Published online Oct 16, 2011. doi: 10.4253/wjge.v3.i10.183
Revised: July 15, 2011
Accepted: August 30, 2011
Published online: October 16, 2011
Currently, in gastrointestinal endoscopy there is increasing interest in high resolution endoscopic technologies that can complement high-definition white light endoscopy by providing real-time subcellular imaging of the epithelial surface. These ‘optical biopsy’ technologies offer the potential to improve diagnostic accuracy and yield, while facilitating real-time decision-making. Although many endoscopic techniques have preliminarily shown high accuracy rates, these technologies are still evolving. This review will provide an overview of the most promising high-resolution imaging technologies, including high resolution microendoscopy, optical coherence tomography, endocytoscopy and confocal laser endoscopy. This review will also discuss the application and current limitations of these technologies for the early detection of neoplasia in Barrett’s esophagus, ulcerative colitis and colorectal cancer.
- Citation: Shukla R, Abidi WM, Richards-Kortum R, Anandasabapathy S. Endoscopic imaging: How far are we from real-time histology? World J Gastrointestinal Endoscopy 2011; 3(10): 183-194
- URL: https://www.wjgnet.com/1948-5190/full/v3/i10/183.htm
- DOI: https://dx.doi.org/10.4253/wjge.v3.i10.183
Endoscopic surveillance is an important tool in the management and detection of many pre-malignant diseases throughout the gastrointestinal (GI) tract, including Barrett’s esophagus, ulcerative colitis and atrophic gastritis. The key to the early detection and treatment of GI cancer lies in the ability to detect and delineate intraepithelial neoplasia. Although the endoscopic treatment of many GI mucosal lesions by endoscopic resection or ablation is very effective, dysplasia and neoplasia are not always apparent on conventional white light endoscopy. As such, it is important to develop technologies that can better define subtle mucosal changes, offering a more targeted approach to current endoscopic surveillance protocols and the potential for real-time decision making [e.g., immediate endoscopic mucosal resection (EMR)]. Many wide-field imaging technologies, such as high-definition white light endoscopy and autofluorescence imaging (AFI), have been shown to enhance diagnostic sensitivity; however, the lack of spatial resolution results in limited specificity and high false-positive rates[1-3]. Because of these limitations, there has been emerging interest in the development of complementary technologies that provide a high-spatial resolution, thus enhancing specificity. Examples of these high-resolution technologies include confocal microendoscopy, optical coherence tomography (OCT) and endocytoscopy (EC) (Table 1). By providing subcellular imaging of the epithelial surface, these ‘optical biopsy’ technologies have the potential to revolutionize our approach to endoscopic surveillance, potentially offering a more targeted, efficient and cost-effective approach to endoscopic surveillance.
| Technology | Contrast | Field of view | Lateral resolution | Clinical results | |||
| Trial | Patients(n) | No. of examined areas | Results | ||||
| Confocal laser endoscopy (Pentax-eCLE) | IV fluorescein | 475 μm | 0.7 μm | Kiesslich et al[36] | 63 | 156 | Barrett’s: Sensitivity 98.1%; Specificity 94% |
| Topical acriflavine 0.05% | Esophageal neoplasia: Sensitivty 92.9%; Specificity 98.4% | ||||||
| Confocal laser endoscopy (Mauna Kea-pCLE) | IV fluorescein | 240-600 μm | 1 μm | Bajbouj et al[51] | 39 | 670 | Specificity: 97% (blinded), 95% (on-site); |
| Topical acriflavine 0.05% | Sensitivity: 28% (blinded), 12% (on-site) | ||||||
| Endocytoscopy (Olympus) | Absorptive contrast (i.e. Methylene Blue or Toludine Blue) | < 0.5 mm | 1.7-4 μm | Pohl et al[33] | 16 | 166 | PPV/NPV (HGIN or cancer in Barrett’s patients)-0.29/0.87 (× 450)-0.44/0.83 (× 1125) |
| Sasajima et al[35] | 6 | 7 | 93.3% accuracy in colon cancer detection | ||||
| HRME | Topical proflavine 0.01% | 750 μm | < 4 μm | N/A→Currently going to clinical trial | |||
| Optical coherence tomography | None | Traditional systems: 7-20 μm | Poneros et al[52 | 121 | 288 | Diagnosis of intestinal metaplasia: 97% sensitive, 92% specific | |
| Catheter-based: 1-2 μm | |||||||
Multiple “wide-field” technologies, including narrow-band imaging (NBI), Fujinon Intelligent Color Enhancement system and AFI, have been developed with the goal of highlighting suspicious GI mucosa. These modalities can enhance the macroscopic view of the GI mucosa to theoretically improve diagnostic sensitivity of standard endoscopy. There are no large randomized trials showing an advantage of these technologies over high-definition white light endoscopy. However, these techniques serve an important, complementary role by “red-flagging” suspicious mucosa which can then be better characterized by imaging modalities with greater spatial resolution. This “combination strategy” offers the potential to better identify and characterize lesions at the point of care. Such an approach may enhance detection and treatment strategies by preventing the unnecessary removal of benign lesions and facilitating margin determination during endoscopic therapy.
Resolution of an image, a separate quality from magnification, is defined as the ability to optically distinguish two closely approximated objects or points[4]. High resolution endoscopy augments the ability to perceive detail within an image while magnification endoscopy enlarges an image. For endoscopic video imaging, resolution is a function of pixel density; the higher the pixel density, the better the resolution. At this time, standard definition endoscopes offer a pixel density of 100 000 to 400 000 pixels, meaning that each image is built from 100 000 to 400 000 pixels. High definition or high resolution endoscopes produce images with resolutions ranging from 850 000 to 1 million pixels[4]. High resolution endoscopes are able to detect objects 10-71 microns in diameter, compared with the naked eye which can discriminate objects 125-165 microns in diameter[5].
Magnification endoscopy usually involves the use of a movable lenses that allows the endoscopist to zoom in on a small area of mucosa and magnify it up to 150 times[6]. With greater magnification and resolution, greater image detail can be detected compared to white-light endoscopy. Often, these techniques are used in conjunction with other endoscopic imaging tools, such as chromoendoscopy, to improve detection of mucosal abnormalities. A novel high resolution microscope described by Muldoon et al[7] involves inexpensive components capable of fluorescence microscopy. This system (Figure 1) consists of a small caliber fiberoptic 3-m image-guide bundle that is made of 30 000 individual fibers and totals to 1 mm in diameter. The center-to-center spacing between the fibers is 4 μm; this spacing largely determines the resolution of the endoscopic microscope[8].
