Published online Feb 16, 2011. doi: 10.4253/wjge.v3.i2.40
Revised: December 19, 2010
Accepted: December 6, 2010
Published online: February 16, 2011
A 52-year-old white woman had suffered from intermittent gastrointestinal (GI) bleeding for one year. Upper GI endoscopy, colonoscopy and peroral double-balloon enteroscopy (DBE) did not detect any bleeding source, suggesting obscure GI bleeding. However, in videocapsule endoscopy a jejunal ulceration without bleeding signs was suspected and this was endoscopically confirmed by another peroral DBE. After transfusion of packed red blood cells, the patient was discharged from our hospital in good general condition. Two weeks later she was readmitted because of another episode of acute bleeding. Multi-detector row computed tomography with 3D reconstruction was performed revealing a jejunal tumor causing lower gastrointestinal bleeding. The patient underwent exploratory laparotomy with partial jejunal resection and end-to-end jejunostomy for reconstruction. Histological examination of the specimen confirmed the diagnosis of a low risk gastrointestinal stromal tumor (GIST). Nine days after surgery the patient was discharged in good health. No signs of gastrointestinal rebleeding occurred in a follow-up of eight months. We herein describe the complex presentation and course of this patient with GIST and also review the current approach to treatment.
- Citation: Reuter S, Bettenworth D, Mees ST, Neumann J, Beyna T, Domschke W, Wessling J, Ullerich H. A typical presentation of a rare cause of obscure gastrointestinal bleeding. World J Gastrointest Endosc 2011; 3(2): 40-45
- URL: https://www.wjgnet.com/1948-5190/full/v3/i2/40.htm
- DOI: https://dx.doi.org/10.4253/wjge.v3.i2.40
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors in the GI tract. The clinicopathology and appearance of GISTs vary considerably and symptoms might result from both small incidental nodules and large tumors. Symptomatic GISTs have often grown large before they are discovered and that is why their diagnosis frequently results from emergency surgery for gastrointestinal (GI) perforation or GI bleeding. Small GISTs often form solid subserosal or intramural masses, sometimes ulcerating or eroding vessels but rarely growing into the lumen. This is why the GISTs are sometimes hard to diagnose. However, GI bleeding (acute or chronic) is the most common clinical presentation of GISTs.
We report the case of a 52-year-old female who presented with intermittent GI bleeding for one year. Due to its submucosal location, multiple endoscopic approaches failed to diagnose the tumor correctly. However, a multi-detector row computed tomography (MDCT) study with 3D reconstruction disclosed a homogeneous, hypervascularized abdominal mass showing arterial contrast enhancement and extravasation of contrast media into the intestines. This striking case illustrates that MDCT is a useful tool for diagnosis and localization in cases of acute obscure GI bleeding when diagnosis may be missed by endoscopy.
