Published online Jul 16, 2025. doi: 10.4253/wjge.v17.i7.108541
Revised: April 23, 2025
Accepted: June 9, 2025
Published online: July 16, 2025
Processing time: 83 Days and 20 Hours
Endoscopic ultrasound (EUS) has evolved from a diagnostic tool to a management technique for various gastroenterological conditions, including biliary strictures.
To summarize the current evidence on EUS’s role in diagnosing and managing biliary strictures.
Two independent reviewers searched five electronic databases (PubMed, CENTRAL, Science Direct, Google Scholar, and EMBASE) for articles published up to January 2025. Included articles met specific criteria, and statistical software was used to analyze reported outcomes.
Of 935 articles, 19 met the inclusion criteria. Ten articles focused on diagnostic EUS, while nine focused on EUS-guided therapeutic interventions. EUS fine-needle aspiration demonstrated superior sensitivity [0.43-1.00; 95% confidence interval (CI): 0.24-1.00] compared to conventional techniques (0.36-0.96; 95%CI: 0.19-0.99) for diag
EUS is a promising tool for diagnosing and managing biliary strictures. Combining EUS-guided and conventional interventions improves diagnostic performance. Further research is needed to investigate the feasibility and use of EUS-guided interventions in this field.
Core Tip: Endoscopic ultrasound (EUS) has emerged as a valuable tool for diagnosing and managing biliary strictures. This systematic review and meta-analysis found that EUS-guided fine-needle aspiration is superior to conventional techniques for diagnosing malignant biliary strictures, with higher sensitivity while maintaining high specificity. For management, EUS-guided interventions showed significantly higher clinical success rates compared to conventional methods, though technical success rates were similar. EUS-guided biliary drainage and stenting appear promising for cases where endoscopic retrograde cholangiopancreatography fails. However, more research is needed on the efficacy of various EUS-guided therapies for biliary strictures. Overall, EUS demonstrates an expanding and important role in biliary stricture diagnosis and treatment.
- Citation: Gadour E, Miutescu B, Okasha HH, Albeshir M, Alamri T, Ghoneem E, Burciu C, Popa A, Koppandi O, AlQahtani MS. Evolving role of endoscopic ultrasound in biliary stricture management: A meta-analysis and systematic review. World J Gastrointest Endosc 2025; 17(7): 108541
- URL: https://www.wjgnet.com/1948-5190/full/v17/i7/108541.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i7.108541
Endoscopic ultrasound (EUS) is one of the most significant innovations in gastroenterology and related disciplines[1]. EUS has evolved as a critical tool for the diagnosis and management of various conditions[2]. Furthermore, its use has advanced to include the management of complications that may arise during endoscopic procedures. EUS has emerged as a pivotal tool for the diagnosis and management of various biliary pathologies[3].
Biliary strictures are areas of narrowing in both the intrahepatic and extrahepatic biliary systems. This condition results in biliary obstruction, which often leads to antegrade bile flow and pathological manifestations of the disease[4]. The clinical manifestations of biliary strictures are often variable depending on the severity of the disease and underlying pathologies. These manifestations include pruritus and jaundice, with other patients often being asymptomatic and strictures being diagnosed only on routine imaging studies[5].
Various tools are used to diagnose biliary strictures, of which the most common is magnetic resonance cholangiopancreatography (MRCP). It is the most sensitive non-invasive diagnostic tool for biliary pathologies, including biliary strictures[6]. However, EUS has emerged as an alternative and potential choice for diagnosing and managing biliary strictures[7]. In this article, we aimed to summarize the current evidence regarding the utility of EUS in both the diagnosis and management of biliary strictures. EUS is an advanced imaging technique combining endoscopy with high-frequency ultrasound, providing detailed visualization of the gastrointestinal tract and adjacent structures. It involves inserting a flexible endoscope with an ultrasound transducer into the gastrointestinal tract, allowing close proximity imaging of organs like the pancreas and biliary system. EUS offers advantages for biliary strictures, including high-resolution imaging, tissue sampling capability through EUS-guided fine needle aspiration or biopsy, and interventional applications. Compared to MRCP, EUS is minimally invasive, allows direct tissue sampling, provides real-time imaging, and typically offers higher spatial resolution. However, EUS is more operator-dependent and less widely available than MRCP. While MRCP remains the initial non-invasive imaging modality for suspected biliary strictures, EUS has emerged as a valuable complementary tool, particularly for tissue acquisition and detailed characterization of strictures.
This systematic review was conducted according to the PRISMA 2020 guidelines[8]. The protocol for this review was not registered in any database.