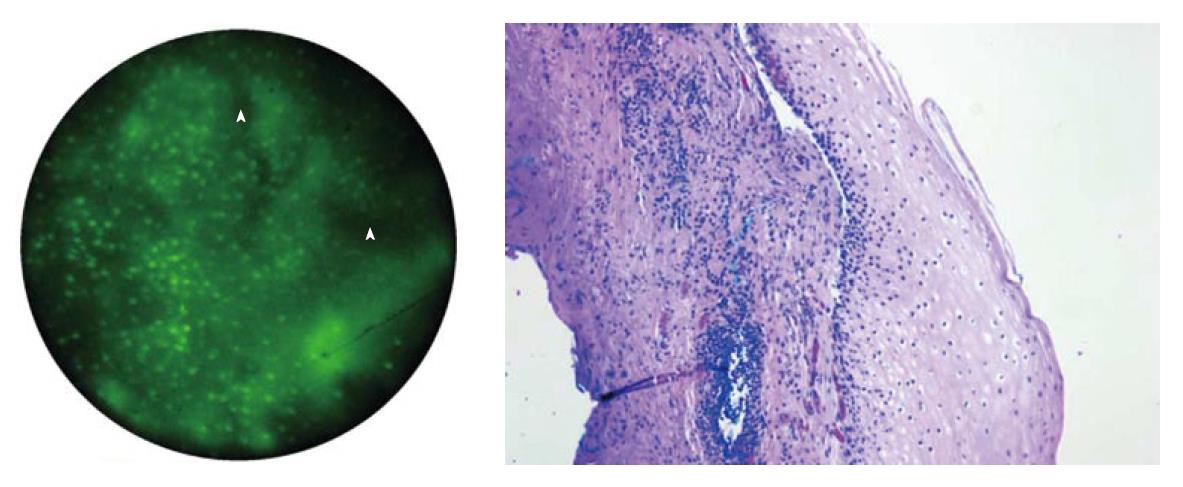
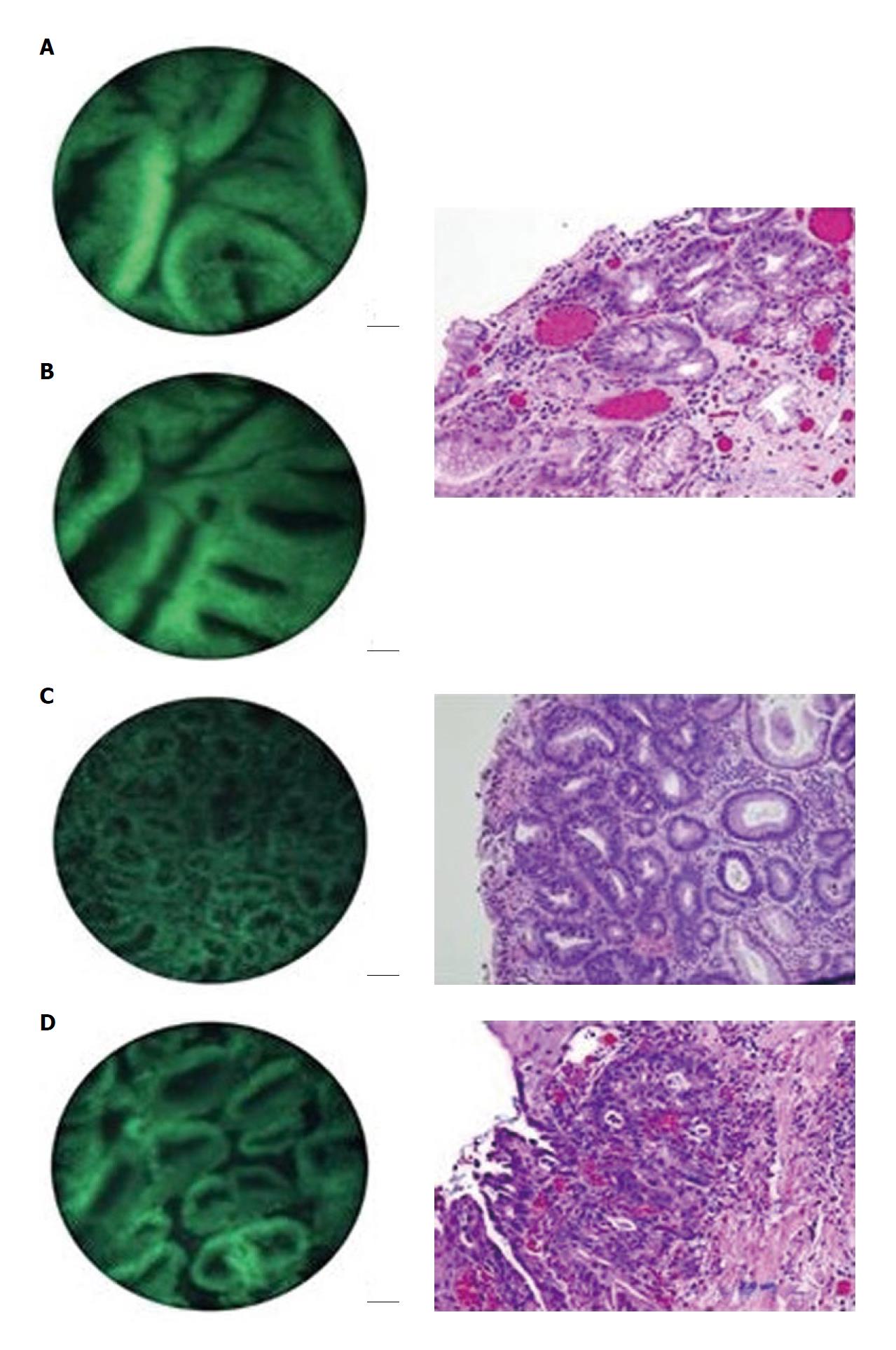
In preliminary studies by the authors, a small amount of acriflavine hydrochloride, a nuclear-specific fluorescent contrast agent, was applied to the tissue surface prior to imaging. Images were produced by placing the distal surface of the fiberoptic bundle in to direct contact with the tissue to be examined. Illumination was provided using a 455 nm blue light emitting diode that produced a light intensity of approximately 1 W. The fluorescent light returning to the bundle is directed to a scientific grade charged-coupled device that transmitted images to a personal computer. This imaging technique allows for in vivo visualization of normal mucosa, such as the normal squamous tissue of the esophagus (Figure 2). When used on in vitro biopsy and EMR specimens, the device was able to effectively delineate esophageal intraepithelial neoplasia from Barrett’s metaplasia and low-grade dysplasia (Figure 3)[7,8].
This microscope is particularly appealing as the cost of the current prototype consists of less than $3500 in components and uses a 1-2 mm (outer diameter) probe, which can be inserted into the biopsy channel of any endoscope. In addition, the system is capable of fluorescence imaging at a subcellular resolution, thus allowing targeted endomicroscopy as more sophisticated molecularly targeted fluorescent contrast agents become available.
OCT is the endoscopic, optical analogue of high frequency B-mode ultrasonography[9]. However, instead of using sound waves to generate images, OCT uses light waves. OCT is an optical signal acquisition and processing method that can capture high resolution, three dimensional images within any optical scattering media, such as biological tissue. Cross-sectional images are obtained by measuring the intensity of back-scattered light from various depths of tissue using a technique called low-coherence interferometry. Just as in B-mode ultrasonography, a quantitative measurement of backscattering is performed at each axial depth and these measurements are repeated at different transverse positions. In this manner, a linear or radial two-dimensional map of backscattering strength is acquired and used to produce a high resolution image[10,11]. OCT has attracted interest in a variety of medical applications, including ophthalmic scanning[12], diagnosis of epithelial lesions[13], bronchoscopy[14] and evaluation of atherosclerosis[15].