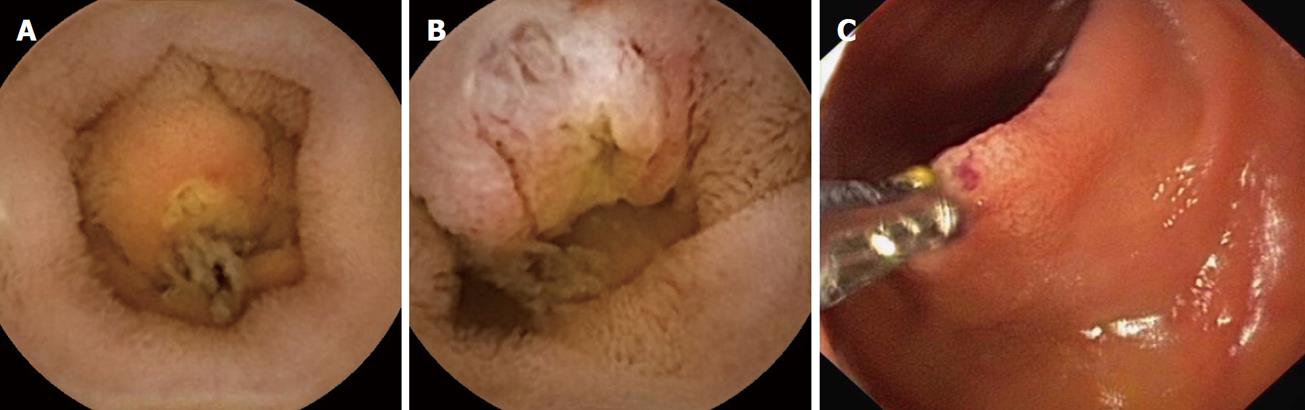
A 52-year-old female patient had suffered from intermittent GI bleeding (melena) for one year. Therefore, she underwent upper GI endoscopy and colonoscopy and peroral double-balloon enteroscopy (DBE) in a community hospital, the results of which were unremarkable. In video capsule endoscopy (PillCamTM SB2 and Rapid 5 workstation, Given Imaging, Hamburg, Germany), a jejunal ulceration without bleeding signs was suspected (Figure 1). However, the blood hemoglobin level dropped slowly from 140 g/L to 92 g/L over 12 mo.
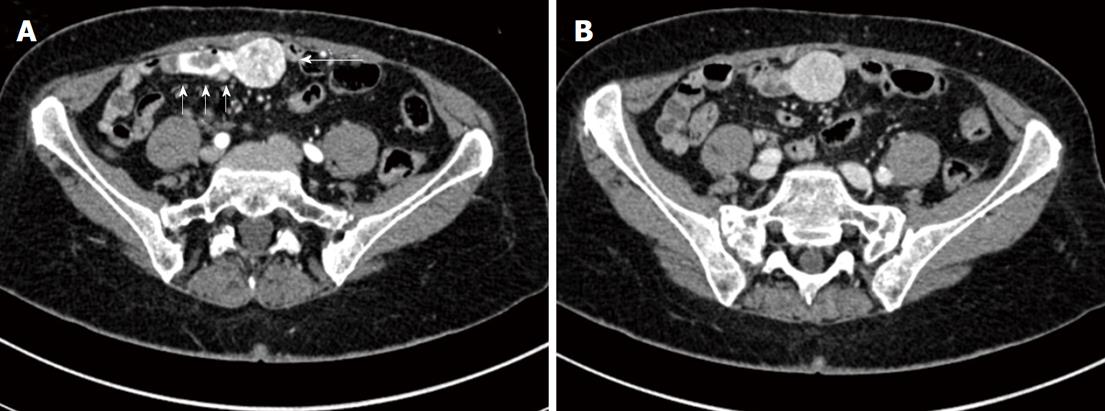
On admission to our hospital, physical examination of the hemodynamically stable patient (blood pressure 110/70 mmHg) showed regular motility of the gut without evidence of tenderness or any pathological abdominal mass. There was slight percussion pain in the right upper abdominal quadrant and melena on rectal exam. Her past medical history revealed uterine myomas, a diaphragmatic hernia, an iron deficiency anemia, and an appendectomy in her twenties. Laboratory findings included a white cell count of 4.5 × 109/L, a red cell count of 4.0 × 1012/L, hemoglobin 92 g/L, hematocrit 32%, mean corpuscular volume 79.2 fl, ferritin 4 μg/L, blood iron 120 μg/L, transferrin 3 g/L, and transferrin saturation index 3%. Serum creatinine was 61 μl/L. Abdominal sonography results were unremarkable. Upper GI endoscopy and colonoscopy did not detect any bleeding source, suggesting obscure GI-bleeding. Peroral DBE was performed revealing a small ulceration (6-8 mm) without bleeding signs at 260 cm post-pylorus, confirming the jejunal ulceration previously suspected from video capsule endoscopy (Figure 1). Following negative biopsy results, the patient received packed red blood cells and was discharged from our hospital in good general condition. A second peroral and peranal DBE (insertion depth around 200 cm post-pylorus and peranal), conducted two weeks later because of persistent melena, did not reveal any significant findings. After a further two weeks later she was readmitted because of another episode of acute bleeding. An MDCT study (Figure 2) with 3D reconstruction was then performed (Figure 3).
