Published online Jul 16, 2010. doi: 10.4253/wjge.v2.i7.237
Revised: May 28, 2010
Accepted: June 4, 2010
Published online: July 16, 2010
Contrast enhanced endoscopic ultrasound (CEUS) is a new modality that takes advantage of vascular structure and blood flow to distinguish different clinical entities. Contrast agents are microbubbles that oscillate when exposed to ultrasonographic waves resulting in characteristic acoustic signals that are then converted to colour images. This permits exquisite imaging of macro- and microvasculature, providing information to help delineate malignant from non-malignant processes. The use of CEUS may significantly increase the sensitivity and specificity over conventional endoscopic ultrasound. Currently available contrast agents are safe, with infrequent adverse effects. This review summarizes the theory and technique behind CEUS and the current and future clinical applications.
- Citation: Mohamed RM, Yan BM. Contrast enhanced endoscopic ultrasound: More than just a fancy Doppler. World J Gastrointest Endosc 2010; 2(7): 237-243
- URL: https://www.wjgnet.com/1948-5190/full/v2/i7/237.htm
- DOI: https://dx.doi.org/10.4253/wjge.v2.i7.237
Endoscopic ultrasound (EUS) was a revolutionary development, which allowed gastroenterologists to see within the layers and beyond the gastrointestinal (GI) luminal tract. Since its beginnings in the early 1980s[1,2], developments have largely involved improvement in image quality, and the introduction of the curvilinear echoendoscope to allow for tissue sampling and “interventional” EUS[3-6]. EUS has established roles in the diagnosis of and therapy for a variety of gastrointestinal disorders, in particular cancer staging and pancreaticobiliary disorders.
The use of fine needle aspiration (FNA) provides a cytologic diagnosis which immensely improves the diagnostic accuracy over imaging alone[7-11]. This modality, however, comes with increased time for procedure, cost and risk[12-17]. New imaging modalities have been developed with the aim of improving imaging diagnostic capabilities, one of which is contrast enhanced ultrasound (CEUS). CEUS and endoscopic ultrasound (CE-EUS) use microbubble agents to enhance vascular patterns. In this article, we review the basic concepts of contrast enhancement and its clinical applications.
Major vasculature structures are easily identifiable on standard B-Mode imaging, and the addition of color and power Doppler helps to confirm vascular flow, along with direction and velocity of flow within the vessel. Capillary flow with low volume and very slow velocities cannot be seen with this standard imaging. The addition of “contrast” amplifies microvasculature flow to help define the vascular architecture, and hence to characterize the nature of a specific lesion.
Contrast enhancement involves the administration of an intravenous agent during the (endoscopic) ultrasound study. Contrast agents are microbubbles that respond to energy from sound waves in characteristic ways which aid in enhancing the distinctions between tissue types. First generation agents were agitated normal saline, radiologic contrast agents or the patient’s own blood that was injected into a peripheral vein. These substances were limited in their clinical utility for two main reasons[18,19]. First, the larger size of the microbubbles formed with saline or blood were too large to cross the pulmonary circulation vessels (capillaries approximately 7 μm) thereby making them ineffective in the assessment of abdominal organs. Second, the rapid diffusion of air from the microbubble into the plasma resulted in a very short lifespan, thus limiting the time for tissue examination. These unfavorable properties fueled the development of second generation agents, designed to overcome the limitations of their early counterparts. Currently, several different agents are used (Table 1), all of which employ similar principles. All contain a shell designed to trap the gas and resist degradation or dissolution, resulting in a longer and more stable half-life. Heavier gases such as perfluorocarbons, as opposed to air, reduce leakage out of the shell into the surrounding plasma. Finally, microbubble size is decreased (range 1-7 μm) allowing their passage through the pulmonary circulation to the abdominal organs.
| Contrast agent | Shell components | Gas | Mechanical index |
| Definity® | Phospholipid | Perfluoropropane | Low |
| Imagent® | Phospholipid | Perfluorohexane | Low |
| SonoVue® | Phospholipid | Sulfur hexafluoride | Low |
| Sonavist® | Polymer | Sulfur hexafluoride | Low |
| Sonazoid® | Lipid | Perfluorocarbon | Low |
| Optison® | Albumin | Perfluoropropane | Low |
| Sonogen® | Surfactant | Perfluoropentane | Low |
| Levovist® | Galactose/Palmitic acid | Air | High |
| Albunex® | Albumin | Air | High |
When microbubbles are exposed to ultrasound waves, they undergo compression and expansion that correlates with the peak and trough of the ultrasound wave[18,20]. This “oscillation” produces a strong acoustic signal that is recognized and represented as hyperechogenicity on the ultrasound image. This is in stark contrast to tissue, which is largely incompressible. The vibratory properties of microbubbles are dependent on its physical properties, including the type of gaseous agent used, and the surrounding shell. In addition to microbubble vibration, the significant impedimental difference between the bubble and surrounding tissue reflects the ultrasound wave back at this interface, thus permitting differentiation.