OCT is usually performed by introducing a linear or radial catheter into the accessory channel of a standard endoscope, duodenoscope or colonoscope. As in ultrasound, a water interface or tissue contact is not required to produce an image. Newer OCT systems are capable of producing a 512 × 512 pixel image within one-quarter of a second. Not only are OCT images obtained in real-time, but these images also have a 10-fold greater resolution (approximately 7 to 20 μm) than modalities such as endoscopic ultrasound[16]. This increased resolution allows for visualization of microscopic mucosal features such as villi, crypts and glands. Although these tissue elements can be viewed with high resolution, the sampling depth of OCT is limited to 1-2 mm due to scattering of light by tissue. Additionally, the resolution of OCT is not sufficient to visualize abnormalities such as nuclear dysplasia. Characteristics visualized with OCT that may be indicative of dysplasia or regenerative changes include incomplete surface maturation, with a high-surface OCT signal compared with the subsurface signal, and irregular glands[6].
Currently, research is being done to improve the image-acquisition capabilities of OCT. For example, there are reports of a catheter-based OCT system utilizing femtosecond laser pulses to improve imaging capabilities in the GI system; this system can achieve a resolution of approximately 1-2 μm, 10 times better than current OCT systems. In addition to visualizing mucosal structures such as crypts, villi and glands, newer OCT systems are more capable of identifying architectural distortion associated with inflammatory and neoplastic processes. High-resolution OCT also improves the quality of images with sharper images and reduction of image speckling[17].
Other advances in OCT include spectroscopic OCT, which uses the spectrum of back-scattered light to obtain diagnostic tissue information, and doppler OCT, which can potentially be used to detect differences in microvascular networks between dysplastic and non-dysplastic epithelium[9]. Recently, Winkler et al[18] have coupled OCT imaging with laser induced fluorescence imaging (LIF). Their system provides 18 μm OCT resolution while also providing low resolution laser-induced fluorescence. While the LIF component provides little spatial resolution, the addition of fluorescence microscopy allows targeted molecular targets to be used to further characterize dysplastic mucosa. In addition, modifications are being made to their LIF system to increase their resolution.
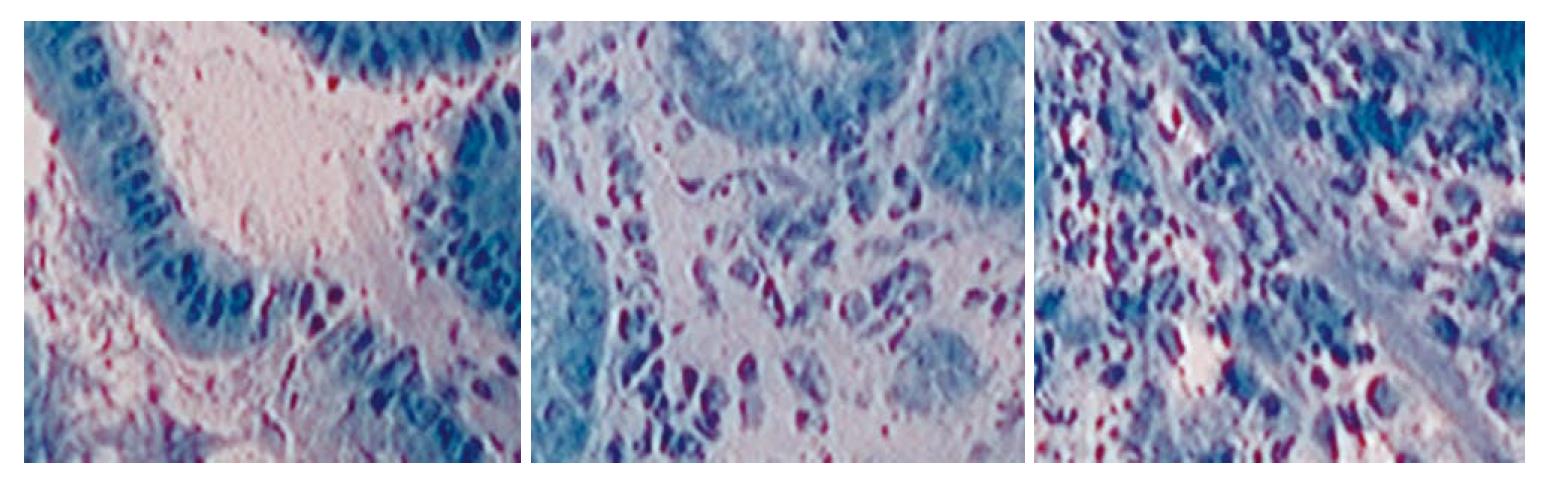
EC is based on the principle of light contact microscopy. This imaging technology enables real-time visualization of the cellular structures of the superficial epithelial layer in a plane parallel to the mucosal surface (Figure 4). In order to visualize cellular and subcellular structures (e.g., nuclei) with EC, it is necessary to prestain the mucosa with an absorptive contrast agent, such as 0.5% to 1% methylene blue, cresyl violet or 0.25% toluidine blue. Often, the mucosa is treated with a mucolytic such as N-acetylcysteine prior to staining. After the stain is applied to the tissue surface, the tip of the endoscope is placed in direct contact with the dye-stained surface mucosa and then the target mucosa is scanned with condensed normal white light. A fixed-focus, high-power objective lens projects highly magnified images from a minute sampling site (< 0.5 mm diameter) on to a charge-coupled device[19-21]. With this method, cytological details can be directly visualized, making direct observation of living cells feasible.
The first EC system was utilized in otolaryngology and consisted of a rigid instrument which was not practical for GI use. Now, a novel endocytoscope system, Endocytoscope, has been developed by Olympus instruments (Tokyo, Japan) which consists of two flexible endoscopes with a diameter of 3.2 that can be passed through the accessory channel with a diameter of 3.7 or larger of an endoscope. The Endocytoscope system is comprised of one low-magnification (× 450) endocytoscope and one high-magnification (× 1100) endocytoscope[20]. In addition to the probe-based endocytoscope system described above, there are integrated endocytoscope system prototypes available as well.
Endocytoscopic diagnosis is based on the assessment of several cytological and architectural features, such as the density, size and arrangement of cells; the size and shape of nuclei; the nucleus-to-cytoplasm ratio; and the staining pattern. For example, squamous cell carcinoma of the esophagus is distinguished by increased density of cells and marked heterogeneity in staining in nuclear staining and size, as opposed to the orderly cellular arrangement and homogenous staining pattern seen in normal squamous esophageal epithelium[19,22].
Confocal laser endoscopy (CLE) is one of the newest endoscopic technologies. While some endoscopic techniques such as NBI and chromoendoscopy can be used effectively to highlight suspicious areas of mucosa for biopsy and histological analysis, CLE offers the endoscopist the ability to make a real-time, in vivo histological assessment of GI mucosa. Confocal laser microscopy is based on the excitation of a fluorophore in a specimen using a laser which sequentially scans specific points on the specimen in a raster pattern whereby the focused spot traverses a line rapidly from left to right and the line is swept top to bottom, mapping out a square[23]. The emission of the fluorophore at the point of illumination is recorded to create the pixels of an image. Emitted light is also channeled through a pinhole such that light that is not focused at a specific depth is unable to reach the detector. Thus, confocal microscopy is able to scan specific optical depths within the specimen and provide a clear, two-dimensional image of the tissue sample[9].