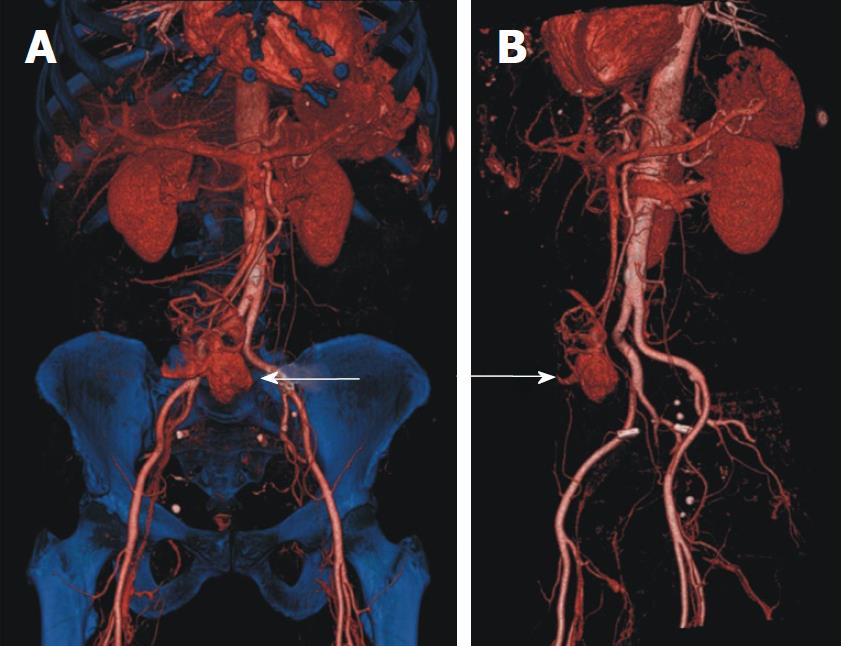
Biphasic MDCT findings disclosed a homogeneous, hypervascularized, smoothly outlined abdominal mass (transverse diameter of about 2.8 cm × 3 cm, Figure 1: large arrow) showing arterial contrast enhancement and extravasation of contrast media into the intestines (Figure 2: small arrows) with washout in the venous phase. There were no signs of additional lesions or suspicious abdominal lymph nodes. 3D reconstruction of CT data was instrumental in determining site, size and vascularization of the bleeding origin (Figure 3). The adjacent intestinal wall for 3-5 cm on either side of the mass appeared to be hyperaemic. Due to multiple arteries arising from A. mesenterica superior and A. iliaca communis dextra feeding the tumor, arterial embolisation which might reduce the risk of intraoperative bleeding was not indicated (Figure 3, arrows). Carcinoembryonic antigen was within the normal physiological range.
