Published online Sep 16, 2025. doi: 10.4253/wjge.v17.i9.111734
Revised: August 2, 2025
Accepted: August 20, 2025
Published online: September 16, 2025
Processing time: 66 Days and 11.3 Hours
Conventional endoscopic submucosal dissection (c-ESD) is a widely used technique for rectal neuroendocrine tumors (NETs), but it poses certain cha
To compare the efficacy and safety of p-ESD and c-ESD for rectal NETs.
This retrospective study included consecutive patients with rectal NETs mea
In total, 103 patients were enrolled (49 in the p-ESD group and 54 in the c-ESD group). The p-ESD group exhibited a significantly shorter median dissection time (9.3 minutes vs 14.9 minutes; P < 0.001) and a higher R0 resection rate (100% vs 88.9%; P = 0.028), while en bloc resection rates were comparable. Rates of minor intraoperative bleeding (10.2% vs 25.9%; P = 0.040) and major intraoperative bleeding (4.1% vs 18.5%; P = 0.030) were lower in the p-ESD group. No muscularis propria injuries occurred in the p-ESD group vs 16.7% in the c-ESD group (P = 0.003). Other adverse events did not differ significantly.
p-ESD is safe and effective for treating rectal NETs. Compared with c-ESD, it is technically easier, requires less dissection time, achieves higher R0 resection rates, reduces intraoperative bleeding, and lowers the risk of muscularis propria injury.
Core Tip: This study compares pretraction-assisted endoscopic submucosal dissection (p-ESD) with conventional endoscopic submucosal dissection for rectal neuroendocrine tumors ≤ 15 mm. The results show that p-ESD significantly shortens dissection time, improves R0 resection rates, and reduces intraoperative bleeding and muscularis propria injury. These findings suggest that p-ESD is a safe, efficient, and technically advantageous technique for treating rectal neuroendocrine tumors.
- Citation: Guo XX, Zhang SH, Chen AJ, Chen YL, Chen FL. Efficacy and safety of pretraction-assisted endoscopic submucosal dissection for treating rectal neuroendocrine tumors. World J Gastrointest Endosc 2025; 17(9): 111734
- URL: https://www.wjgnet.com/1948-5190/full/v17/i9/111734.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i9.111734
Rectal neuroendocrine tumors (NETs) are common in the gastrointestinal tract and make up 12%-27% of all digestive system NETs[1-3]. However, they usually do not cause clear symptoms, making early detection difficult. Recently, as more people undergo endoscopy, the detection rate of these tumors has increased, and they are often small. Approximately 80% of rectal NETs measure 5-10 mm in size, whereas only about 2.3% are larger than 20 mm, and most occur in the lower part of the rectum[4]. Currently, endoscopic resection is considered an appropriate treatment for rectal NETs that are smaller than 15 mm and have good differentiation, no distant metastasis, and no invasion into the muscular layer[5].
Endoscopic submucosal dissection (ESD) provides a higher en bloc resection rate and a lower risk of residual tumor than endoscopic mucosal resection (EMR) and its modified forms[6]. Nonetheless, the key to successful ESD is adequate exposure of the submucosal plane, ensuring good dissection visualization. Inadequate visualization during the procedure can prolong the dissection time, increase the risk of adverse events, and potentially affect the R0 resection rate[7]. To address this challenge, we previously developed the technique of pretraction-assisted ESD (p-ESD) for treating rectal NETs, which makes the procedure technically easier to perform[8]. However, this technique has only been presented in a technical case report and has not yet been compared with conventional ESD (c-ESD). Therefore, this retrospective cohort study aimed to evaluate the therapeutic efficacy and safety of p-ESD compared with c-ESD for rectal NETs.