CLE integrates a confocal laser microscope either in the tip of a video endoscope or as a probe than can be passed through the channel of any endoscope[5]. One widely used CLE system consists of an endoscope with embedded CLE technology manufactured by Pentax (Tokyo, Japan). This system is available both for upper GI endoscopy and colonoscopy[6]. In the embedded CLE technology (eCLE), a confocal laser microscope is integrated directly into the end of a video endoscope. Images are obtained by using a laser which delivers an excitation wavelength of 488 nm. The pixels are collected every 0.7 μm (to give 0.7 μm lateral resolution) for a total field of view of 475 μm and can be collected through various sections of the mucosa in 7 μm increments to a depth of 250 μm. Current equipment is able to achieve this at a rate of 0.8 to 1.6 frames/s. This system is capable of providing both excellent image clarity and the ability to use the confocal endomicroscope just like a conventional endoscope. In order to produce quality images, image stabilization is necessary; this is achieved using suction. Consequently, a “suction polyp” is created that helps to target tissue biopsy to the imaged area[24].
Alternately, a confocal microscope can be introduced as a mini-probe into the working/accessory channel of the endoscope; a probe-based CLE system (pCLE), Cellvizio-GI, has been created by Mauna Kea Technologies (Paris, France). The advantage of this system is that, unlike the embedded CLE technology, the confocal microscopy probe can be used with any commercial endoscope and, subsequently, a variety of wide-field technologies (Narrow Band Imaging, etc.). The probe is placed into the biopsy channel of a standard endoscope and then retracted to allow for tissue biopsy[24]. Depending on the system used, pCLE can scan with 1-3.5 μm lateral resolution for a total field of view of 240-600 μm, which does not compare well with the embedded systems. In addition, pCLE systems are unable to vary the depth at which they collect data and thus lack axial resolution. However, these tradeoffs allow the probe based system to operate with a much faster scanning rate (12 frames/s compared to the 0.8-1.2 frames/s in eCLE).
In order to obtain images using either confocal endomicroscopic tool, the patient must be given a fluorescent contrast agent. This can either be given intravenously or sprayed onto the mucosa directly. The most common contrast agent is IV fluorescein 10%. Fluorescein aptly highlights the vasculature, lamina propria and intracellular spaces, allowing visualization of vessel pattern and cellular architecture[25]. Fluorescein and its metabolites are excreted renally. Adverse side effects of fluorescein are rare but may include hypotension without shock (0.5%), nausea (0.39%), injection site erythema (0.35%), diffuse self-limited rash (0.04%) or mild epigastric pain (0.09%). Anaphylaxis has been reported but is uncommon[23,26]. Of note, fluorescein does not provide direct nuclear visualization and the appreciation of nucleus-to-cytoplasm ratio cannot be used for the diagnosis and grading of intraepithelial neoplasias.
Acriflavine hydrochloride (0.05% in saline) and cresyl violet (0.13% in acetic acid) are two contrast agents that are applied topically to the mucosa during CLE. Acriflavine is used to stain cellular nuclei which fluorescein is unable to do. However, since acriflavine accumulates in the nuclei, there is a potential mutagenic risk associated with it. Cresyl violet causes cytoplasmic enrichment which in turn leads to negative visualization of nuclear morphology. It also provides pit pattern characterization of mucosal lesions and so can be used for chromoendoscopy as well[23].
The aforementioned endoscopic techniques offer a more accurate and in-depth view of GI mucosa. Most studies to date have used these advanced endoscopic techniques in detecting GI lesions to improve early detection of cancer in Barrett’s esophagus, colon cancer screening and ulcerative colitis. The advantages of imaging the mucosa are particularly apparent in clinical scenarios where a large number of random biopsies could be replaced with a targeted biopsy of suspicious lesions.
Barrett’s esophagus (BE) is the most important risk factor for esophageal adenocarcinoma. The purpose of endoscopic surveillance in monitoring BE is to detect early neoplastic lesions [high grade dysplasia (HGD) or intramucosal cancer] and utilize less invasive, endoscopic treatments. Although guidelines recommend surveillance for Barrett’s metaplasia every 3 years, the current practice involves endoscopic white light examination with random four-quadrant biopsy, a procedure that has been shown to miss neoplasia in up to 57% of cases[27,28].
The overall incidence of colorectal cancer (CRC) is increasing in Western populations; CRC now represents the most fatal malignancy in non-smokers in Europe and North America[29]. Although CRC carries a high mortality rate, this malignancy can be treated or prevented with early detection. The use of in vivo histology may improve our ability to differentiate neoplastic from non-neoplastic lesions and facilitate the detection of cancer at an earlier stage.
In an ex vivo study conducted by Muldoon et al[7], high resolution microendoscopy (HRME) imaging was conducted on several specimens obtained by EMR after the application of topical acriflavine. The microendoscopic images were compared to histopathological analysis of the same site. The device was able to delineate normal squamous mucosa from Barrett’s metaplasia/low-grade dysplasia (LGD) and HGD/cancer (Figure 3).
In another study by the same authors, nine patients with pathologically confirmed Barrett’s esophagus underwent endoscopic examination with biopsies or EMR[8]. Resected fresh tissue was imaged with fiber bundle microendoscopy/HRME. The images were then analyzed, either visually or with quantitative computer analysis, to predict whether the imaged sites were non-neoplastic or neoplastic. Predictions were compared to the gold standard of histopathology. Subjective analysis of the images by expert clinicians, including two gastroenterologists and two pathologists, achieved average sensitivity and specificity of 87% and 61% respectively. Subjective image analysis, however, was subject to some intra-observer variability.
In the same study, a quantitative, computer-based algorithm was developed to analyze images obtained with HRME. This algorithm was developed using 59 distinct image features as input; this helped to classify an image as neoplastic or non-neoplastic. Histopathology again was used as the gold standard; sites with a pathological diagnosis of Barrett’s metaplasia or Barrett’s metaplasia with low-grade dysplasia were considered to be non-neoplastic while sites with a pathological diagnosis of Barrett’s metaplasia with high-grade dysplasia or esophageal adenocarcinoma were considered to be neoplastic. The best performing quantitative classification algorithm relied on two image textural features, frequency content and pixel-pair correlation, and achieved a sensitivity and specificity of 87% and 85% respectively.
The greatest advantage of the HRME, as mentioned previously, is its cost, portability and flexibility. The limitations are the axial resolution and poor depth penetration. However, to further assess the feasibility of this technology, clinical trials are currently in progress.
OCT and Barrett’s esophagus: OCT has been widely studied for use in examining the esophagus and the esophago-gastric junction. Normal esophageal tissue on OCT imaging shows an easily recognizable horizontal, layered structure. OCT features predictive for the presence of intestinal metaplasia are: (1) the absence of the layered structure of the normal squamous epithelium and the presence of the vertical crypt-and-pit morphology of normal gastric mucosa; (2) a disorganized architecture with inhomogeneous back-scattering of the signal and an irregular mucosal surface; and (3) the presence of submucosal glands characterized at the OCT imaging as pockets of low reflectance below the epithelial surface[30,31]. When these OCT criteria were applied to images acquired prospectively, the criteria were found to be 97% sensitive and 92% specific for specialized intestinal metaplasia, with a PPV of 84%. Use of these simple criteria is limited, however, as the presence of the crypt-and-pit architecture in normal or inflamed gastric mucosa may make it difficult to discriminate between esophageal metaplasia and normal or inflamed gastric mucosa. Also, it is difficult to identify high-grade dysplasia utilizing OCT; the increased nuclear-to-cytoplasmic ratio occurring in dysplasia can alter the light reflection characteristics, giving a more inhomogeneous back-scattering of the signal. Poneros[30] were able to improve diagnosis of high grade dysplasia by using two parameters of tissue reflectivity as an indicator of dysplasia. These criteria retrospectively diagnosed high-grade dysplasia with 100% sensitivity and 85% specificity.
