Pietro SD, Attili F, Larghi A. Endoscopic ultrasound fine needle aspiration: Technique and applications in clinical practice. World J Gastrointest Endosc. 2012;4:532-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Klapman JB, Chang KJ. Endoscopic ultrasound-guided fine-needle injection. Gastrointest Endosc Clin N Am. 2005;15:169-177, x. [PubMed] |
| 10. | Tarantino I, Barresi L, Fabbri C, Traina M. Endoscopic ultrasound guided biliary drainage. World J Gastrointest Endosc. 2012;4:306-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Ashida R, Chang KJ. Interventional EUS for the treatment of pancreatic cancer. J Hepatobiliary Pancreat Surg. 2009;16:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Perez-Miranda M, de la Serna C, Diez-Redondo P, Vila JJ. Endosonography-guided cholangiopancreatography as a salvage drainage procedure for obstructed biliary and pancreatic ducts. World J Gastrointest Endosc. 2010;2:212-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Tarantino I, Barresi L. Interventional endoscopic ultrasound: Therapeutic capability and potential. World J Gastrointest Endosc. 2009;1:39-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 14. | Matthes K, Mino-Kenudson M, Sahani DV, Holalkere N, Brugge WR. Concentration-dependent ablation of pancreatic tissue by EUS-guided ethanol injection. Gastrointest Endosc. 2007;65:272-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Sun S, Wang S, Ge N, Lei T, Lu Q, Zhou Z, Yang A, Wang Z, Sun M. Endoscopic ultrasound-guided interstitial chemotherapy in the pancreas: results in a canine model. Endoscopy. 2007;39:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Giday SA, Magno P, Gabrielson KL, Buscaglia JM, Canto MI, Ko CW, Clarke JO, Kalloo AN, Jagannath SB, Shin EJ. The utility of contrast-enhanced endoscopic ultrasound in monitoring ethanol-induced pancreatic tissue ablation: a pilot study in a porcine model. Endoscopy. 2007;39:525-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Wu Y, Tang Z, Fang H, Gao S, Chen J, Wang Y, Yan H. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2006;94:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (1)] |
| 18. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. High operative risk of cool-tip radiofrequency ablation for unresectable pancreatic head cancer. J Surg Oncol. 2007;96:89-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Matsui Y, Nakagawa A, Kamiyama Y, Yamamoto K, Kubo N, Nakase Y. Selective thermocoagulation of unresectable pancreatic cancers by using radiofrequency capacitive heating. Pancreas. 2000;20:14-20. [PubMed] |
| 20. | Elias D, Baton O, Sideris L, Lasser P, Pocard M. Necrotizing pancreatitis after radiofrequency destruction of pancreatic tumours. Eur J Surg Oncol. 2004;30:85-87. [PubMed] |
| 21. | Siriwardena AK. Radiofrequency ablation for locally advanced cancer of the pancreas. JOP. 2006;7:1-4. [PubMed] |
| 22. | Spiliotis JD, Datsis AC, Michalopoulos NV, Kekelos SP, Vaxevanidou A, Rogdakis AG, Christopoulou AN. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbecks Arch Surg. 2007;392:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Girelli R, Frigerio I, Salvia R, Barbi E, Tinazzi Martini P, Bassi C. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surg. 2010;97:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 24. | Rösch T, Braig C, Gain T, Feuerbach S, Siewert JR, Schusdziarra V, Classen M. Staging of pancreatic and ampullary carcinoma by endoscopic ultrasonography. Comparison with conventional sonography, computed tomography, and angiography. Gastroenterology. 1992;102:188-199. [PubMed] |
| 25. | Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4:717-25; quiz 664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 26. | Agarwal B, Abu-Hamda E, Molke KL, Correa AM, Ho L. Endoscopic ultrasound-guided fine needle aspiration and multidetector spiral CT in the diagnosis of pancreatic cancer. Am J Gastroenterol. 2004;99:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 217] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 27. | Van Goethem BE, Rosenveldt KW, Kirpensteijn J. Monopolar versus bipolar electrocoagulation in canine laparoscopic ovariectomy: a nonrandomized, prospective, clinical trial. Vet Surg. 2003;32:464-470. [PubMed] |