The oscillation of the microbubble is also directly dependent on the properties of the incident sound wave, of which the most important are the frequency and intensity of the incident wave. Microbubbles smaller than 7 μm oscillate most readily at 2-10 MHz, which serendipitously are the frequencies most often used in EUS. The mechanical index (MI) is a measure of the pressure fluctuations within an ultrasound pulse, and can be thought of as the power of the pulse. It is mathematically derived by dividing the maximum negative sound pressure by the square root of the sound frequency. The effect on microbubbles varies with the mechanical index (Table 2). With very low mechanical indices (< 0.1), microbubbles oscillate symmetrically resulting in a linear relationship between the signal and the emitted sound waves. At low mechanical indices (0.1-0.6), microbubbles resist compression more than expansion, thereby oscillating asymmetrically. This creates a non-linear relationship with the emitted sound waves and the detected signal is shown as multiples of the fundamental vibratory frequency. Similar to overtones on musical instruments, this is known as harmonics. Manipulating these harmonics allows for the differentiation of perfused from non-perfused tissue. With high MI (> 0.6), microbubbles are unable to resist compression and are destroyed. The release of the gas from the bubbles at this high MI results in a transiently intense echo signal.
| Mechanical index | Effect on microbubble oscillation | Relationship between emitted sound waves and detected signal |
| Low (< 0.1) | Symmetrical | Linear |
| Moderate (0.1-0.6) | Asymmetrical | Non-linear |
| High (> 0.6) | Destruction | N/A |
Distinguishing the harmonics created by the microbubbles from those of the surrounding tissue can prove challenging. One method uses the instability of microbubbles at higher mechanical indices. Using color Doppler ultrasound, the disappearance of a previous signal at a high MI can be visualized and has been used to detect abnormalities such as metastatic liver lesions[21]. Specialized Doppler software known as Stimulated Acoustic Emission increases the resolution of lesions within the liver by demonstrating a color defect in areas of microbubble uptake against a highlighted normal liver and spleen. It is mainly used with more fragile contrast agents such as Levovist and is limited by its inability to perform real time scanning due to rapid destruction of the agent[18]. The stability of newer generation microbubbles permits the formation of harmonics at lower frequencies. This helps distinguish the microbubbles from the surrounding tissue without destroying the microbubble at high MIs[22]. Filters are usually required to remove background signals at the expense of reduced spatial resolution.
Phase inversion mode (PIM) was developed in an attempt to maintain spatial resolution while detecting the harmonics of the microbubbles at low mechanical indices. In this modality, two impulses are sent, one being phase-inverted, and the returning emitted signals are summed. Linear signals (i.e. from surrounding tissue) are eliminated as signals received from the two impulses are 180° out of phase with a summation signal of 0, leaving only the non-linear signal of the microbubble to form the image[23,24]. Summing of multiple PIM signals is often required to account for increased noise at lower mechanical indices[25]. Phase inversion can be combined with traditional B-mode ultrasound such that the microbubble signal is displayed over a background B-mode image. Phase inversion mode with conventional Doppler (so called “Vascular Recognition Imaging”) allows for visualization of flow through larger vessels simultaneously with slow-moving microbubbles in smaller vessels[26].
While the second generation agents are generally safe, their administration does involve important potential risks and complications. The use of synthetic molecular components in the shells of these contrast agents poses a potential allergic or anaphylactic risk. In vitro studies have demonstrated a phenomenon termed “cavitation”, whereby adjacent tissue is damaged with very high contrast agent concentrations and high sound energies[27]. Initially, during the low pressure phase of the ultrasound wave, fluid in the blood is pulled away from the microbubbles, creating a free air bubble. This bubble then collapses (“cavitates”) in the high pressure phase of the wave releasing a large amount of energy resulting in increased the local temperature, release of free radicals, and lysis of neighboring cells. Importantly, this effect has not been demonstrated with the conventional concentrations and sound energies used. Finally, caution should be exercised in patients with ischemic heart disease with specific contrast agents (SonoVue®, Definity®, Optison®), as there have been reported cases of cardiac deaths during contrast echocardiogram studies[19].