OCT and the colon polyps: In studies undertaken at Eppendorf Clinic University of Hamburg (Germany) and Cleveland Clinic Foundation (United States), endoscopic OCT was tested as a possible tool for in vivo endoscopic differential diagnosis of colon polyps and assessing the need of their removal during colonoscopy[22]. OCT features of adenomas and hyperplastic polyps were developed, based on which the diagnostic accuracy of OCT was statistically analyzed. 48 tubular adenomas, 12 tubulovillous adenomas and 56 hyperplastic polyps were studied. It was found that the hyperplastic polyp is characterized by a three-layer “benign” OCT image demonstrating thickening of the upper layer (glandular mucus membrane) and clear border between glandular mucus membrane and submucous layer. Additionally, adenoma is characterized by an image that does not demonstrate layers. This study showed that, of 116 polyps studied, OCT can differentiate adenomas from hyperplastic polyps with good sensitivity (92%) and specificity (84%).
EC and Barrett’s esophagus: Pohl et al[33] conducted a study examining the utility of EC in the surveillance of patients with Barrett’s esophagus. In this in vivo study, 166 biopsy sites from 16 patients (13 male, mean age 62.1 years) were examined; these patients had no visible lesions and were presenting for Barrett’s surveillance endoscopy. EC images were recorded from pre-marked areas in the Barrett’s segment using magnification × 1125 or × 450. Biopsies were taken from the same area to allow precise comparison with histology. The images of each area were individually reviewed by both a pathologist and a gastroenterologist in a blinded fashion. In the study, the pathologist noted that features of neoplasia could be evaluated in only 0%-19% of image sequences with magnification × 450 and in only 4%-41% of sequences obtained with magnification × 1125. Conversely, the gastroenterologist noted 74% of low-magnification images as interpretable for neoplasia and 78% of high-magnification images were interpretable for neoplasia. Overall, there was poor inter-observer agreement between the gastroenterologist and pathologist and many images were found to have poor quality or could not be examined at all. Positive and negative predictive values for high grade intraepithelial neoplasia (HGIN) or cancer were 0.29 and 0.87 respectively for magnification × 450 and 0.44 and 0.83 respectively for magnification × 1125.
Although this study did not demonstrate good utility for EC in obtaining endoscopic histology, another study by Eberl et al[34] showed that the sensitivity and specificity for the evaluation of 25 patients with neoplastic esophageal lesions by blinded pathologists was 81% and 100% respectively. These improved results are likely due to the presence of identified neoplastic lesions, whereas in the Pohl study there were no visible lesions present. It is important to note that image quality remains an issue with EC. However, advances in this technology are combining EC with other endoscopic tools such as NBI to screen Barrett’s esophagus and isolate suspicious areas of the mucosa for evaluation and biopsy.
EC and colon cancer: Sasajima et al[35] studied the utility of EC in evaluating colorectal lesions; 60 patients were enrolled for examination (43 men, 17 women) and these patients underwent evaluation with EC. The images obtained were compared to histology as the gold standard. A pathologist, who was blinded to the conventional colonoscopic views and the final histological diagnosis, made a diagnosis by assessing the digital EC images. A diagnosis of high-grade adenoma was made by 7 evaluated criteria: (1) disorder of polarity; (2) deformity of nuclei; (3) enlargement of nuclei; (4) various shapes of cells; (5) higher cellular density; (6) increased nuclear:cytoplasmic ratio; and (7) irregular colonic glands. The overall accuracy of EC in this study was 93.3%. According to this study, EC has the capability of providing real-time in vivo images which are nearly as accurate as histology. Additionally, in the study conducted by Eberl et al[34], 28 patients with neoplastic colonic lesions were studied with EC and the obtained images were evaluated by a blinded pathologist. The study found a sensitivity and specificity of 61% and 86% respectively in predicting pathological lesions at × 450 magnification, but increased those results to 83% and 87% respectively at × 1125. Clearly, the higher magnification EC was superior in sensitivity compared to the lower magnification.
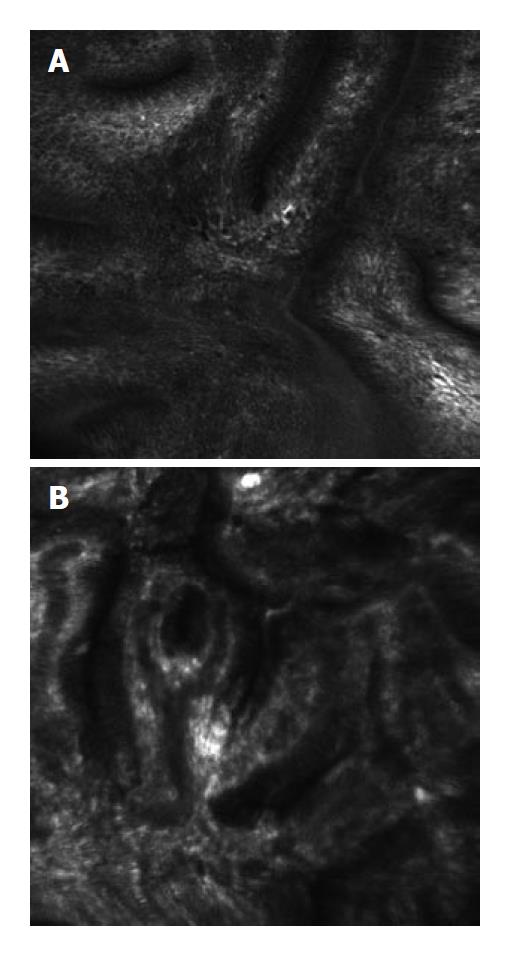
CLE and Barrett’s esophagus: Confocal laser endomicroscopy is a new technology that enables the endoscopist to obtain real-time histological information about the examined GI mucosa. Kiesslich et al[36] demonstrated that the embedded CLE diagnosed Barrett’s-related neoplasia during endoscopy with a sensitivity of 92.9% and a specificity of 98.4%. Additionally, Dunbar et al[37] conducted a prospective, randomized, double-blinded, crossover study analyzing the diagnostic yield of CLE-targeted biopsies. This study compared four-quadrant random biopsies with CLE-targeted biopsies in 39 patients. They demonstrated that CLE improved the diagnostic yield for detecting neoplasia in BE patients, particularly for endoscopically-inapparent neoplastic lesions. CLE detected 33.7% of neoplastic lesions vs 17.2% of lesions detected with random biopsies. Additionally, two-thirds of patients in the routine surveillance group did not need any biopsies when examined with CLE due to absence of neoplasia during in vivo imaging. The Kiesslich et al[36] study also reported a potential reduction in number of mucosal biopsies needed; only 30 of 156 (19.2%) CLE sites in 63 patients examined would have required a mucosal biopsy for confirmation of diagnosis of neoplasia. Ongoing work by us and others continue to study the feasibility of CLE in diagnosing dysplasia in Barrett’s esophagus (Figure 5).