This retrospective study included patients with rectal NETs who underwent ESD at Fujian Medical University Union Hospital, China. All ESD procedures were performed by an expert endoscopist. The exclusion criteria were: Patients showing deep tumor infiltration into the muscularis propria on endoscopic ultrasound, and evidence of lymph node or distant metastasis on preprocedural magnetic resonance imaging or computed tomography (CT) scans. Additionally, we excluded patients with rectal NETs initially misdiagnosed as polyps who underwent endoscopic polypectomy, had positive postoperative margins, and subsequently received salvage ESD. Patients who underwent ESD with dental floss pretraction assistance were categorized into the p-ESD group, while those treated with ESD without traction assistance were assigned to the c-ESD group. The choice between p-ESD and c-ESD was not determined by the time of treatment but was instead made at the discretion of the endoscopist, based on the characteristics and technical demands of each case. This study was approved by the Ethics Committee of Fujian Medical University Union Hospital (2025KY287).
Patient and tumor characteristics, including age, sex, tumor location (upper or lower rectum), tumor size, pathological grade, vascular and lymphatic invasion, and requirement for additional surgery, were recorded. The primary outcomes were dissection time, en bloc resection rate, and R0 resection rate. Dissection time was measured from tumor marking to complete excision, including the time for traction using dental floss and endoclips; R0 resection was defined as the absence of tumor involvement at lateral or basal margins. The secondary outcomes were the rates of adverse events, including bleeding, muscularis propria injury, and perforation. Minor intraoperative bleeding was defined as bleeding controlled solely with a dual knife; major intraoperative bleeding was defined as hemostasis that could not be effectively controlled using the dual knife alone, typically due to arterial bleeding and poor visualization, and necessitating switching to thermal hemostatic forceps for successful hemostasis[9]. Delayed bleeding was defined as a hemoglobin decline ≥ 2 g/dL, or clinically evident hematochezia requiring blood transfusion or endoscopic hemostasis, occurring within 30 days postESD[10]. Muscularis propria injury was characterized by visualization of the muscular layer with partial tearing, in the absence of perforation. Intraoperative perforation was defined as an observed full-thickness defect of the muscularis propria during the procedure. Delayed perforation was defined as the onset of postoperative abdominal pain accompanied by CT findings such as localized exudate, retroperitoneal gas accumulation, or free intraperitoneal gas.
All endoscopic procedures employed a single-channel endoscope (Pentax i-7000 with EC38-i10F; Pentax Medical, Tokyo, Japan) equipped with an endoscopic CO2 regulation unit and water pump (Hangzhou AGS MedTech, Hangzhou, China), a high-frequency generator (ERBE VIO 200S; ERBE, Tübingen, Germany), and essential accessory instruments: A dual knife (KD-650Q; Olympus, Tokyo, Japan), thermal hemostatic forceps (HDBF-2.4-230-S; Cook Medical, Bloomington, IN, United States), a submucosal injection needle (M00518300; Boston Scientific, Marlborough, MA, United States), and endoclips (Hangzhou AGS MedTech). The submucosal injection solution consisted of a mixture of glycerol fructose (10% glycerol and 5% fructose) and 0.1% methylene blue.