A literature search was conducted independently by two reviewers using two approaches. The reviewers defined a comprehensive search criterion for the electronic databases using the first approach. This criterion and the associated keywords were then used for the electronic search of five electronic databases: ScienceDirect, PubMed, Google Scholar, CENTRAL, and EMBASE. The keywords used for the electronic search included (Biliary strictures OR Malignant biliary strictures OR Benign biliary strictures OR Biliary obstruction) AND (Diagnosis) AND (Management) AND (EUS OR Ultrasound AND Endoscopic ultrasound-guided management). These keywords were then modified for each database to maximize the number of results obtained. After the electronic search, the reviewers reviewed the lists of references of the obtained studies to identify additional studies that would have likely been missed in the initial search. Lastly, the reviewers searched for any trials registered in the clinical trial.gov registry for any completed trial with results but lacking available publications.
Once all the articles had been obtained from the databases and registries, the authors utilized the pre specified eligibility criteria to analyze each of the articles before their inclusion in the review.
The included articles were selected based on the following criteria: (1) Studies published in English; (2) Studies that included patients with both benign and malignant biliary strictures; (3) Studies investigating the use of EUS as a diagnostic tool for biliary strictures; (4) Studies investigating various EUS-guided interventions for biliary strictures; and (5) Studies designed as primary interventional studies, such as clinical trials.
Studies were excluded from the review during the eligibility analysis if they met the following criteria: (1) Studies that did not include patients with biliary strictures; (2) Studies that did not include EUS as a diagnostic tool or guide for various interventions; and (3) Secondary studies, including systematic reviews, meta-analyses, and narrative reviews.
Two independent reviewers conducted the study selection process in different phases. The phases entailed the removal of duplicate articles, screening abstracts and titles, and screening the available full texts. For inclusion in the review, the independent authors first screened the abstracts of articles obtained after removing duplicates. Articles that met the inclusion criteria were included in the study; however, if the reviewers could not ascertain their eligibility, they obtained the full text for screening. After completing the study selection process, the reviewers extracted all data and converted them into pilot-tested forms. The information extracted from each study included the study ID, study design, study setting, sample size, intervention, mean age of patients, inclusion criteria, and reported outcomes.