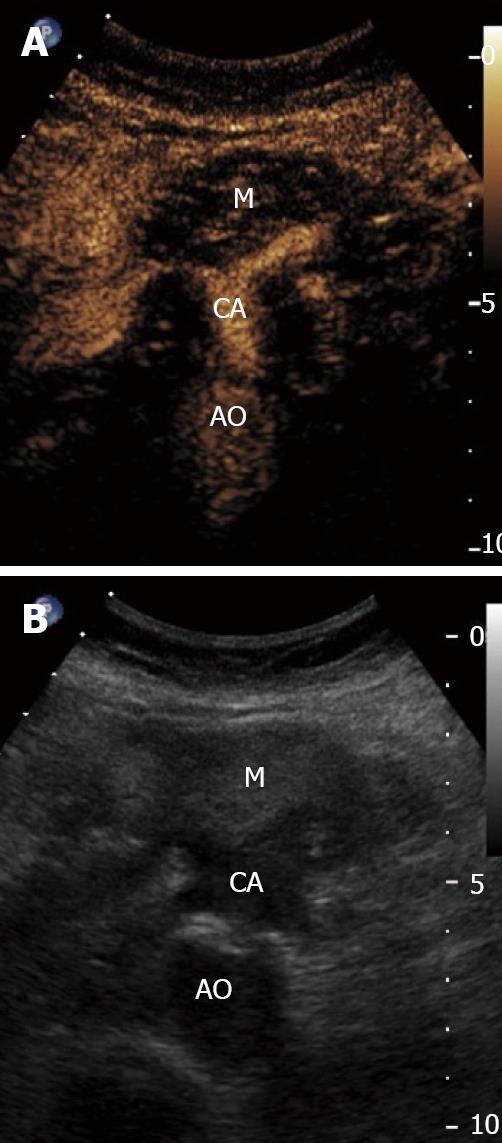
The differentiation of chronic pancreatitis and pancreatic cancer is difficult when using traditional diagnostic tools. As chronic pancreatitis is an established risk factor for pancreatic adenocarcinoma, the differentiation between the two is of added importance in order to avoid unnecessary intervention and to instigate appropriate therapy. Transabdominal ultrasound has been a traditional diagnostic tool but is limited in its ability to differentiate these entities. Transabdominal contrast enhanced ultrasound offers significant advantages in discerning the etiology of pancreatic lesions (Figure 1). In the absence of chronic pancreatitis, conventional endoscopic ultrasound has a diagnostic accuracy of 85%-100% for pancreatic neoplasms[28]. In the presence of chronic pancreatitis the accuracy of EUS is markedly reduced, even in conjunction with FNA[29-31].
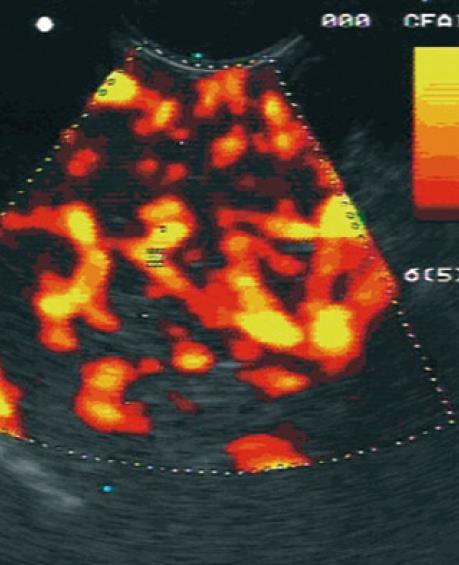
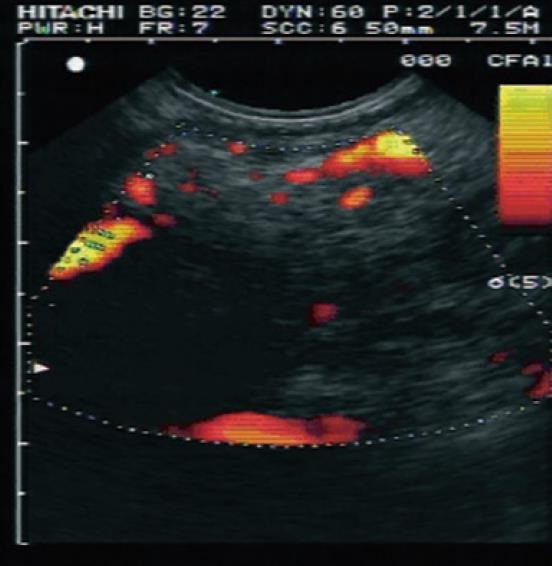
The use of a contrast agent is able to enhance the different vascular patterns of pancreatic neoplasms and chronic pancreatitis (Figures 2 and 3), in particular, more reliable discrimination of arterial and venous blood flow[32,33]. In differentiating focal pancreatitis from pancreatic cancer, contrast enhanced EUS has a sensitivity of 91% and a specificity of 93%, with positive and negative predictive values of 100% and 88% respectively[31,32]. These values are significant improvements over standard EUS imaging in this population. In general, pancreatic cancer is hypovascular on contrast color Doppler imaging whereas focal pancreatitis appears hypervascular. Hocke et al[34] performed a study using SonoVue to differentiate the vascular patterns of focal pancreatitis and pancreatic adenocarcinoma. Malignant lesions demonstrated absence of venous vessels and an irregular appearance to the arterial vessel architecture within the tumor. Vascularization of these malignant foci was visible only after the injection of a contrast agent. Conversely, chronic pancreatitis demonstrated both venous and arterial vessels with regular arterial microvascular architecture. This vascularity was visible on conventional Doppler assessment, prior to administration of a contrast agent. Using these criteria, the addition of contrast enhancement to conventional Doppler EUS improved the sensitivity from 73.2% to 91.1% and the specificity from 83.3% to 93.3%. CEUS offers improved accuracy over conventional imaging methods for the diagnosis of pancreatic neoplasms (Table 3). Levovist has also been used as a contrast agent for the differentiation of pancreatic cancer and chronic pancreatitis. In comparison to power Doppler EUS, contrast enhancement with Levovist has been shown to improve from 11% to 83.3% sensitivity for the detection of lesions smaller than 2 cm[35].