CLE and ulcerative colitis: In a 2007 study by Kiesslich et al[10], it was demonstrated that chromoendoscopy-guided CLE can be very useful in patients with ulcerative colitis (UC) in detecting neoplasia. The study involved 153 patients who were randomized to undergo conventional colonoscopy or chromoscopy (with 0.1% methylene blue) with endomicroscopy. In the conventional group, random biopsy examinations and targeted biopsy examinations were taken. In the endomicroscopy group, circumscribed mucosal lesions were identified by chromoscopy and evaluated for targeted biopsy examination by endomicroscopy. The results of this study showed that for grading of UC activity, the endomicroscope assessment agreed with histology 92.5% of the time. By using the confocal pattern classification, the presence of intraepithelial neoplasia (IN), within the endomicroscopy group, was predicted with a sensitivity of 94.7% and a specificity of 98.3%. By using chromoendoscopy with CLE, 4.75-fold more INs was found compared with conventional colonoscopy with random biopsy specimens. Additionally, similar to the results for studies on Barrett’s, using chromoscopy-guided CLE allowed for 50% fewer biopsies compared with conventional colonoscopy.
The management of dysplasia-associated lesional mass (DALM) and adenoma-like mass (ALM) in chronic ulcerative colitis is significantly different. With dysplasia-associated lesional mass, the treatment involves total proctocolectomy whereas with adenoma-like mass, the treatment is limited to endoscopic resection and surveillance colonoscopy. Conventional colonoscopy has limited utility in differentiating these two lesions. However, confocal endomicroscopy has been shown to be effective in evaluating these lesions. In a study by Hurlstone et al[38], the accuracy of confocal endomicroscopy for in vivo diagnosis of dysplasia-associated lesional mass vs adenoma-like mass was 97% (95% CI: 86%-99%). ALM was defined at endomicroscopy as any lesional morphology (Paris criteria 0-II/I) within or outside of an ulcerative colitis zone with no adjacent flat neoplastic architecture. DALM was defined as any lesional morphology (Paris 0-II/I) within a colitis zone accompanied by adjacent mucosal neoplastic criteria. Neoplastic confocal criteria were defined as mucosa demonstrating either dilated/distorted vascular architecture with or without leakage or crypt/goblet cell depletion with or without irregular epithelial “ridge-lined” crypt architecture.
The ability to differentiate ALM and DALM helps to better manage patients clinically, replacing random biopsies with fewer targeted biopsies. As such, there is decreased development of submucosal desmoplasia that almost inevitably complicate multiple biopsies. Also, this technique helps to target biopsies to areas of high suspicion for dysplasia in vivo, thereby allowing for rapid, high accuracy diagnosis of dysplasia-associated lesional mass. This leads to more appropriate use of endoscopic resection and directs therapy to pan-proctocolectomy when it is indicated.
CLE and colon cancer screening: A study by Polglase et al[39] showed that with either topical acriflavine stain or with IV fluorescein stain, the histological correlates of the mucin-containing goblet cells and the columnar epithelial cells, including those vertically oriented across the surface in contact with the confocal imaging window and those radially oriented within crypts, were readily identifiable within the surface of the colonic mucosa[11]. Confocal endomicroscopy is useful in identifying normal histology of the colonic mucosa and, in addition, this tool can be used to effectively identify abnormal mucosal patterns.
Kiesslich et al[10] described a clinical study aimed at evaluating the role of confocal endomicroscopy in predicting histology in screening colonoscopy for CRC, which showed that the presence of neoplastic changes was predicted with high accuracy (97.4% sensitivity, 99.4% specificity and 99.2% accuracy). Additionally, a study by Buchner et al[40] compared CLE with virtual chromoscopy (Flexible spectral Imaging Color Enhancement) for classification of colonic polyps. This study demonstrated that when compared to the histopathology, endomicroscopy had a higher sensitivity than virtual chromoscopy in classifying colonic polyps, 91% vs 77% respectively. Further multi-center clinical trials are needed to prove the utility of CLE in identifying malignant lesions (Figure 6).
In another study by Kiesslich et al[41], the authors analyzed the predictive power of CLE for diagnosing IN and CRC during ongoing video colonoscopy. In this study, 27 patients underwent CLE evaluation of the colon using either topical acriflavine or IV fluorescein contrast. Additionally, 42 patients underwent observation of standard locations within the colon with CLE (using only IV fluorescein) in addition to CLE observation of specific lesions “unmasked” using adjunctive methylene blue pan-chromoendoscopy. Using both acriflavine hydrochloride and fluorescein sodium, high-quality images were obtained with acriflavine strongly staining the superficial epithelial cells and IV fluorescein permitting deep laminal resolution imaging. Fluorescein-guided CLE was also able to resolve tubular, villous and irregular crypt architecture with an associated loss of goblet cells. Vascular architecture was found to have changed in the context of IN with irregular peri-cryptic vessels (rather than the discrete ‘honeycombing’ observed surrounding the normal crypt), with an invariable accompanying fluorescein capillary leakage characterized by the neo-angiogenic drive associated with overt neoplasia. These initial data subsequently permitted the creation of the now validated Mainz CLE criteria for IN. Thirteen thousand and twenty CLE images from 390 locations were compared with the histopathological reports (the “gold standard”) from 1038 targeted biopsy specimens. IN was subsequently predicted with a high overall accuracy of 99.2% (sensitivity 97.4%, specificity 99.4%)[41].
CLE and celiac disease: Given the great potential to perform in vivo histology, it is not surprising that endomicroscopy has been applied to other disease processes besides neoplasia. Venkatesh et al[42] recently applied CLE to the diagnosis of celiac disease (CD) in children. Nine patients with suspected CD and 10 matched controls were evaluated with eCLE and a scoring system was developed based on the shape of the villi, distortion of the pattern of surface epithelial enterocytes, decrease in goblet cells, presence of infolding villi and presence of intervillous bridging. Based on the evaluation by two trained confocal endoscopists, eCLE was 100% sensitive and 80% specific compared to the diagnosis by a histopathologist. Further studies using greater number of patients and using the scoring system with untrained endoscopists would have to be done to fully evaluate the applicability of CLE on CD.
With the advent of the technologies reviewed above, the tools to allow real-time histology have undoubtedly been developed. Many of the techniques reviewed offer very high sensitivity and specificity in detecting histological abnormalities. Whether the technology can be reasonably used in a wide variety of clinical settings, however, is a far more complicated question.
One limitation to subcellular endoscopy is the vast amount of time that would theoretically be required to analyze large sections of mucosa. Many studies have overcome this problem by complementing microscopic endoscopy with so-called “red flag techniques” such as NBI and chromoendoscopy. These systems can highlight areas of suspicion for further, targeted examination by more advanced endoscopic imaging tools.
As reviewed above, endocystoscopy, HRME and CLE all require some kind of contrast agent. Currently we are limited to IV injection of fluorescein and topical application of methylene blue, toludine blue or acriflavine. These agents rely on the interpretation of the clinician to make the appropriate clinical diagnosis. Thus, one area of development over the next several years will be in the development of more specific, targeted stains and contrast agents.
There are already several studies that have used targeted contrast agents either in ex vivo biopsy samples or in vivo mouse models. Based on the observation that malignant cells upregulate glucose transport into their cytoplasm[43-45], Thekkek et al[46] have used topically applied fluorescent deoxyglucose on ex vivo specimens to differentiate normal from neoplastic specimens[46,47]. One exciting development is the use of contrast agents that bind specific surface proteins that are overexpressed in malignant cells. Hsiung et al[48] have developed a fluorescent heptapeptide isolated from a phage library that binds colonic adenomas with a sensitivity of 81% and specificity of 82%. Foersch et al[49] were able to inject fluorescently tagged antibodies against vascular endothelial growth factor (VEGF) in mouse colon cancer models and perform murine in vivo confocal endomicroscopy with significantly increased staining of malignant cells and clear delineation of the transition zone. Most recently, a fluorescently labeled VEGF was used to highlight VEGF receptors on the surface of mouse colon cancers[18]. Further development of such targeted contrast agents will be needed before opening the realm of endoscopic immunofluorescence.