Owing to the diverse designs of the included studies, we utilized various risk of bias (RoB) and methodological assessment tools to determine the RoB and methodological quality of the included studies. We used the RoB 2 tool provided by the Cochrane Collaboration for randomized controlled trials. For the diagnostic test accuracy (DTA) studies, we used the QUADAS2 to assess their methodological quality. Finally, we utilized the Newcastle-Ottawa scale for cohort and case-control studies to assess their methodological quality.
We used Review Manager Software (RevMan 5.4, The Cochrane Collaboration 2019, The Nordic Cochrane Centre, Copenhagen, Denmark) for quantitative synthesis. DTA meta-analysis was conducted for DTA studies. To facilitate the analysis, we used the data reported in the studies to calculate true positives, true negatives, false positives, and false negatives. To calculate these values from the summarized values provided in the studies, we utilized a web-based calculator provided by “2-way Contingency Table Analysis” (accessed at https://statpages.info/ctab2x2.html). These values were used to estimate the sensitivity and specificity of each diagnostic method. For interventional studies, we used the odds ratio (OR) to analyze the clinical and technical success rates as they were all dichotomous outcomes. We also used the I2 to measure and quantify the heterogeneity of the studies.
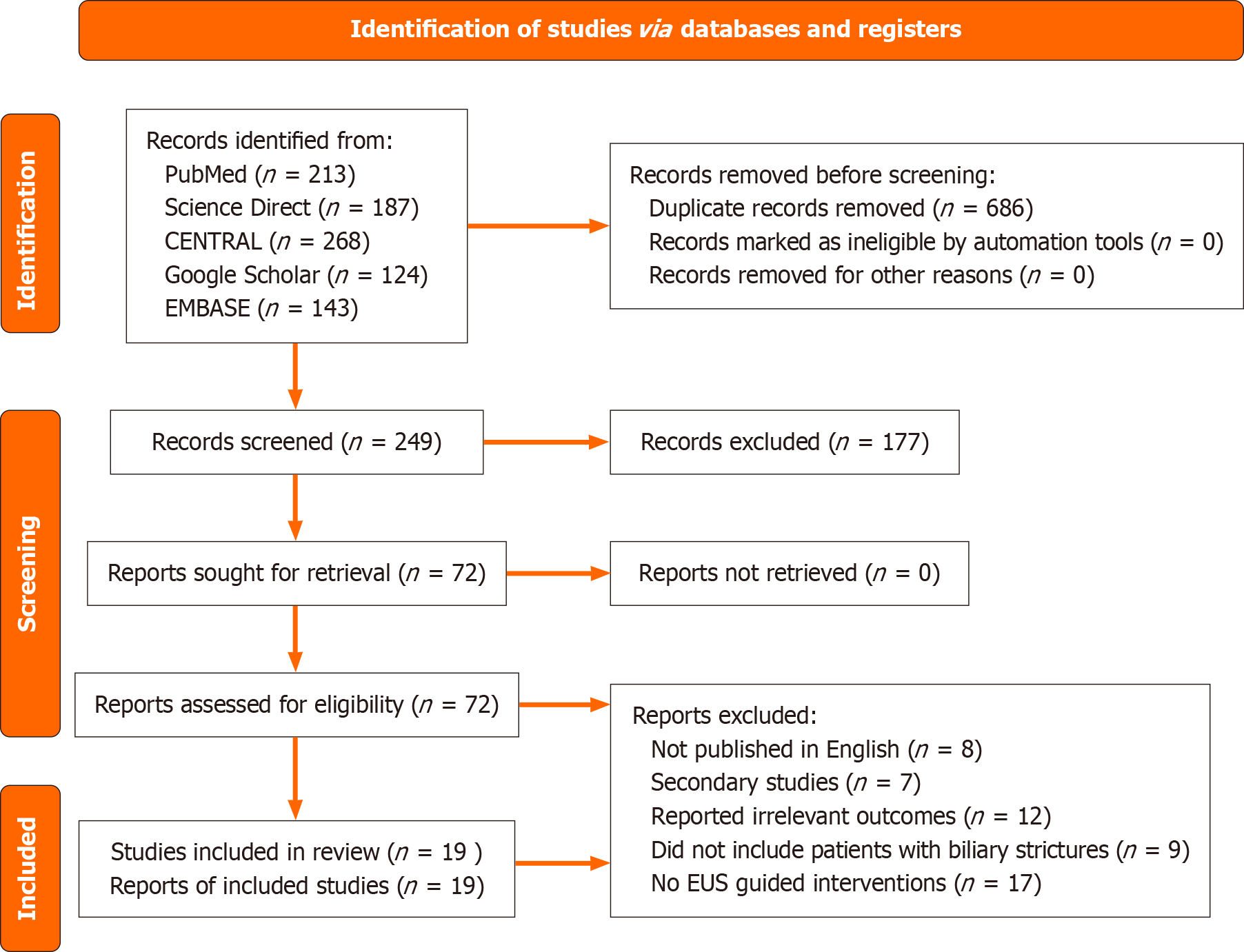
Our electronic search retrieved 935 articles from the databases. Duplication assessment helped remove 686 duplicates. The remaining 249 publications were assessed based on their title and abstract relevance, leading to the removal of 177 irrelevant studies. Seventy-two articles were then retrieved and evaluated based on our eligibility criteria. Finally, 19 articles that met the inclusion criteria were selected. The remaining articles were excluded for the following reasons: 8 published in language other than English, 7 other secondary studies, 12 did not report any of the required outcomes, 9 did not include patients with biliary strictures, and 17 did not include any EUS-guided interventions for either the management or diagnosis of biliary strictures. The PRISMA diagram in Figure 1 presents a detailed summary of the search strategy.
The review included 19 studies, of which 10 were DTA studies[9-18], and 9 were interventional studies[19-27]. Of the nine interventional studies, seven investigated biliary drainage after biliary obstruction secondary to biliary strictures, and two investigated the feasibility of using EUS-guided biliary stenting to manage biliary strictures. The characteristics of the included DTA studies are summarized in Table 1, and those of the included interventional studies are summarized in Table 2.