Detection of neuroendocrine tumors (NETs) of the pancreas may also be improved with contrast enhancement. NETs usually present as a small singular well demarcated lesion with echogenicity ranging from hypo to iso to hyperechoic[36-40]. Detection on standard EUS is at times problematic, in particular if they are isoechoic. Classically, however, these tumors are hypervascular; therefore the use of contrast enhancement would significantly improve EUS diagnostic capabilities[41]. In a small study of 37 patients with pancreatic lesions, Hirooka et al[42] showed contrast enhancement in 100% (n = 4) of islet cell tumors compared to 0% (n = 11) in adenocarcinoma lesions. The ability to distinguish an NET by imaging without the need for FNA is ideal for two reasons. First enucleation procedures for cure may be hampered if FNA is performed. Second, adequate tissue acquisition may be difficult in these small lesions. The decision for or against FNA depends on the individual patient, as there may be prognostic implications to cytology results[43].
The differentiation of malignant from benign cystic lesions of the pancreas is also at times problematic. Traditionally, FNA with fluid analysis for CEA was the single best test for the diagnosis of a mucinous neoplasm[44]. More recently, the addition of DNA analysis further increases the ability to determine a mucinous and/or malignant cyst[45]. Contrast enhancement may help in the diagnosis of mucinous cystic neoplasms, and in particular, help determine if a solid component/mural nodule appears suspicious for adenocarcinoma. In the study by Hirooka et al, 80% of intraductal papillary mucinous tumors displayed enhancement. Unfortunately the authors of this study did not offer pathologic correlation of these cystic lesions nor distinguish the proportion of these tumors with a solid component[42].
While metastases to the pancreas are rare, they remain an important entity in the differential diagnosis of a pancreatic nodule or mass. CE-EUS can play an important complementary role to tissue sampling through FNA. In a small study, 4 of 5 metastatic lesions in the pancreas displayed a hypervascular echogenic signal[46]. Whether metastatic lesions from different primary sites provide different CE-EUS signals remains to be seen.
Fine needle aspiration (FNA) remains a mainstay technique for obtaining tissue for the diagnosis of pancreatic lesions. It is doubtful that contrast enhanced imaging will replace tissue acquisition, and for cancer management in particular. In certain situations, however, contrast enhancement may help decide if FNA is warranted, in particular if surgical treatment and outcomes would be affected. Furthermore, while the positive predictive value of FNA approaches 100%[47], the negative predictive value only reaches 30%-44%[47,48]. This often necessitates a second EUS procedure for repeat FNA or a percutaneous biopsy[9,30,49]. As suggested by Giovanni, the high sensitivity and specificity of CE-EUS may reduce the need for repeat procedures if the initial FNA is negative[50].
EUS plays a pivotal role in the nodal staging of GI and mediastinal malignancies. Major differences in management are dependent on the accurate determination of malignant lymph nodes. In esophageal cancer, EUS has proven to be more accurate than CT scanning in detecting the presence of abnormal lymphadenopathy[51,52] with an overall accuracy for lymph node staging of approximately 75%. Standard EUS criteria for malignancy include size > 1 cm, hypoechoic, round shape, and sharp demarcation[53]. With FNA, the accuracy has been reported to be up to 99.4%[7]. The improved accuracy is important, as the presence of local metastatic lymphadenopathy remains one the most important predictors of survival, and is a determinant for adjuvant therapy and/or resectability. Hocke et al[54] compared contrast enhanced endoscopic ultrasound features of lymph nodes to fine needle aspiration. CE-EUS criteria for malignant lymph nodes were irregular appearance of vessels, and the sole presence of arterial vessels, whereas regular vessel appearance and presence of both arterial and venous vessels were used to identify benign lymph nodes. CE-EUS had a specificity of 91% but a sensitivity of only 60% in the differentiation of benign and malignant mediastinal lymph nodes. While CE-EUS improved the specificity in comparison to traditional EUS, low sensitivity prevents its ability to be used as the sole tool to discern malignant lymph nodes (Table 3). It may, however, be helpful for examining small nodes, or when FNA cannot be done due to intervening vasculature or tumor presence within the needle path.