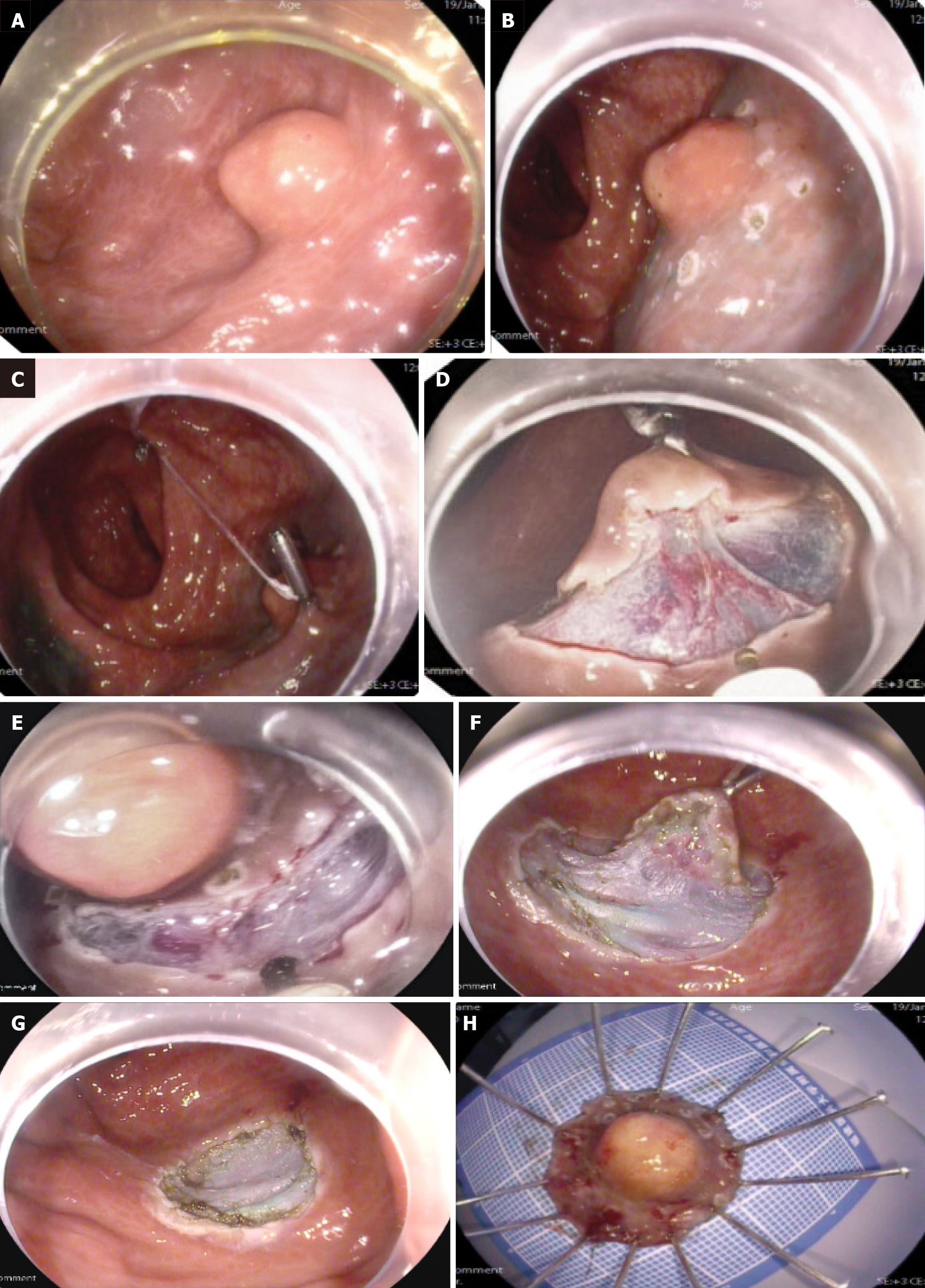
In the c-ESD group, mucosal marking at a distance of 3 mm-5 mm beyond the tumor margins was initially performed using the dual knife. Subsequent submucosal injection was then administered to facilitate dissection within the submucosal plane. Additional injections were applied as necessary to sustain adequate lifting throughout the procedure. The p-ESD procedure began identically to cESD, with tumor margin marking and submucosal injection. Before mucosal incision, a pulley system was created. First, a dental floss-loaded endoclip was placed at the anal-side mark; second, the dental floss was fixed to the opposite intestinal wall with another endoclip. This provided continuous external tension on the lesion, aiding both mucosal incision and submucosal dissection. After complete tumor resection, applying mild pulling force allowed simultaneous removal of the specimen and endoclips (Figure 1).
All patients underwent endoscopic surveillance at 6 and 12 months post-treatment, followed by yearly evaluations to detect local recurrence. Abdominal magnetic resonance imaging or CT scans were performed every 6-12 months to assess lymph node metastasis. Patients with pathological evidence of lymphovascular invasion underwent additional surgery.