| Ref. | Study design | Location of lesion | Sample size | Inclusion criteria | Diagnostic intervention | Gold standard | Findings | Center type |
| Novis et al, 2010[9] | Comparative study | Distal | 46 | Biliary obstruction | EUS-FNA and ERCP | Histology, surgery, and follow-up | Benign lesions (n = 7) and malignant lesions (n = 37) | SC |
| Fritscher-Ravens | Prospective | Proximal | 44 | Patients with obstruc | EUS-FNA | Autopsy, surgery, or follow-up | Benign (n = 12) and malignant (n = 31) | SC |
| Ohshima | Prospective | Biliary | 22 | Patients with suspected malignant biliary stric | EUS-FNA | Histology, surgery, and follow-up | Malignant (n = 16) and benign (n = 6) | SC |
| Weilert | Prospective | Biliary | 51 | Patients with suspected pancreaticobiliary path | EUS-FNA and ERCP | Surgery, definitive findings, and follow-up | Benign (n = 3) and malignant (n = 48) | SC |
| DeWitt | Prospective | Proximal | 24 | Patients with suspected or confirmed proximal biliary strictures | EUS-FNA | Surgical pathology findings and follow-up | Malignant (n = 17) and benign (n = 7) | SC |
| Eloubeidi | Prospective | Biliary | 25 | Patients with common bile duct strictures | EUS-FNA | Follow-up and surgical pathology | Malignant (n = 31) and benign (n = 7) | SC |
| Rösch et al, 2004[14] | Prospective | Biliary | 50 | Patients with indeter | EUS-FNA and ERCP | Follow-up and surgical pathology or other biopsy results | Malignant (n = 28) and benign (n = 22) | SC |
| Lee et al, 2019[15] | Prospective | Distal | 181 | Patients with suspected malignant biliary stric | EUS-FNA, ERCP, and POC-FB | Surgical pathology findings and malignant diagnosis after biopsy or during follow-up | Malignant (n = 51) and benign (n = 8) | MC |
| Yeo et al, 2019[16] | Retrospective | Biliary | 93 | Patients with suspected biliary strictures | EUS-TS and ERCP-TS | Histopathology findings based on the surgical specimen, cytology findings of either EUS-TS or ERCP-TS, or during clinical follow-up | Malignant (n = 70) and benign (n = 16) | MC |