Experience with CE-EUS for examination of biliary tract disorders is very limited. One study showed possible application to differentiating benign sclerosing cholangitis from cholangiocarcinoma[55]. In one small study of 14 patients, CE-EUS improved gall bladder tumor staging (T stage) accuracy from 78.6% to 92.9%[56]. Whether this will change patient management or translate into improved patient outcomes is unknown.
CE-EUS has been explored as a method of assessing treatment response in pancreatic lesions. Giday and colleagues, using a porcine model, demonstrated a marked difference in enhancement in ablated areas of the pancreas (no enhancement) compared to surrounding tissue (increased enhancement)[57]. In a novel experiment, Korpanty et al[58] created targeted microbubbles to vascular endothelial growth factor activated blood vessels that are seen in pancreatic neoplasms. The enhancement by these targeted microbubbles was significantly reduced with the use of anti-angiogenesis therapy, thereby providing a method of monitoring response to these agents.
The ability to target microbubbles allows the focused delivery of therapeutic substances within the bubbles to a specific site, which can then be released by a targeted ultrasound wave. Chemotherapeutic drugs within microbubbles can be released in a specific concentrated area by destroying the bubbles using high mechanical indices under real time ultrasound guidance[59]. Delivery in this fashion would provide more uniform delivery to specific, actively perfused areas of the tumor, compared to fine needle injection. Animal in vivo studies are still lacking. This technique is likely to gain popularity in the near future, given its specificity for the target tissue.
Contrast enhanced ultrasound is a newer technique that is gaining favor in the diagnosis and delivery of therapy in a variety of gastrointestinal disorders. Its ability to accurately differentiate diseased tissue from surrounding normal tissue will facilitate more accurate identification of lesions that were traditionally difficult to characterize. Multiple technological advances, including second generation microbubbles and phase inversion mode allow for improved spatial resolution, thereby increasing the accuracy of this modality in smaller and smaller lesions. Future directions for contrast enhanced endoscopic ultrasound will include complementary use with endoscopic elastography, which is another rapidly expanding field in endoscopic imaging.
| 1. | DiMagno EP, Buxton JL, Regan PT, Hattery RR, Wilson DA, Suarez JR, Green PS. Ultrasonic endoscope. Lancet. 1980;1:629-631. |
| 2. | Strohm WD, Phillip J, Hagenmüller F, Classen M. Ultrasonic tomography by means of an ultrasonic fiberendoscope. Endoscopy. 1980;12:241-244. |
| 3. | Matsumoto K, Yamao K, Okubo K, Hara K, Sawaki A, Mizuno N, Tajika M, Kawai H, Ashida R. Endoscopic ultrasound-guided ethanol injection in the pancreas in a porcine model: a preliminary study. J Gastroenterol Hepatol. 2008;23:e1-e6. |
| 4. | Yan BM, Van Dam J. Endoscopic ultrasound-guided intratumoural therapy for pancreatic cancer. Can J Gastroenterol. 2008;22:405-410. |
| 5. | Yamao K, Sawaki A, Mizuno N, Shimizu Y, Yatabe Y, Koshikawa T. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): past, present, and future. J Gastroenterol. 2005;40:1013-1023. |
| 6. | Klapman JB, Chang KJ. Endoscopic ultrasound-guided fine-needle injection. Gastrointest Endosc Clin N Am. 2005;15:169-177, x. |
| 7. | Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine needle aspiration is superior to lymph node echofeatures: a prospective evaluation of mediastinal and peri-intestinal lymphadenopathy. Am J Gastroenterol. 2004;99:628-633. |
| 8. | Chang KJ, Nguyen P, Erickson RA, Durbin TE, Katz KD. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387-393. |
| 9. | Savides TJ, Donohue M, Hunt G, Al-Haddad M, Aslanian H, Ben-Menachem T, Chen VK, Coyle W, Deutsch J, DeWitt J. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: a benchmark for quality performance measurement. Gastrointest Endosc. 2007;66:277-282. |
| 10. | Turner BG, Cizginer S, Agarwal D, Yang J, Pitman MB, Brugge WR. Diagnosis of pancreatic neoplasia with EUS and FNA: a report of accuracy. Gastrointest Endosc. 2010;71:91-98. |