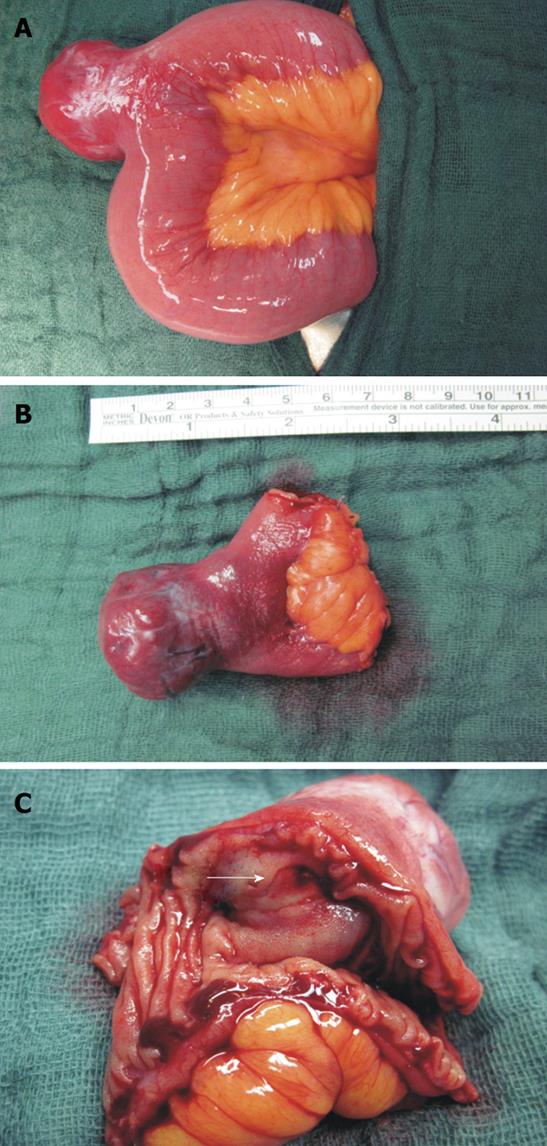
For definite histopathological diagnosis, surgery was indicated. On exploration, a mass (3.6 cm × 3.2 cm × 2.8 cm) arising from the jejunum (240 cm behind the plica duodenalis superior/Treitz´s ligament) (Figure 4A) was revealed. A partial jejunal resection (Figure 4B) with end-to-end jejunostomy for reconstruction was performed showing a transmurally growing and bleeding tumor. There was no evidence of pathological lymph nodes or metastases.
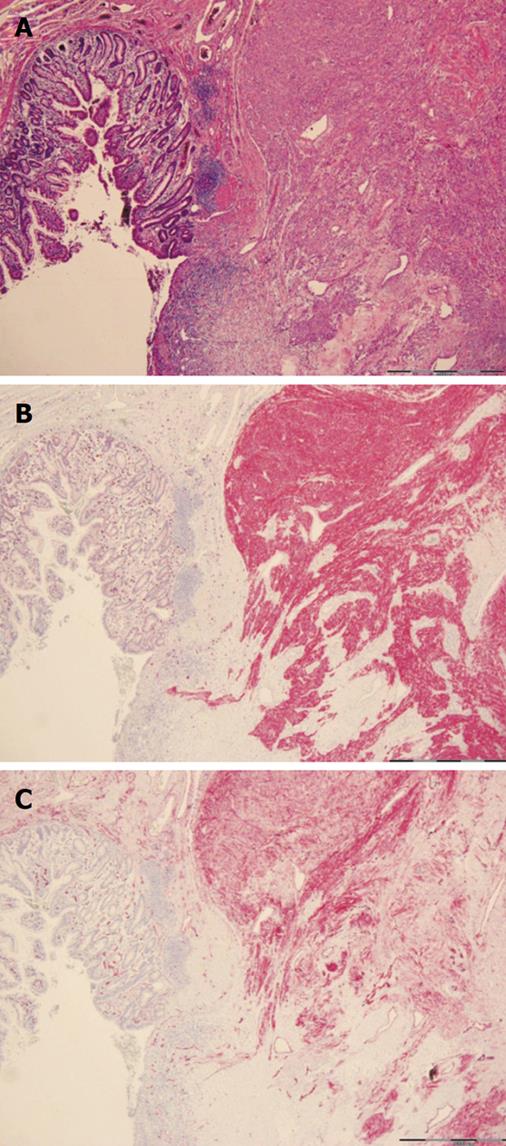
Macroscopically, the tumor appeared as a lobulated, hypervascularized red-white mass infiltrating and ulcerating the intestinal wall (Figure 4C, arrow). Histological assessment revealed proliferation of whorls of spindle cells (uniform elongated cells with syncytial-appearing eosinophilic cytoplasm and uniform ovoid nuclei) with fibers, vessels and a mononuclear inflammatory infiltrate (Figure 5A). Using immunohistochemical staining techniques, nearly all tumor cells showed a positive reactivity for CD117 (c-kit) (Figure 5B) and CD34 (Figure 5C). Analysis by PCR amplification revealed a c-kit gene mutation in the exon 9. Staining against smooth muscle antigen (SMA) was negative and less than 5% of cells were positive for Ki-67 protein (cells expressing this protein are thought to be actively dividing). Because of the low mitotic rate [number of mitoses per 50 high-power fields (HPF): 5] and a size between 2 and 5 cm, the neoplasm was classified as a low risk gastrointestinal stromal tumor (GIST)[1]. Thus, therapy was exclusively surgical. Nine days after surgery the patient was discharged in good health. No signs of gastrointestinal rebleeding occurred in a follow-up of eight months.