| Lee | Prospective | Biliary | 178 | Patients with suspected malignant biliary stric | ERCP with TPB and EUS-FNA | Histopathologic findings of the surgical specimens, diagnosis based on TPB or EUS-FNAB, or during the clinical imaging on follow-up | Malignant (n = 171) and benign (n = 7) | MC |
| Ref. | Study design | Study setting | Study group | Sample size | Mean age (years) | Inclusion criteria | Reported outcomes | Center type |
| Bang et al, 2018[19] | RCT | United States | EUS-BD | 33 | 69.4 ± 12.6 | Patients with obstructive jaundice and a pancreatic head mass | Rate of adverse events, technical success, treatment success, re-interventions, and procedural duration | MC |
| Paik et al, 2018[20] | RCT | South Korea | EUS-BD | 64 | 64.8 | Adult patients with unresectable malignant biliary strictures | Technical success rates, clinical success rates, median hospital stay, and early adverse events | MC |
| Sharaiha | Retrospective case-control study | United States | EUS-BD | 47 | - | Patients with either malignant or benign biliary obstruction | Technical and clinical success, post-procedural pain, and incidence of adverse events | SC |
| Bapaye | Retrospective case-control study | India | EUS-BD | 25 | 59.9 ± 13.3 | Patients with unresectable malignancies causing biliary obstruction | Successful stent placement and incidence of complications | SC |
| Huang et al, 2017[23] | Retrospective case-control study | China | EUS-BD | 36 | 68 ± 4.62 | Patients with failed ERCP for the management of obstructive jaundice secondary to malignancy | Technical and clinical success rates, complications, and length of hospital stay | SC |
| Lee et al, 2016[24] | RCT | South Korea | EUS-BDS | 34 | 66.5 | Patients with distal MBOs | Technical and functional success, procedure-related adverse events, reintervention rate, and hospital stay | MC |
| Khashab | Retrospective case-control study | UnitedStates | EGBD | 64 | 64.9 ± 12.5 | Patients with distal MBOs with at least one failed ERCP session | Adverse events, procedure-related costs, and reintervention rates | SC |