| 11. | Gleeson FC, Clain JE, Papachristou GI, Rajan E, Topazian MD, Wang KK, Levy MJ. Prospective assessment of EUS criteria for lymphadenopathy associated with rectal cancer. Gastrointest Endosc. 2009;69:896-903. |
| 12. | Eloubeidi MA, Tamhane A, Varadarajulu S, Wilcox CM. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: a prospective evaluation. Gastrointest Endosc. 2006;63:622-629. |
| 13. | O’Toole D, Palazzo L, Arotçarena R, Dancour A, Aubert A, Hammel P, Amaris J, Ruszniewski P. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470-474. |
| 14. | Mahnke D, Chen YK, Antillon MR, Brown WR, Mattison R, Shah RJ. A prospective study of complications of endoscopic retrograde cholangiopancreatography and endoscopic ultrasound in an ambulatory endoscopy center. Clin Gastroenterol Hepatol. 2006;4:924-930. |
| 15. | Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690-695. |
| 16. | Chen VK, Arguedas MR, Kilgore ML, Eloubeidi MA. A cost-minimization analysis of alternative strategies in diagnosing pancreatic cancer. Am J Gastroenterol. 2004;99:2223-2234. |
| 17. | Chang KJ, Soetikno RM, Bastas D, Tu C, Nguyen PT. Impact of endoscopic ultrasound combined with fine-needle aspiration biopsy in the management of esophageal cancer. Endoscopy. 2003;35:962-966. |
| 18. | Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol. 2006;60:324-330. |
| 19. | Raisinghani A, DeMaria AN. Physical principles of microbubble ultrasound contrast agents. Am J Cardiol. 2002;90:3J-7J. |
| 20. | Phillips P, Gardner E. Contrast-agent detection and quantification. Eur Radiol. 2004;14 Suppl 8:P4-P10. |
| 21. | Albrecht T, Urbank A, Mahler M, Bauer A, Doré CJ, Blomley MJ, Cosgrove DO, Schlief R. Prolongation and optimization of Doppler enhancement with a microbubble US contrast agent by using continuous infusion: preliminary experience. Radiology. 1998;207:339-347. |
| 22. | Burns PN. Harmonic imaging with ultrasound contrast agents. Clin Radiol. 1996;51 Suppl 1:50-55. |
| 23. | Burns PN, Wilson SR, Simpson DH. Pulse inversion imaging of liver blood flow: improved method for characterizing focal masses with microbubble contrast. Invest Radiol. 2000;35:58-71. |
| 24. | Burns PN, Hope Simpson D, Averkiou MA. Nonlinear imaging. Ultrasound Med Biol. 2000;26 Suppl 1:S19-S22. |
| 25. | Goertz DE, Wong SWS, Chin CT, Cherin E, Burns PN, Foster FS. Non-linear scattering from microbubble contrast agents in the 14-40 MHz range. Ultrasonics Symposium. 2001;2:1747-1750. |
| 26. | Mine Y. [Harmonic imaging]. Nippon Rinsho. 1998;56:881-885. |
| 27. | ter Haar GR. Ultrasonic contrast agents: safety considerations reviewed. Eur J Radiol. 2002;41:217-221. |
| 28. | Iglesias GJ, Lariño NJ, Domínguez MJE. Endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. Rev Esp Enferm Dig. 2009;101:631-638. |
| 29. | Maluf-Filho F, Dotti CM, Halwan B, Queiros AF, Kupski C, Chaves DM, Nakao FS, Kumar A. An evidence-based consensus statement on the role and application of endosonography in clinical practice. Endoscopy. 2009;41:979-987. |
| 30. | Bhutani MS. Endoscopic ultrasonography--new developments and interesting trends. Endoscopy. 2004;36:950-956. |
| 31. | Becker D, Strobel D, Bernatik T, Hahn EG. Echo-enhanced color- and power-Doppler EUS for the discrimination between focal pancreatitis and pancreatic carcinoma. Gastrointest Endosc. 2001;53:784-789. |
| 32. | Hocke M, Menges M, Topalidis T, Dietrich CF, Stallmach A. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J Cancer Res Clin Oncol. 2008;134:473-480. |
| 33. | Schmidt J, Ryschich E, Daniel V, Herzog L, Werner J, Herfarth C, Longnecker DS, Gebhard MM, Klar E. Vascular structure and microcirculation of experimental pancreatic carcinoma in rats. Eur J Surg. 2000;166:328-335. |
| 34. | Hocke M, Schulze E, Gottschalk P, Topalidis T, Dietrich CF. Contrast-enhanced endoscopic ultrasound in discrimination between focal pancreatitis and pancreatic cancer. World J Gastroenterol. 2006;12:246-250. |
| 35. | Sakamoto H, Kitano M, Suetomi Y, Maekawa K, Takeyama Y, Kudo M. Utility of contrast-enhanced endoscopic ultrasonography for diagnosis of small pancreatic carcinomas. Ultrasound Med Biol. 2008;34:525-532. |