| 28. | Lee JM, Han JK, Choi SH, Kim SH, Lee JY, Shin KS, Han CJ, Choi BI. Comparison of renal ablation with monopolar radiofrequency and hypertonic-saline-augmented bipolar radiofrequency: in vitro and in vivo experimental studies. AJR Am J Roentgenol. 2005;184:897-905. [PubMed] |
| 29. | Hines-Peralta A, Hollander CY, Solazzo S, Horkan C, Liu ZJ, Goldberg SN. Hybrid radiofrequency and cryoablation device: preliminary results in an animal model. J Vasc Interv Radiol. 2004;15:1111-1120. [PubMed] |
| 30. | Carrara S, Arcidiacono PG, Albarello L, Addis A, Enderle MD, Boemo C, Campagnol M, Ambrosi A, Doglioni C, Testoni PA. Endoscopic ultrasound-guided application of a new hybrid cryotherm probe in porcine pancreas: a preliminary study. Endoscopy. 2008;40:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Petrone MC, Arcidiacono PG, Carrara S, Albarello L, Enderle MD, Neugebauer A, Boemo C, Doglioni C, Testoni PA. US-guided application of a new hybrid probe in human pancreatic adenocarcinoma: an ex vivo study. Gastrointest Endosc. 2010;71:1294-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 32. | Arcidiacono PG, Carrara S, Reni M, Petrone MC, Cappio S, Balzano G, Boemo C, Cereda S, Nicoletti R, Enderle MD. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. 2012;76:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Di Matteo F, Grasso R, Pacella CM, Martino M, Pandolfi M, Rea R, Luppi G, Silvestri S, Zardi E, Costamagna G. EUS-guided Nd: YAG laser ablation of a hepatocellular carcinoma in the caudate lobe. Gastrointest Endosc. 2011;73:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 34. | Gan SI, Thompson CC, Lauwers GY, Bounds BC, Brugge WR. Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. 2005;61:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Oh HC, Seo DW, Lee TY, Kim JY, Lee SS, Lee SK, Kim MH. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | DeWitt J, DiMaio CJ, Brugge WR. Long-term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc. 2010;72:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Goodman AJ, Gress FG. EUS-guided ethanol lavage for pancreatic cysts: is it ready for prime time? Gastrointest Endosc. 2010;72:867-869. [PubMed] |
| 39. | Chang KJ, Nguyen PT, Thompson JA, Kurosaki TT, Casey LR, Leung EC, Granger GA. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. 2000;88:1325-1335. [PubMed] |
| 40. | Chang KJ, Irisawa A. EUS 2008 Working Group document: evaluation of EUS-guided injection therapy for tumors. Gastrointest Endosc. 2009;69:S54-S58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | Chang KJ, Lee JG, Holcombe RF, Kuo J, Muthusamy R, Wu ML. Endoscopic ultrasound delivery of an antitumor agent to treat a case of pancreatic cancer. Nat Clin Pract Gastroenterol Hepatol. 2008;5:107-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, Kirn DH, Freeman SM. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555-561. [PubMed] |
| 43. | Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, McGrath K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | DiMaio CJ, Nagula S, Goodman KA, Ho AY, Markowitz AJ, Schattner MA, Gerdes H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos). Gastrointest Endosc. 2010;71:1204-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Ammar T, Coté GA, Creach KM, Kohlmeier C, Parikh PJ, Azar RR. Fiducial placement for stereotactic radiation by using EUS: feasibility when using a marker compatible with a standard 22-gauge needle. Gastrointest Endosc. 2010;71:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Ghassemi S, Faigel DO. EUS-guided placement of fiducial markers using a 22-gauge needle. Gastrointest Endosc. 2009;69:AB337-AB338. [DOI] [Full Text] |
| 49. | Sun S, Xu H, Xin J, Liu J, Guo Q, Li S. Endoscopic ultrasound-guided interstitial brachytherapy of unresectable pancreatic cancer: results of a pilot trial. Endoscopy. 2006;38:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 127] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
P- Reviewers Sahu RP, Izbicki JR, Baba H, Di Matteo F S- Editor Song XX L- Editor A E- Editor Zhang DN