Statistical analyses were conducted using SPSS version 27.0 (IBM Corp., Armonk, NY, United States). Continuous variables demonstrating a normal distribution were reported as mean ± SD, whereas non-normally distributed variables were reported as median (range). Group comparisons were performed using Student’s t-test for normally distributed data and the Mann-Whitney U test for non-normally distributed data. Categorical variables were expressed as number (%) and analyzed using Pearson’s χ2 test or Fisher’s exact test, as appropriate. Statistical significance was defined as P < 0.05.
We initially included 113 patients diagnosed with rectal NETs who underwent ESD. A total of 10 patients who were preoperatively misdiagnosed with rectal polyps and underwent endoscopic resection, and whose subsequent pathological diagnosis revealed NETs with positive margins, thus necessitating salvage ESD, were excluded. Ultimately, a total of 103 patients with rectal NETs were included, among whom 49 underwent p-ESD, and 54 underwent c-ESD. In both groups, there were no statistically significant differences in general descriptive parameters including age, sex, tumor location, tumor size, pathological grade, or the presence of vascular and lymphatic invasion. In the p-ESD group, additional surgical intervention was required for one patient due to vascular invasion (Table 1).
| p-ESD group (n = 49) | c-ESD group (n = 54) | P value | |
| Age (years) | 48.9 ± 11.5 | 50.2 ± 9.9 | 0.1011 |
| Sex | 0.4812 | ||
| Male | 27 (55.1) | 26 (48.1) | |
| Female | 22 (44.9) | 28 (51.9) | |
| Tumor location | 0.2822 | ||
| Upper rectum | 10 (20.4) | 16 (29.6) | |
| Lower rectum | 39 (79.6) | 38 (70.4) | |
| Tumor size, cm | 0.6 (0.3-1.4) | 0.5 (0.2-1.3) | 0.1233 |
| Pathological grade | |||
| G1 | 46 (93.9) | 49 (90.7) | 0.8222 |
| G2 | 3 (6.1) | 5 (9.3) | |
| Vascular invasion | 1 (2.0) | 0 (0.0) | 0.4764 |
| Lymphatic invasion | 0 (0.0) | 0 (0.0) | - |
| Additional surgery | 1 (2.0) | 0 (0.0) | 0.4764 |
Regarding secondary outcome measures, the incidence of minor intraoperative bleeding was significantly lower in the p-ESD group than in the c-ESD group [4.1% (2/49) vs 18.5% (10/54); P = 0.030]. The pESD group had significantly lower rates of both minor [5/49 (10.2%) vs 14/54 (25.9%); P = 0.040] and major [2/49 (4.1%) vs 10/54 (18.5%); P = 0.030] intraoperative bleeding compared with the cESD group. Delayed bleeding occurred in two patients (3.7%) in the c-ESD group but in none of the patients in the p-ESD group; however, this difference was not statistically significant (P = 0.496). Muscularis propria injury was reported in nine patients (16.7%) in the c-ESD group, whereas no such injuries occurred in the p-ESD group, representing a significant difference (P = 0.003). Intraoperative perforation was observed in three patients (5.6%) in the c-ESD group, all successfully managed with endoclips, but none in the p-ESD group. This difference was not statistically significant (P = 0.244). No cases of delayed perforation occurred in either group (Table 2).