GIST are extremely rare neoplasms, with an incidence of 10-15 per million people per year, which usually occur in adults in their fifth or sixth decade (median age 55-60 years). They occur throughout the gastrointestinal tract with 60%-70% in the stomach, 25%-35% in small intestine, and less than 5% in rectum, esophagus, omentum, and mesentery[2,3]. GISTs are the most common mesenchymal tumors in the GI tract and comprise about 1%-3% of all malignant GI tumors. Interestingly, GIST can occur as classical familial GIST syndrome, GIST or as part of multi-neoplastic disease[4]. A debate on nomenclature, cell types of origin, and pathological subclassification was recently published by Miettinen and Lasota[2,3] and Fletcher et al[1].
The clinicopathology and appearance of GISTs vary considerably and as symptoms might result from both small incidental nodules and large tumors. Interestingly, up to 80% of patients with GISTs are without any symptoms at the time of diagnosis as smaller GISTs are frequently asymptomatic and are identified incidentally during surgery, radiologic or endoscopic studies[3]. Thus, symptomatic GISTs have often grown large before they are discovered and that is why their diagnosis frequently occurs following emergency surgery for GI perforation or GI bleeding. Small GISTs often form solid subserosal or intramural masses, sometimes ulcerating or eroding vessels but rarely growing into the lumen. Therefore, GI bleeding (acute or chronic) is the most common clinical presentation of GISTs while nonspecific symptoms, such as obstruction, invagination, perforation or anemia occur in approximately 20% of cases[5]. It is most likely that the jejunal ulceration seen on VCE and DBE is part of the GIST. The location of the endocopically detected lesion (distal part of the jejunum, insertion depth 260 cm post-pylorus) is consistent with the MDCT data and surgical resection specimen.
Recently, the diagnostic role of MDCT in upper and lower GI bleeding has been markedly extended due to its high spatial and temporal resolution, acquisition of arterial- and venous phase images as well as depiction of active extravasation of contrast medium. A presumed bleeding site or potential causative pathology was detected and localized by MDCT in > 80% of patients and active contrast media extravasation was apparent during most examinations[6-8]. Thus, in addition to the endoscopic standard work-up, MDCT seems to be recommended for obscure bleeding indications[9]. It is currently the imaging modality of choice for patients with suspected abdominal mass or biopsy-proven GIST[10]. Differential diagnoses of GIST include lymphoma, leiomyosarcoma, adenocarcinoma or metastases. Unlike the latter tumor entities, lymphadenopathy is not a common sign of GISTs. Metastases, if they occur, have been described as multiple smooth and not calcified (prior to therapy) masses with features distinguishing them from carcinoids. As luminal obstruction frequently occurs in adenocarcinomas (including clinical signs of constipation or paradoxical diarrhea) but not in GISTs (excepting large tumors), this might also help in differential diagnosis[11].