| Artifon | RCT | Brazil | EUS-CD | 13 | 63.4 ± 11.1 | Patients with unresectable MBOs | Success rate, quality of life outcomes, and incidence of adverse events | SC |
| Bill et al, 2016[27] | Retrospective case–control study | United States | EUSr | 25 | 65.4 ± 11.6 | Patients with MDBO with a previous failed ERCP | Technical and clinical success, length of hospital stay, and repeat procedure rate | SC |
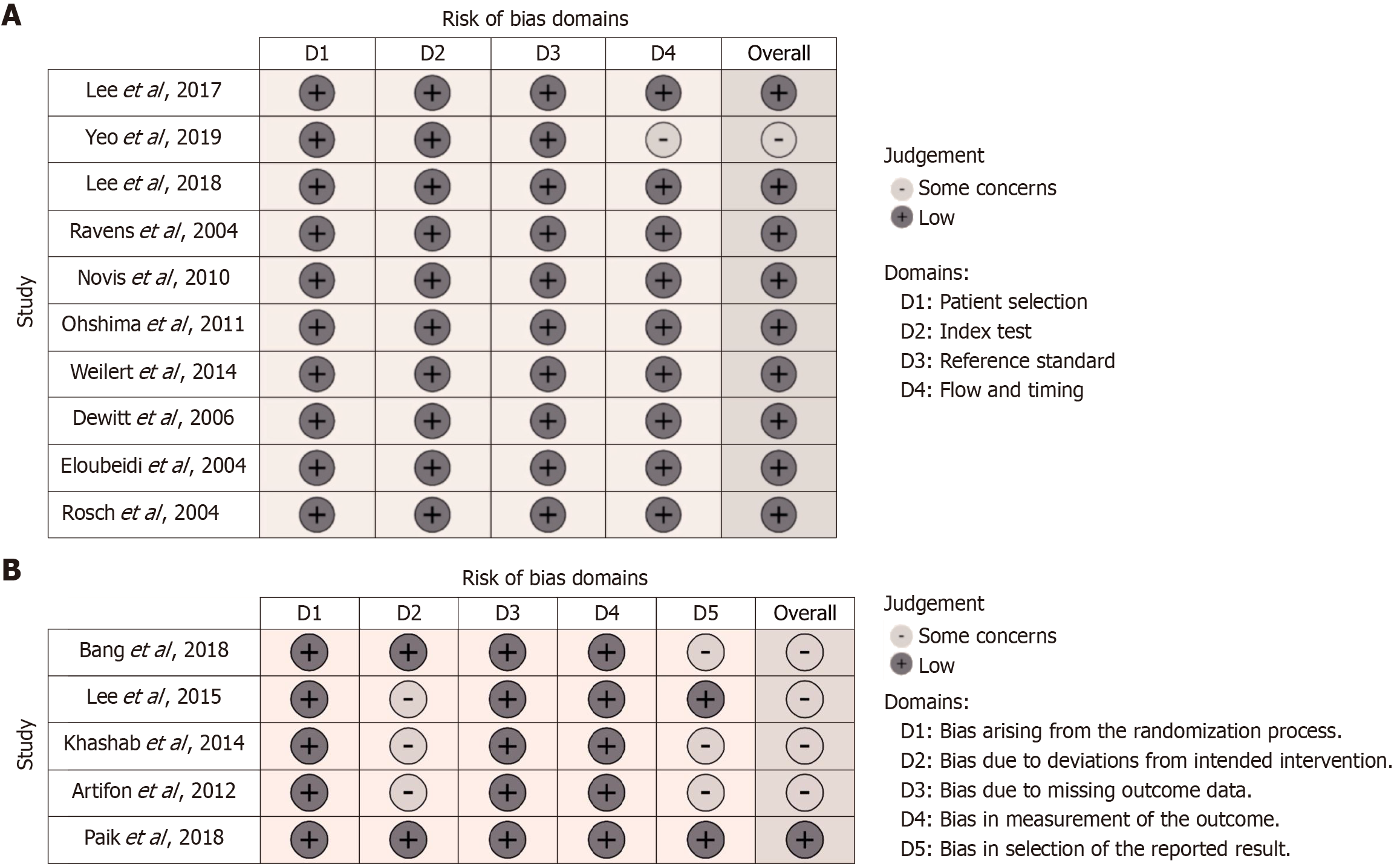
Most of the included DTA studies had good methodological quality with a low RoB (Figure 2). In the study by Yeo et al[16], there were some concerns due to a lack of blinding and the absence of a registered protocol. All cohort and case-control studies had good Agency for Healthcare Research and Quality standards for methodological quality. The studies were noted as having a good methodology for the selection of participants and reporting of outcomes (Table 3). Of the five randomized controlled trials, only one had a low RoB[20]. The remainder had “some concerns” attributed to bias in deviations from the intended intervention and selection of the reported results (Figure 2B)[19,24-26].
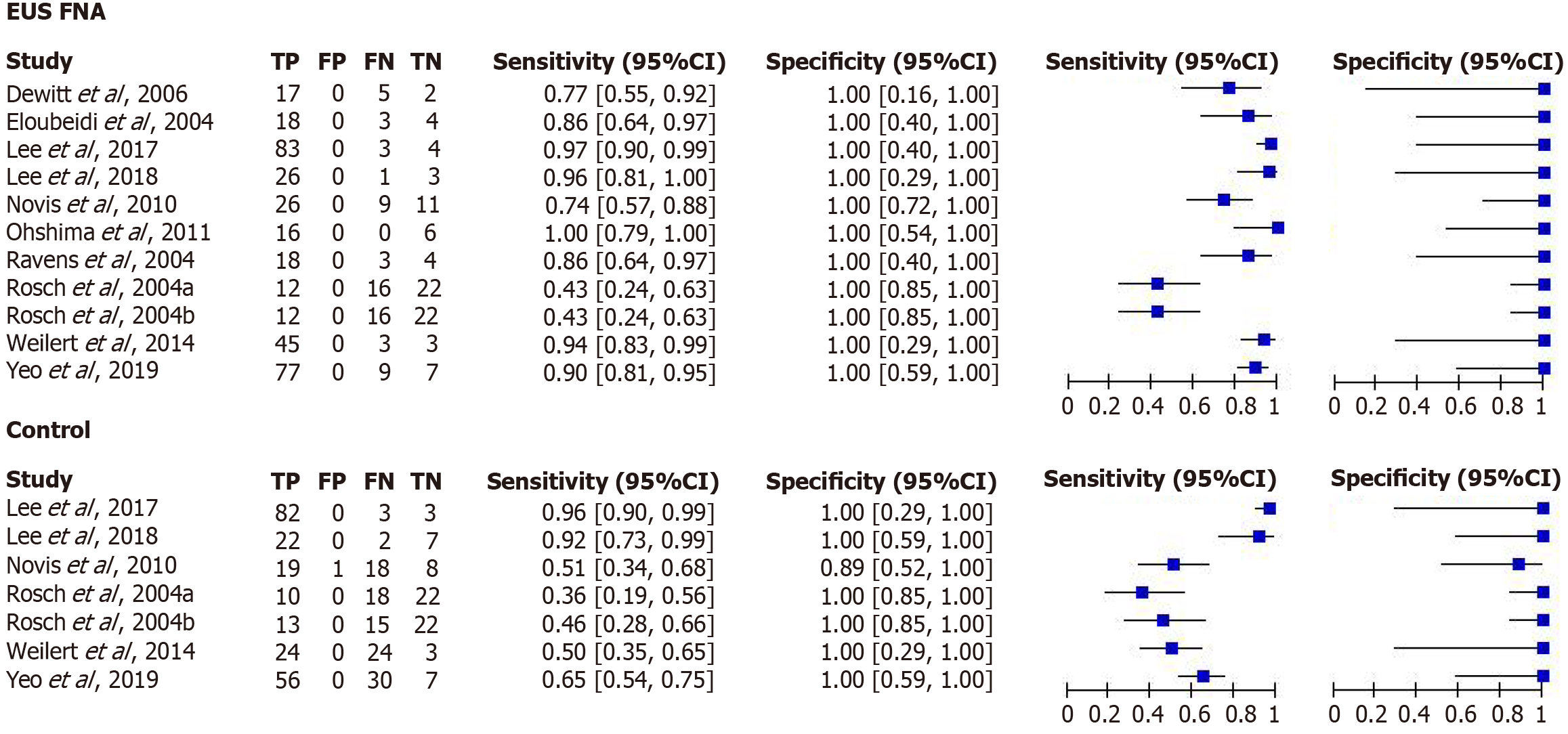
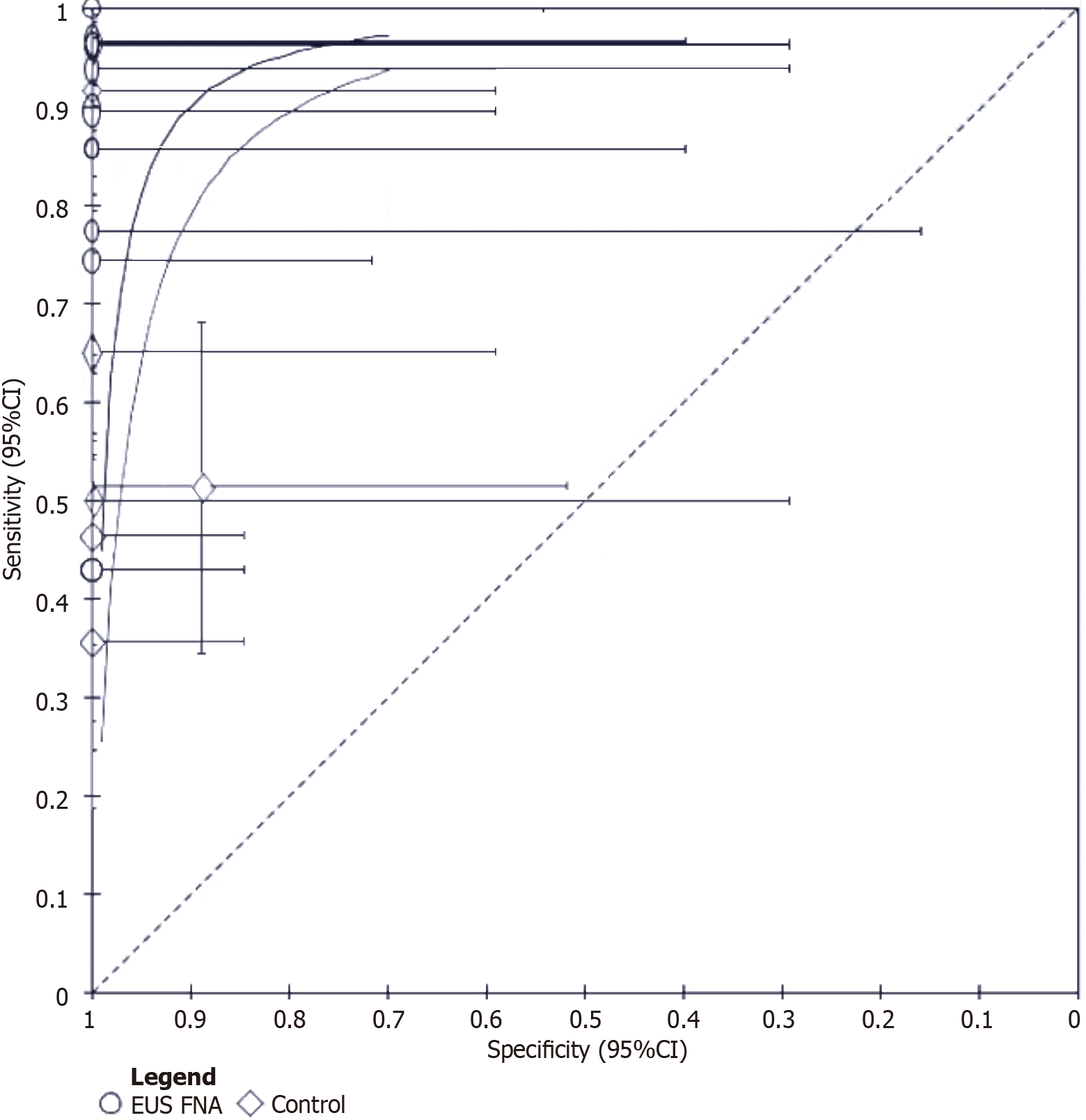
Diagnostic accuracy of EUS-fine-needle aspiration for malignant biliary strictures: The meta-analysis was performed separately for DTA and interventional studies. Meta-analysis showed that the sensitivity of EUS-fine-needle aspiration (FNA) in the diagnosis of malignant biliary strictures ranged from 0.43 [95% confidence interval (CI): 0.24-0.63] to 1.00 (95%CI: 0.79-1.00), with a very high specificity of 1.00 (95%CI: 0.16-1.00) (Figure 3). In contrast, the conventional diagnostic methods had a sensitivity ranging from 0.36 (95%CI: 0.19-0.56) to 0.96 (95%CI: 0.90-0.99). These methods also had high specificity for the detection of malignant biliary strictures, with specificity ranging from 0.89 (95%CI: 0.89) (95%CI: 0.52-1.00) to 1.00 (95%CI: 