In addition to a lack of good contrast agents, another significant limitation of these technologies is that they require significant training[50]. Each specialized endoscope requires familiarity with the basic operation of the system and technical training to obtain the best pictures. For example, despite measures to stabilize the endoscope against the mucosa in many of the above technologies, the stability of the endoscope remains of crucial importance when looking at subcellular architecture; excessive movements can create distorted pictures. The largest amount of time, however, will likely be spent on training clinicians to interpret the endomicroscopic images. Preliminary studies show that training of clinicians can be done with significantly high accuracy and interobserver agreement. Dunbar et al[24] show that after training 11 endoscopists on pCLE using 20 images with known histology followed by a blinded review of 20 unknown images, the overall sensitivity for obtaining the correct diagnosis was 88% and specificity 96%, with substantial interobserver agreement (86%). The conclusions, however, are limited by the small number of endoscopists tested and the presence of clinicians with prior pCLE experience which may have biased the results.
There are several ways the issue of training can be addressed. As mentioned above, Muldoon et al[8] offer an interesting quantitative computer-based algorithm that would make interpretation of images from Barrett’s esophagus much simpler, with good sensitivity and specificity. Such systems would complement the interpretation of a trained clinician. In addition, the development of new contrast agents, if specific to malignant cells, has the potential to simplify interpretation to merely the differentiation of “bright vs dark”.
Lastly, the most significant limitation of currently available technology is cost. Given some of the sophisticated electronics and engineering involved in these systems, it is not surprising that current systems range from $150 000-$300 000. While the cost of unnecessary biopsies and pathological interpretation is saved, the price of these platforms makes real-time histology advantageous only in specific clinical scenarios where large numbers of biopsies are usually performed, as in Barrett’s esophagus or familial adenomatous polyposis. Alternatively, these microscopic images could be invaluable in situations where the immediate histological information can significantly change clinical care, such as identifying and delineating the margins of neoplasia for EMR. Finally, cheaper solutions such as the HRME may provide an adequate degree of resolution to facilitate real-time decision making in lower resource settings where confocal systems may be not be feasible. These are all questions that are in the process of being answered.
The past several years have been marked by the emergence of several exciting and innovative “optical biopsy” technologies that provide real-time subcellular imaging of the GI tract. The ability to make a real-time histopathological diagnosis is potentially invaluable in enhancing the detection of early neoplasia and in facilitating minimally invasive therapies, such as EMR. Widespread application of these technologies, however, is limited by the current cost of these platforms, the lack of targeted contrast agents and the learning curve associated with interpretation of the microendoscopic images. Increased clinical and translational research in these areas will likely ensure that many of these issues are resolved over the next decade, making real-time histology a reality for today’s endoscopist.
Peer reviewers: Claudio Rolim Teixeira, MD, PhD, Foundation of Gastroenterology of Rio Grande do Sul, FUGAST, Av. Silva So 255, Porto Alegre, Brazil; Dimitris K Iakovidis, PhD, Assistant Professor, Technological Educational Institute of Lamia, 3rd km Old National Road Lamias-Athinas, Lamia GR 35100, Greece; Shinji Tanaka, MD, PhD, Professor, Department of Endoscopy, Hiroshima University Hospital, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan
S- Editor Zhang SJ L- Editor Roemmele A E- Editor Zheng XM
| 1. | Curvers WL, Bergman JJ. Multimodality imaging in Barrett's esophagus: looking longer, seeing better, and recognizing more. Gastroenterology. 2008;135:297-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Curvers WL, Kiesslich R, Bergman JJ. Novel imaging modalities in the detection of oesophageal neoplasia. Best Pract Res Clin Gastroenterol. 2008;22:687-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Falk GW. Autofluorescence endoscopy. Gastrointest Endosc Clin N Am. 2009;19:209-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Kwon RS, Adler DG, Chand B, Conway JD, Diehl DL, Kantsevoy SV, Mamula P, Rodriguez SA, Shah RJ, Wong Kee Song LM. High-resolution and high-magnification endoscopes. Gastrointest Endosc. 2009;69:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Reddymasu SC, Sharma P. Advances in endoscopic imaging of the esophagus. Gastroenterol Clin North Am. 2008;37:763-74, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Waxman I, Konda VJ. Endoscopic techniques for recognizing neoplasia in Barrett's esophagus: which should the clinician use? Curr Opin Gastroenterol. 2010;26:352-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Muldoon TJ, Anandasabapathy S, Maru D, Richards-Kortum R. High-resolution imaging in Barrett's esophagus: a novel, low-cost endoscopic microscope. Gastrointest Endosc. 2008;68:737-744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Muldoon TJ, Thekkek N, Roblyer D, Maru D, Harpaz N, Potack J, Anandasabapathy S, Richards-Kortum R. Evaluation of quantitative image analysis criteria for the high-resolution microendoscopic detection of neoplasia in Barrett's esophagus. J Biomed Opt. 2010;15:026027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Wong Kee Song LM, Wilson BC. Endoscopic detection of early upper GI cancers. Best Pract Res Clin Gastroenterol. 2005;19:833-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Kiesslich R, Goetz M, Lammersdorf K, Schneider C, Burg J, Stolte M, Vieth M, Nafe B, Galle PR, Neurath MF. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007;132:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 379] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 11. | Toyoda H, Rubio C, Befrits R, Hamamoto N, Adachi Y, Jaramillo E. Detection of intestinal metaplasia in distal esophagus and esophagogastric junction by enhanced-magnification endoscopy. Gastrointest Endosc. 2004;59:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Chen J, Lee L. Clinical applications and new developments of optical coherence tomography: an evidence-based review. Clin Exp Optom. 2007;90:317-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Mogensen M, Thrane L, Jørgensen TM, Andersen PE, Jemec GB. OCT imaging of skin cancer and other dermatological diseases. J Biophotonics. 2009;2:442-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 116] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Williamson JP, McLaughlin RA, Phillips MJ, Armstrong JJ, Becker S, Walsh JH, Sampson DD, Hillman DR, Eastwood PR. Using optical coherence tomography to improve diagnostic and therapeutic bronchoscopy. Chest. 2009;136:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Farooq MU, Khasnis A, Majid A, Kassab MY. The role of optical coherence tomography in vascular medicine. Vasc Med. 2009;14:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Das A, Sivak MV, Chak A, Wong RC, Westphal V, Rollins AM, Willis J, Isenberg G, Izatt JA. High-resolution endoscopic imaging of the GI tract: a comparative study of optical coherence tomography versus high-frequency catheter probe EUS. Gastrointest Endosc. 2001;54:219-224. [PubMed] [DOI] [Full Text] |