| p-ESD group (n = 49) | c-ESD group (n = 54) | P value | |
| Dissection time, minutes | 9.03 (7.0-12.3) | 14.9 (11.0-20.5) | < 0.0011 |
| En bloc resection | 49 (100) | 54 (100) | 1.000 |
| R0 resection | 49 (100) | 48 (88.9) | 0.0282 |
| Adverse events | |||
| Minor intraoperative bleeding | 5 (10.2) | 14 (25.9) | 0.040 |
| Major intraoperative bleeding | 2 (4.1) | 10 (18.5) | 0.0302 |
| Delayed bleeding | 0 (0.0) | 2 (3.7) | 0.4962 |
| Muscularis propria injury | 0 (0.0) | 9 (16.7) | 0.0032 |
| Intraoperative perforation | 0 (0.0) | 3 (5.6) | 0.2442 |
| Delayed perforation | 0 (0.0) | 0 (0.0) | - |
| Tumor recurrence | 0 (0.0) | 0 (0.0) | - |
Postoperative follow-up was completed for all 49 patients (100%) in the p-ESD group, with no loss to follow-up. In the c-ESD group, 4 patients (7.4%) were lost to follow-up, resulting in 50 patients (92.6%) under follow-up. The median follow-up period was 22 months (range: 12–54 months). During follow-up, no recurrences or metastases were detected in either group.
P-ESD significantly improved clinical outcomes by achieving higher R0 resection rates, shorter dissection times, reduced intraoperative bleeding, and minimization of the risk of muscularis propria injury or perforation. These findings suggest that early traction facilitates submucosal exposure and enhances procedural control, thereby improving both the safety and efficiency of rectal NETs resection.
Although modified forms of EMR, including EMR with a ligation device and capassisted EMR, are widely used for rectal NETs less than 10 mm in diameter and have demonstrated high en bloc and complete resection rates[11,12], their efficacy may decline as lesion size increases. For intermediatesized lesions, particularly those measuring 7 mm-16 mm, several studies have shown that ESD may achieve higher complete resection rates than capassisted EMR[13,14]. Unlike EMR, ESD facilitates precise dissection within the submucosal plane and is not limited by lesion size, providing a distinct advantage for achieving en bloc R0 resection of larger or complex lesions[15]. Despite its efficacy, ESD remains technically demanding and has a steep learning curve. Consequently, traction-assisted techniques are essential for overcoming procedural limitations and optimizing clinical outcomes by minimizing technical difficulties.
Traction-assisted ESD has consistently demonstrated enhancements in procedural efficiency and safety. A recent meta-analysis of randomized controlled trials revealed that traction-ESD significantly reduces total procedure time for both esophageal and colorectal neoplasms, without compromising en bloc resection, bleeding, or perforation rates compared to c-ESD[16]. Likewise, research has reported that applying traction techniques to colorectal lesions accelerated procedure times and increased resection speeds, while maintaining en bloc resection and adverse event profiles equivalent to standard ESD[17]. In a prospective randomized trial evaluating an endoclip-and-thread traction device for large colorectal tumors, findings indicated significantly decreased dissection time and higher completion rates among operators with intermediate experience[18]. Consistent with these findings, our pilot study on rectal NETs showed substantial benefits of p-ESD over c-ESD. However, several studies have indicated that traction may not provide equivalent benefits for rectal lesions or small (< 20 mm) colorectal lesions. In the multicenter randomized trial, while traction significantly reduced procedure time for colon lesions, it did not shorten overall procedure time for rectal lesions[19]. Similarly, rubber band traction-assisted ESD did not significantly reduce overall procedure time compared with c-ESD for rectal NETs, as reported in another study[20].
These seemingly contradictory findings highlight how the traction technique can markedly influence outcomes across different lesion locations and sizes. In most studies, tractionassisted ESD involves applying traction only after the mucosal incision[21]. In contrast, our p-ESD technique establishes continuous submucosal tension before mucosal incision, offering several key advantages. First, by placing the traction device prior to dissection, we can capture more mucosa, ensuring the endoclip anchors securely in normal mucosa and avoiding grasping of the lesion or muscularis propria, thereby making subsequent dissection easier. Second, pretraction places the mucosa under sustained tension prior to incision. Upon incision, this tension induces immediate mucosal retraction, rapidly exposing the submucosal plane. The maintenance of tension throughout the procedure ensures stable and uniform exposure of the submucosal plane, allowing clear visualization of the vasculature and muscularis propria. This reduces the risk of blind dissection, intraoperative bleeding, and inadvertent muscular injury or perforation, while facilitating precise dissection along the deep margin to achieve higher R0 resection rates. Third, following en bloc resection of the lesion, gentle traction on the dental floss allows simultaneous retrieval of both the intact specimen and the endoclips, avoiding the need for additional instruments such as the snares or forceps used in conventional internal traction methods.