| 36. | Patel KK, Kim MK. Neuroendocrine tumors of the pancreas: endoscopic diagnosis. Curr Opin Gastroenterol. 2008;24:638-642. |
| 37. | Zimmer T, Scherübl H, Faiss S, Stölzel U, Riecken EO, Wiedenmann B. Endoscopic ultrasonography of neuroendocrine tumours. Digestion. 2000;62 Suppl 1:45-50. |
| 38. | Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, Scheiman JM. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271-2277. |
| 39. | Ardengh JC, Rosenbaum P, Ganc AJ, Goldenberg A, Lobo EJ, Malheiros CA, Rahal F, Ferrari AP. Role of EUS in the preoperative localization of insulinomas compared with spiral CT. Gastrointest Endosc. 2000;51:552-555. |
| 40. | Schumacher B, Lübke HJ, Frieling T, Strohmeyer G, Starke AA. Prospective study on the detection of insulinomas by endoscopic ultrasonography. Endoscopy. 1996;28:273-276. |
| 41. | Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590-597.e1. |
| 42. | Hirooka Y, Goto H, Ito A, Hayakawa S, Watanabe Y, Ishiguro Y, Kojima S, Hayakawa T, Naitoh Y. Contrast-enhanced endoscopic ultrasonography in pancreatic diseases: a preliminary study. Am J Gastroenterol. 1998;93:632-635. |
| 43. | Fasanella KE, McGrath KM, Sanders M, Brody D, Domsic R, Khalid A. Pancreatic endocrine tumor EUS-guided FNA DNA microsatellite loss and mortality. Gastrointest Endosc. 2009;69:1074-1080. |
| 44. | Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-1336. |
| 45. | Khalid A, Zahid M, Finkelstein SD, LeBlanc JK, Kaushik N, Ahmad N, Brugge WR, Edmundowicz SA, Hawes RH, McGrath KM. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest Endosc. 2009;69:1095-1102. |
| 46. | Giovannini M. Endosonography: new developments in 2006. Scientific World Journal. 2007;7:341-363. |
| 47. | Raut CP, Grau AM, Staerkel GA, Kaw M, Tamm EP, Wolff RA, Vauthey JN, Lee JE, Pisters PW, Evans DB. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118-126; discussion 127-128. |
| 48. | Giovannini M, Seitz JF, Monges G, Perrier H, Rabbia I. Fine-needle aspiration cytology guided by endoscopic ultrasonography: results in 141 patients. Endoscopy. 1995;27:171-177. |
| 49. | LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475-481. |
| 50. | Giovannini M. Contrast-enhanced endoscopic ultrasound and elastosonoendoscopy. Best Pract Res Clin Gastroenterol. 2009;23:767-779. |
| 51. | Lightdale CJ, Kulkarni KG. Role of endoscopic ultrasonography in the staging and follow-up of esophageal cancer. J Clin Oncol. 2005;23:4483-4489. |
| 52. | Takizawa K, Matsuda T, Kozu T, Eguchi T, Kato H, Nakanishi Y, Hijikata A, Saito D. Lymph node staging in esophageal squamous cell carcinoma: a comparative study of endoscopic ultrasonography versus computed tomography. J Gastroenterol Hepatol. 2009;24:1687-1691. |
| 53. | Catalano MF, Sivak MV Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442-446. |
| 54. | Hocke M, Menges M, Topalidis T, Dietrich CF, Stallmach A. Contrast-enhanced endoscopic ultrasound in discrimination between benign and malignant mediastinal and abdominal lymph nodes. J Cancer Res Clin Oncol. 2008;134:473-480. |
| 55. | Hyodo T, Hyodo N, Yamanaka T, Imawari M. Contrast-enhanced intraductal ultrasonography for thickened bile duct wall. J Gastroenterol. 2001;36:557-559. |
| 56. | Hirooka Y, Naitoh Y, Goto H, Ito A, Hayakawa S, Watanabe Y, Ishiguro Y, Kojima S, Hashimoto S, Hayakawa T. Contrast-enhanced endoscopic ultrasonography in gallbladder diseases. Gastrointest Endosc. 1998;48:406-410. |
| 57. | Giday SA, Magno P, Gabrielson KL, Buscaglia JM, Canto MI, Ko CW, Clarke JO, Kalloo AN, Jagannath SB, Shin EJ. The utility of contrast-enhanced endoscopic ultrasound in monitoring ethanol-induced pancreatic tissue ablation: a pilot study in a porcine model. Endoscopy. 2007;39:525-529. |
| 58. | Korpanty G, Carbon JG, Grayburn PA, Fleming JB, Brekken RA. Monitoring response to anticancer therapy by targeting microbubbles to tumor vasculature. Clin Cancer Res. 2007;13:323-330. |
| 59. | Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1153-1166. |
| 60. | Giovannini M. Contrast-enhanced endoscopic ultrasound and elastosonoendoscopy. Best Pract Res Clin Gastroenterol. 2009;23:767-779. |