0.85-1.00) (Figure 3). To further elucidate the results, we generated standardized receiver operating curves for the two diagnostic methods. While RevMan 5.4 did not allow us to generate the value of the area under the curve for each diagnostic method, a visual inspection of the curve indicated that the area under the curve of EUS-FNA was greater than that of conventional methods (Figure 4). Thus, we concluded that EUS-FNA has superior diagnostic efficacy.
Efficacy of EUS-guided therapies for biliary strictures: We analyzed the efficacy of two EUS-guided therapies for managing biliary strictures: EUS-guided biliary drainage and EUS-guided biliary stent placement.
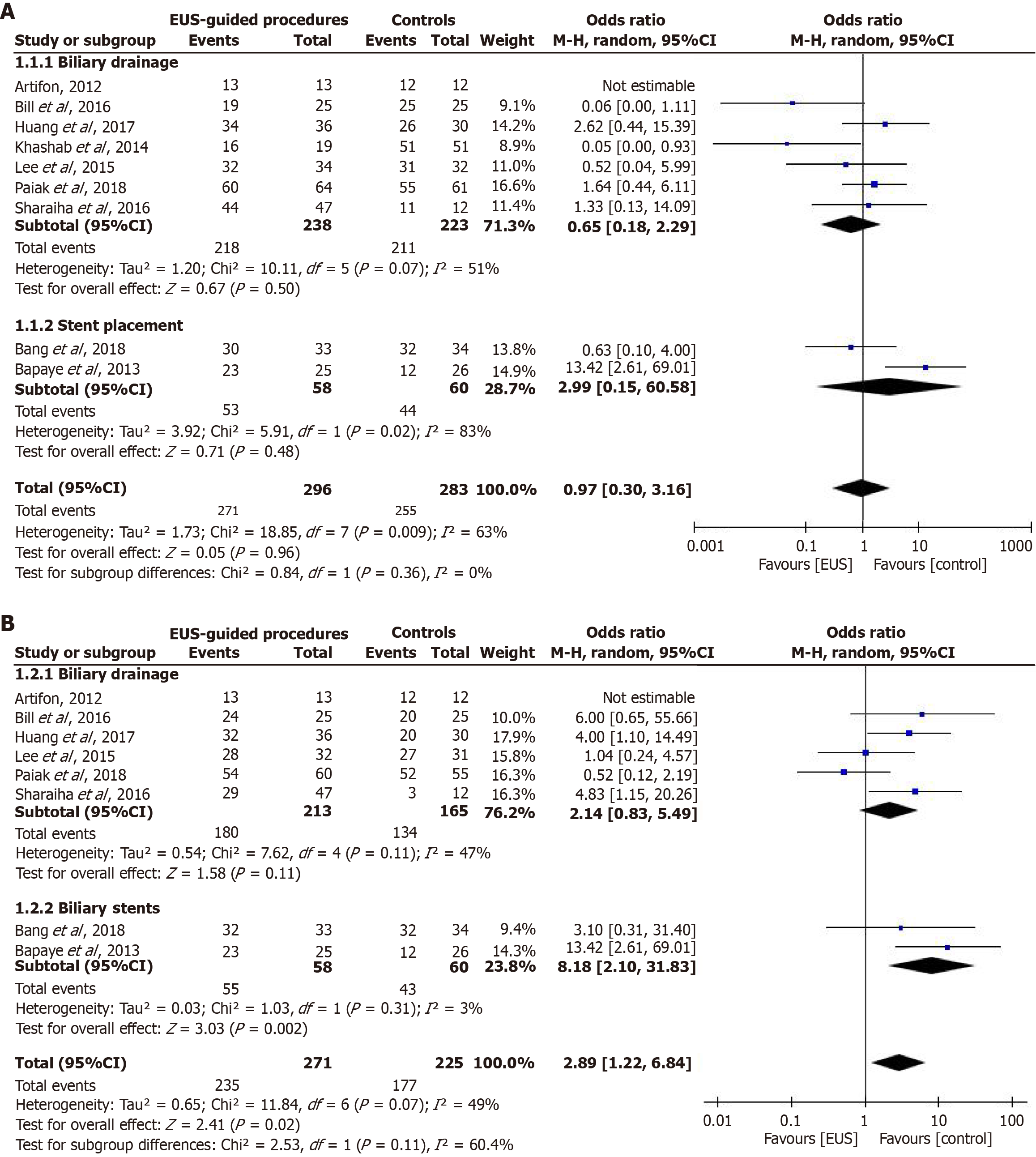
Technical success rate: Technical success rates were reported in nine studies. We pooled the reported results and found no significant difference in the technical success rate of EUS-guided procedures and conventional methods (OR = 0.97; 95%CI: 0.30-3.16; P = 0.96) (Figure 5A). In the included studies, two interventions were investigated; therefore, we conducted a subgroup analysis to determine the comparative efficacy of each intervention. Similarly, the technical success rates of EUS-guided biliary drainage and EUS-guided biliary stent placement were comparable to those of conventional interventions (OR = 0.65; 95%CI: 0.18-2.29; P = 0.50) and (OR = 2.99; 95%CI: 0.15-60.58; P = 0.48), respectively (Figure 5A).
The distinction between technical and clinical success rates in EUS-guided interventions is critical, with technical success referring to the successful completion of the procedure itself, while clinical success relates to the achievement of the desired clinical outcome or symptom resolution. Limitations due to heterogeneity include patient population variability, different control interventions, sample size limitations, study design variability, and subgroup analysis limitations. The inclusion of both malignant and benign biliary strictures introduces variability affecting result interpretation and