Limitations of this study must be acknowledged. First, its retrospective, single-center design carries an inherent risk of selection bias regarding how lesions were assigned to the p-ESD or c-ESD group, despite comparable baseline characteristics between groups. Although the choice of technique was made on a case-by-case basis according to the lesion characteristics and procedural considerations, at the endoscopist's discretion, this approach may have introduced unmeasured confounding factors independent of the treatment period. Second, the study was constrained by a limited sample. Third, although all procedures were performed by the same expert endoscopist, which reduces interoperator variability, we cannot completely exclude temporal bias from the learning curve effect.
p-ESD represents an effective and safe approach for treating rectal NETs. To further validate its efficacy and generalizability, future studies should employ prospective designs, involve larger sample sizes, and be conducted across multiple centers.
| 1. | Gallo C, Rossi RE, Cavalcoli F, Barbaro F, Boškoski I, Invernizzi P, Massironi S. Rectal neuroendocrine tumors: Current advances in management, treatment, and surveillance. World J Gastroenterol. 2022;28:1123-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (8)] |
| 2. | Osagiede O, Habermann E, Day C, Gabriel E, Merchea A, Lemini R, Jabbal IS, Colibaseanu DT. Factors associated with worse outcomes for colorectal neuroendocrine tumors in radical versus local resections. J Gastrointest Oncol. 2020;11:836-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Wang XY, Chai NL, Linghu EQ, Li HK, Zhai YQ, Feng XX, Zhang WG, Zou JL, Li LS, Xiang JY. Efficacy and safety of hybrid endoscopic submucosal dissection compared with endoscopic submucosal dissection for rectal neuroendocrine tumors and risk factors associated with incomplete endoscopic resection. Ann Transl Med. 2020;8:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | van Rees JM, Elferink MAG, Tanis PJ, de Wilt JHW, Burger JWA, Verhoef C. The incidence, treatment and survival of patients with rare types of rectal malignancies in the Netherlands: A population-based study between 1989 and 2018. Eur J Cancer. 2021;152:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 5. | Park SS, Kim BC, Lee DE, Chang HJ, Han KS, Kim B, Hong CW, Sohn DK, Lee DW, You K, Park SC, Oh JH. Stratification of risk for lymph node metastasis and long-term oncologic outcomes in patients initially treated by endoscopic resection for rectal neuroendocrine tumors. Gastrointest Endosc. 2025;101:1222-1232.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | He L, Deng T, Luo H. Efficacy and safety of endoscopic resection therapies for rectal carcinoid tumors: a meta-analysis. Yonsei Med J. 2015;56:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Park HW, Byeon JS, Park YS, Yang DH, Yoon SM, Kim KJ, Ye BD, Myung SJ, Yang SK, Kim JH. Endoscopic submucosal dissection for treatment of rectal carcinoid tumors. Gastrointest Endosc. 2010;72:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Guo X, Chen Y, Liu M, Zhang S, Zhong C, Chen A, Chen F. Pretraction-assisted endoscopic submucosal dissection for the treatment of a rectal neuroendocrine tumor. Endoscopy. 2023;55:E400-E401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Horikawa Y, Fushimi S, Sato S. Hemorrhage control during gastric endoscopic submucosal dissection: Techniques using uncovered knives. JGH Open. 2020;4:4-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Takada K, Yoshida N, Hayashi Y, Togo D, Oka S, Fukunaga S, Morita Y, Hayashi T, Kozuka K, Tsuji Y, Murakami T, Yamamura T, Komeda Y, Takeuchi Y, Shinmura K, Fukuda H, Yoshii S, Ono S, Katsuki S, Kawashima K, Nemoto D, Yamamoto H, Saito Y, Tamai N, Tamura A; ABCD-J Working Group. Prophylactic clip closure in preventing delayed bleeding after colorectal endoscopic submucosal dissection in patients on anticoagulants: a multicenter retrospective cohort study in Japan. Endoscopy. 