| 17. | Hsiung PL, Pantanowitz L, Aguirre AD, Chen Y, Phatak D, Ko TH, Bourquin S, Schnitt SJ, Raza S, Connolly JL. Ultrahigh-resolution and 3-dimensional optical coherence tomography ex vivo imaging of the large and small intestines. Gastrointest Endosc. 2005;62:561-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Winkler AM, Rice PF, Weichsel J, Watson JM, Backer MV, Backer JM, Barton JK. In Vivo, Dual-Modality OCT/LIF Imaging Using a Novel VEGF Receptor-Targeted NIR Fluorescent Probe in the AOM-Treated Mouse Model. Mol Imaging Biol. 2010;Epub ahead of print. [PubMed] |
| 19. | Fujishiro M, Kodashima S, Takubo K, Kakushima N, Omata M. Detailed comparison between endocytoscopy and horizontal histology of an esophageal intraepithelial squamous cell carcinoma. Dis Esophagus. 2008;21:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Inoue H, Kudo SE, Shiokawa A. Technology insight: Laser-scanning confocal microscopy and endocytoscopy for cellular observation of the gastrointestinal tract. Nat Clin Pract Gastroenterol Hepatol. 2005;2:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Kwon RS, Wong Kee Song LM, Adler DG, Conway JD, Diehl DL, Farraye FA, Kantsevoy SV, Kaul V, Kethu SR, Mamula P. Endocytoscopy. Gastrointest Endosc. 2009;70:610-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Zagaynova E, Gladkova N, Shakhova N, Gelikonov G, Gelikonov V. Endoscopic OCT with forward-looking probe: clinical studies in urology and gastroenterology. J Biophotonics. 2008;1:114-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Neumann H, Kiesslich R, Wallace MB, Neurath MF. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology. 2010;139:388-92, 392.e1-2. [PubMed] |
| 24. | Dunbar K, Canto M. Confocal endomicroscopy. Curr Opin Gastroenterol. 2008;24:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Kiesslich R, Goetz M, Vieth M, Galle PR, Neurath MF. Confocal laser endomicroscopy. Gastrointest Endosc Clin N Am. 2005;15:715-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 26. | Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr-Locke D, Löhr M. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 27. | van Sandick JW, van Lanschot JJ, Kuiken BW, Tytgat GN, Offerhaus GJ, Obertop H. Impact of endoscopic biopsy surveillance of Barrett's oesophagus on pathological stage and clinical outcome of Barrett's carcinoma. Gut. 1998;43:216-222. [PubMed] |
| 28. | Dellon ES, Shaheen NJ. Does screening for Barrett's esophagus and adenocarcinoma of the esophagus prolong survival? J Clin Oncol. 2005;23:4478-4482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Herszényi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249-258. [PubMed] |
| 30. | Poneros JM. Diagnosis of Barrett's esophagus using optical coherence tomography. Gastrointest Endosc Clin N Am. 2004;14:573-88, x. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Testoni PA, Mangiavillano B. Optical coherence tomography in detection of dysplasia and cancer of the gastrointestinal tract and bilio-pancreatic ductal system. World J Gastroenterol. 2008;14:6444-6452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Faruqi SA, Arantes V, Bhutani MS. Barrett's esophagus: current and future role of endosonography and optical coherence tomography. Dis Esophagus. 2004;17:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Pohl H, Koch M, Khalifa A, Papanikolaou IS, Scheiner K, Wiedenmann B, Rösch T. Evaluation of endocytoscopy in the surveillance of patients with Barrett's esophagus. Endoscopy. 2007;39:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Eberl T, Jechart G, Probst A, Golczyk M, Bittinger M, Scheubel R, Arnholdt H, Knuechel R, Messmann H. Can an endocytoscope system (ECS) predict histology in neoplastic lesions? Endoscopy. 2007;39:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Sasajima K, Kudo SE, Inoue H, Takeuchi T, Kashida H, Hidaka E, Kawachi H, Sakashita M, Tanaka J, Shiokawa A. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest Endosc. 2006;63:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR. In vivo histology of Barrett's esophagus and associated neoplasia by confocal laser endomicroscopy. Clin Gastroenterol Hepatol. 2006;4:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 322] [Article Influence: 16.1] [Reference Citation Analysis (1)] |
| 37. | Dunbar KB, Okolo P, Montgomery E, Canto MI. Confocal laser endomicroscopy in Barrett's esophagus and endoscopically inapparent Barrett's neoplasia: a prospective, randomized, double-blind, controlled, crossover trial. Gastrointest Endosc. 2009;70:645-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Hurlstone DP, Thomson M, Brown S, Tiffin N, Cross SS, Hunter MD. Confocal endomicroscopy in ulcerative colitis: differentiating dysplasia-associated lesional mass and adenoma-like mass. Clin Gastroenterol Hepatol. 2007;5:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Polglase AL, McLaren WJ, Skinner SA, Kiesslich R, Neurath MF, Delaney PM. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc. 2005;62:686-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 270] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 40. | Buchner AM, Shahid MW, Heckman MG, Krishna M, Ghabril M, Hasan M, Crook JE, Gomez V, Raimondo M, Woodward T. Comparison of probe-based confocal laser endomicroscopy with virtual chromoendoscopy for classification of colon polyps. Gastroenterology. 2010;138:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 41. | Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 563] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 42. | Venkatesh K, Abou-Taleb A, Cohen M, Evans C, Thomas S, Oliver P, Taylor C, Thomson M. Role of confocal endomicroscopy in the diagnosis of celiac disease. J Pediatr Gastroenterol Nutr. 2010;51:274-279. [PubMed] |
| 43. | Larson SM. Positron emission tomography-based molecular imaging in human cancer: exploring the link between hypoxia and accelerated glucose metabolism. Clin Cancer Res. 2004;10:2203-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Rajendran JG, Mankoff DA, O'Sullivan F, Peterson LM, Schwartz DL, Conrad EU, Spence AM, Muzi M, Farwell DG, Krohn KA. Hypoxia and glucose metabolism in malignant tumors: evaluation by [18F]fluoromisonidazole and [18F]fluorodeoxyglucose positron emission tomography imaging. Clin Cancer Res. 2004;10:2245-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 300] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 45. | Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 420] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 46. | Nitin N, Carlson AL, Muldoon T, El-Naggar AK, Gillenwater A, Richards-Kortum R. Molecular imaging of glucose uptake in oral neoplasia following topical application of fluorescently labeled deoxy-glucose. Int J Cancer. 2009;124:2634-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Thekkek N, Maru DM, Polydorides AD, Bhutani MS, Anandasabapathy S, Richards-Kortum R. Pre-Clinical Evaluation of Fluorescent Deoxyglucose as a Topical Contrast Agent for the Detection of Barrett's-Associated Neoplasia During Confocal Imaging. Technol Cancer Res Treat. 2011;10:431-441. [PubMed] |
| 48. | Hsiung PL, Hardy J, Friedland S, Soetikno R, Du CB, Wu AP, Sahbaie P, Crawford JM, Lowe AW, Contag CH. Detection of colonic dysplasia in vivo using a targeted heptapeptide and confocal microendoscopy. Nat Med. 2008;14:454-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 324] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 49. | Foersch S, Kiesslich R, Waldner MJ, Delaney P, Galle PR, Neurath MF, Goetz M. Molecular imaging of VEGF in gastrointestinal cancer in vivo using confocal laser endomicroscopy. Gut. 2010;59:1046-1055. [PubMed] |
| 50. | Dunbar K, Canto M. Confocal endomicroscopy. Techniques in Gastrointestinal Endoscopy. 2010;12:90-99. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Neumann H, Neurath MF, Mudter J. New endoscopic approaches in IBD. World J Gastroenterol. 2011;17:63-68. [PubMed] |