Up to one fourth of gastric GISTs and half of all small intestinal GISTs are clinically malignant with metastases commonly occurring intra-abdominally, i.e. preferentially in the liver; rarely in soft tissues, bones or skin; and even less frequent in lymph nodes and lungs. Metastases may develop a long time (> 15 years) after primary surgery and long-term follow-up is,therefore, encouraged[3]. Morphological features such as tumor size and mitotic activity (Ki-67 staining) have gained greatest acceptance for predicting outcome and distinguishing benign from malignant GISTs[1]. Histological features are site dependent with a majority being spindle cell tumors (70%) and a minority presenting with an epithelioid (20%) or mixed spindle, epithelioid, or a nested paraganglioma-like or carcinoid-like growth pattern or,rarely, a cytological pleomorphism (2%-3%)[1]. Further immunohistochemical characterization of the tumor in this case revealed a key feature of GISTs (Figure 5B, C) - positivity for CD117 (c-kit or KIT) and CD34.
It is generally accepted that all GISTs are malignant regardless of tumor size or mitotic index[1]. Usually, the primary therapy of localized GISTs is surgical. Nevertheless, about 50% of patients treated by resection relapse within 5 years. Using the most important prognostic risk factors, tumor size and mitotic index, a system for classification of patients has been validated[1]. At present, further prognostic factors such as localisation, presence and type of c-kit mutation have been suggested for stratification of relapse risk (overview in[12] and[13]).
In cases of very low and low/intermediate grade GISTs, surgical resection has a good prognosis[14,15]. In patients with intermediate/high-risk GISTs increased recurrence and decreased survival rates occur despite complete surgical resection[16]. GIST is considered to be an extensively chemotherapy-resistant soft-tissue sarcoma subtype[17]. The standard systemic treatment for soft-tissue sarcomas is a Doxorubicin-based chemotherapy, which achieves a 2-year survival rate of only 20% in patients with GIST[18]. For these patients, the development of imatinib, a tyrosine kinase inhibitor targeting both c-kit and platelet-derived growth factor alpha (PDGFRA), has considerably improved the outcome. In advanced disease this is now the standard firstline therapy[19].
Interestingly, due to secondary resistance often related to secondary c-kit or PDGFRA mutations, most patients initially responding to imatinib treatment will eventually develop tumor progression[20,21]. Nevertheless, for patients requiring cytoreductive therapy before resection, neo-adjuvant treatment with imatinib is an emerging option[20]. There are established guidelines for the follow-up of a patient, such as ours, after resection with curative intent. According to the GIST Consensus Conference for low- or very low risk GIST, i.e. tumors < 5 cm and with a mitotic index < 5/50 high power fields, a systematic follow-up with CT scan every 6 mo for 5 years would be reasonable. At present, however, there is no evidence indicating that these are the optimal time intervals, and whether follow-up with CT is beneficial or not in these patients[10].
In conculsion, findings of the present report indicate that GI-bleeding is a typical presentation of GIST. In these cases MDCT is a useful tool for (initial) diagnosis and quick localization of submucosal GI tumors, since endoscopic diagnostic tools such as VCE and DBE may miss these lesions. In cases of very low and low/intermediate grade GISTs surgical resection has a good prognosis. However, novel therapeutic targets have been identified which may lead to potential new treatment options in the future.