2025;57:631-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 11. | Park SB, Kim HW, Kang DH, Choi CW, Kim SJ, Nam HS. Advantage of endoscopic mucosal resection with a cap for rectal neuroendocrine tumors. World J Gastroenterol. 2015;21:9387-9393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Lim HK, Lee SJ, Baek DH, Park DY, Lee BE, Park EY, Park JW, Kim GH, Song GA. Resectability of Rectal Neuroendocrine Tumors Using Endoscopic Mucosal Resection with a Ligation Band Device and Endoscopic Submucosal Dissection. Gastroenterol Res Pract. 2019;2019:8425157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Wang X, Xiang L, Li A, Han Z, Li Y, Wang Y, Guo Y, Zuang K, Yan Q, Zhong J, Xiong J, Yang H, Liu S. Endoscopic submucosal dissection for the treatment of rectal carcinoid tumors 7-16 mm in diameter. Int J Colorectal Dis. 2015;30:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Yang DH, Park Y, Park SH, Kim KJ, Ye BD, Byeon JS, Myung SJ, Yang SK. Cap-assisted EMR for rectal neuroendocrine tumors: comparisons with conventional EMR and endoscopic submucosal dissection (with videos). Gastrointest Endosc. 2016;83:1015-22; quiz 1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Arezzo A, Passera R, Marchese N, Galloro G, Manta R, Cirocchi R. Systematic review and meta-analysis of endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal lesions. United European Gastroenterol J. 2016;4:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Giacobo Nunes F, Gomes ILC, De Moura DTH, Dominguez JEG, Fornari F, Ribeiro IB, Peixoto de Oliveira GH, de Figueiredo SMP, Bernardo WM, Hourneaux de Moura EG. Conventional Versus Traction-Assisted Endoscopic Submucosal Dissection for Esophageal, Gastric, and Colorectal Neoplasms: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cureus. 2024;16:e55645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Singh S, Mohan BP, Chandan S, Sharma N, Vinayek R, Dutta S, Kantsevoy SV, Le M, Adler DG. Conventional Versus Traction Endoscopic Submucosal Dissection for Colorectal Tumors: A Meta-analysis of Randomized Controlled Trials. J Clin Gastroenterol. 2024;58:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Yamasaki Y, Takeuchi Y, Uedo N, Kanesaka T, Kato M, Hamada K, Tonai Y, Matsuura N, Akasaka T, Hanaoka N, Higashino K, Ishihara R, Okada H, Iishi H. Efficacy of traction-assisted colorectal endoscopic submucosal dissection using a clip-and-thread technique: A prospective randomized study. Dig Endosc. 2018;30:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 19. | Ito M, Miura Y, Mizuguchi Y, Furuhashi H, Tsuji Y, Takamaru H, Tamai N, Fujishiro M, Saito Y, Sumiyama K. Efficacy and safety of multi-loop traction device-assisted colorectal endoscopic submucosal dissection: Multicenter randomized clinical trial. Endosc Int Open. 2025;13:a24660718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Peng J, Lin J, Fang L, Zhou J, Song Y, Yang C, Zhang Y, Gu B, Ji Z, Lu Y, Mao X, Yan L. Conventional versus rubber band traction-assisted endoscopic submucosal dissection for rectal neuroendocrine tumors: a single-center retrospective study (with video). Surg Endosc. 2024;38:6485-6492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Nagata M. Advances in traction methods for endoscopic submucosal dissection: What is the best traction method and traction direction? World J Gastroenterol. 2022;28:1-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/