| 61. | Kinney T. Evidence-based imaging of pancreatic malignancies. Surg Clin North Am. 2010;90:235-249. |
| 62. | Hunt GC, Faigel DO. Assessment of EUS for diagnosing, staging, and determining resectability of pancreatic cancer: a review. Gastrointest Endosc. 2002;55:232-237. |
| 63. | Mertz HR, Sechopoulos P, Delbeke D, Leach SD. EUS, PET, and CT scanning for evaluation of pancreatic adenocarcinoma. Gastrointest Endosc. 2000;52:367-371. |
| 64. | Delbeke D, Pinson CW. Pancreatic tumors: role of imaging in the diagnosis, staging, and treatment. J Hepatobiliary Pancreat Surg. 2004;11:4-10. |
| 65. | Papanikolaou IS, Adler A, Neumann U, Neuhaus P, Rösch T. Endoscopic ultrasound in pancreatic disease--its influence on surgical decision-making. An update 2008. Pancreatology. 2009;9:55-65. |
| 66. | Săftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1-17. |
| 67. | Wiersema MJ, Vilmann P, Giovannini M, Chang KJ, Wiersema LM. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087-1095. |
| 68. | Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663-2668. |
| 69. | Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, Abdulkader I, Larino-Noia J, Antunez J, Forteza J. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol. 2007;13:289-293. |
| 70. | Ardengh JC, Lopes CV, de Lima LF, de Oliveira JR, Venco F, Santo GC, Modena JL. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112-3116. |
| 71. | Bronstein YL, Loyer EM, Kaur H, Choi H, David C, DuBrow RA, Broemeling LD, Cleary KR, Charnsangavej C. Detection of small pancreatic tumors with multiphasic helical CT. AJR. 2004;182:619-623. |
| 72. | Legmann P, Vignaux O, Dousset B, Baraza AJ, Palazzo L, Dumontier I, Coste J, Louvel A, Roseau G, Couturier D. Pancreatic tumors: comparison of dual-phase helical CT and endoscopic sonography. AJR. 1998;170:1315-1322. |
| 73. | Bipat S, Phoa SS, van Delden OM, Bossuyt PM, Gouma DJ, Laméris JS, Stoker J. Ultrasonography, computed tomography and magnetic resonance imaging for diagnosis and determining resectability of pancreatic adenocarcinoma: a meta-analysis. J Comput Assist Tomogr. 2005;29:438-445. |
| 74. | Johnson PT, Outwater EK. Pancreatic carcinoma versus chronic pancreatitis: dynamic MR imaging. Radiology. 1999;212:213-218. |
| 75. | Rieber A, Tomczak R, Nüssle K, Klaus H, Brambs HJ. MRI with mangafodipir trisodium in the detection of pancreatic tumours: comparison with helical CT. Br J Radiol. 2000;73:1165-1169. |
| 76. | Kanamori A, Hirooka Y, Itoh A, Hashimoto S, Kawashima H, Hara K, Uchida H, Goto J, Ohmiya N, Niwa Y. Usefulness of contrast-enhanced endoscopic ultrasonography in the differentiation between malignant and benign lymphadenopathy. Am J Gastroenterol. 2006;101:45-51. |
| 77. | Giovannini M, Thomas B, Erwan B, Christian P, Fabrice C, Benjamin E, Geneviève M, Paolo A, Pierre D, Robert Y. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: a multicenter study. World J Gastroenterol. 2009;15:1587-1593. |
| 78. | Romagnuolo J, Scott J, Hawes RH, Hoffman BJ, Reed CE, Aithal GP, Breslin NP, Chen RY, Gumustop B, Hennessey W. Helical CT versus EUS with fine needle aspiration for celiac nodal assessment in patients with esophageal cancer. Gastrointest Endosc. 2002;55:648-654. |
| 79. | Zhang X, Watson DI, Lally C, Bessell JR. Endoscopic ultrasound for preoperative staging of esophageal carcinoma. Surg Endosc. 2005;19:1618-1621. |
| 80. | Kelly S, Harris KM, Berry E, Hutton J, Roderick P, Cullingworth J, Gathercole L, Smith MA. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut. 2001;49:534-539. |
| 81. | Botet JF, Lightdale CJ, Zauber AG, Gerdes H, Urmacher C, Brennan MF. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology. 1991;181:419-425. |
| 82. | Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474-479. |
| 83. | Catalano MF, Alcocer E, Chak A, Nguyen CC, Raijman I, Geenen JE, Lahoti S, Sivak MV Jr. Evaluation of metastatic celiac axis lymph nodes in patients with esophageal carcinoma: accuracy of EUS. Gastrointest Endosc. 1999;50:352-356. |
Peer reviewer: Yutaka Saito, MD, PhD, Head, Division of Endoscopy, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo 104-0045, Japan