| 1. | Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, Miettinen M, O'Leary TJ, Remotti H, Rubin BP. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33:459-465. |
| 2. | Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer. 2002;38 Suppl 5:S39-S51. |
| 3. | Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466-1478. |
| 4. | Beghini A, Tibiletti MG, Roversi G, Chiaravalli AM, Serio G, Capella C, Larizza L. Germline mutation in the juxtamembrane domain of the kit gene in a family with gastrointestinal stromal tumors and urticaria pigmentosa. Cancer. 2001;92:657-662. |
| 5. | Ghanem N, Altehoefer C, Furtwängler A, Winterer J, Schäfer O, Springer O, Kotter E, Langer M. Computed tomography in gastrointestinal stromal tumors. Eur Radiol. 2003;13:1669-1678. |
| 6. | Frattaroli FM, Casciani E, Spoletini D, Polettini E, Nunziale A, Bertini L, Vestri A, Gualdi G, Pappalardo G. Prospective study comparing multi-detector row CT and endoscopy in acute gastrointestinal bleeding. World J Surg. 2009;33:2209-2217. |
| 7. | Jaeckle T, Stuber G, Hoffmann MH, Jeltsch M, Schmitz BL, Aschoff AJ. Detection and localization of acute upper and lower gastrointestinal (GI) bleeding with arterial phase multi-detector row helical CT. Eur Radiol. 2008;18:1406-1413. |
| 8. | Scheffel H, Pfammatter T, Wildi S, Bauerfeind P, Marincek B, Alkadhi H. Acute gastrointestinal bleeding: detection of source and etiology with multi-detector-row CT. Eur Radiol. 2007;17:1555-1565. |
| 9. | Aschoff AJ. MDCT of the abdomen. Eur Radiol. 2006;16 Suppl 7:M54-M57. |
| 10. | Blay JY, Bonvalot S, Casali P, Choi H, Debiec-Richter M, Dei Tos AP, Emile JF, Gronchi A, Hogendoorn PC, Joensuu H. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST Consensus Conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005;16:566-578. |
| 11. | Tateishi U, Miyake M, Maeda T, Arai Y, Seki K, Hasegawa T. CT and MRI findings in KIT-weak or KIT-negative atypical gastrointestinal stromal tumors. Eur Radiol. 2006;16:1537-1543. |
| 12. | Sleijfer S, Wiemer E, Verweij J. Drug Insight: gastrointestinal stromal tumors (GIST)--the solid tumor model for cancer-specific treatment. Nat Clin Pract Oncol. 2008;5:102-111. |
| 13. | Liegl-Atzwanger B, Fletcher JA, Fletcher CD. Gastrointestinal stromal tumors. Virchows Arch. 2010;456:111-127. |
| 14. | Das A, Wilson R, Biankin AV, Merrett ND. Surgical therapy for gastrointestinal stromal tumours of the upper gastrointestinal tract. J Gastrointest Surg. 2009;13:1220-1225. |
| 15. | Hassan I, You YN, Shyyan R, Dozois EJ, Smyrk TC, Okuno SH, Schleck CD, Hodge DO, Donohue JH. Surgically managed gastrointestinal stromal tumors: a comparative and prognostic analysis. Ann Surg Oncol. 2008;15:52-59. |
| 16. | Reichardt P, Hogendoorn PC, Tamborini E, Loda M, Gronchi A, Poveda A, Schöffski P. Gastrointestinal stromal tumors I: pathology, pathobiology, primary therapy, and surgical issues. Semin Oncol. 2009;36:290-301. |
| 17. | Van Glabbeke M, Verweij J, Judson I, Nielsen OS. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer. 2002;38:543-549. |
| 18. | Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, Issels R, van Oosterom A, Hogendoorn PC, Van Glabbeke M. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127-1134. |
| 19. | Blanke CD, Demetri GD, von Mehren M, Heinrich MC, Eisenberg B, Fletcher JA, Corless CL, Fletcher CD, Roberts PJ, Heinz D. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625. |
| 20. | Lopes LF, Bacchi CE. Imatinib treatment for gastrointestinal stromal tumour (GIST). J Cell Mol Med. 2010;14:42-50. |
| 21. | Gramza AW, Corless CL, Heinrich MC. Resistance to Tyrosine Kinase Inhibitors in Gastrointestinal Stromal Tumors. Clin Cancer Res. 2009;15:7510-7518. |
Peer reviewer: Michal Procke, MD, Department of Internal Medicine - Gastroenterology and Endoscopy, Charles University, 2nd Medical School, Motol University Hospital, Prague, Czech Republic
S- Editor Zhang HN L- Editor Hughes D E- Editor Liu N