generalizability. Different comparators complicate the assessment of comparative effectiveness, while limited studies for some interventions potentially affect conclusion reliability. Differences across included studies contribute to heterogeneity, and the inability to conduct detailed subgroup analyses due to heterogeneity limits insights into various factors' impact on outcomes. These limitations should be acknowledged as they affect the strength and generalizability of conclusions.
Clinical success rate: The clinical success rate was reported in eight of the included studies. A pooled analysis of these results showed that the clinical success rate of EUS-guided procedures was significantly higher than that of conventional methods (OR = 2.89; 95%CI: 1.22-6.84; P = 0.02) (Figure 5B). Similarly, when we conducted a subgroup analysis, we found that the clinical success rate of EUS-guided biliary stent placement was significantly higher than that of endoscopic retrograde cholangiopancreatography-guided stent placement (OR = 8.18; 95%CI: 2.10-31.83; P = 0.002). However, the clinical success rate of EUS-guided biliary drainage was comparable to that of ERCP-guided biliary drainage (OR = 2.14; 95%CI: 0.83-5.49; P = 0.11) (Figure 5B).
Our results show that EUS-FNA is superior to other diagnostic methods for malignant biliary strictures. In addition, EUS-guided interventions have inconsistent efficacy in managing biliary strictures compared with conventional diagnostic methods. Although the clinical success of EUS-guided procedures is significantly higher than that of conventional diagnostic methods, the technical success rate is still comparable.
While EUS has shown promising results in the diagnosis of biliary strictures, the current diagnostic approach does not focus solely on one diagnostic method. Instead, most clinicians advocate a multimodal approach for diagnosing most biliary strictures[28,29]. Notably, in most of the studies included in this review, EUS-FNA was considered after an initial diagnosis of ERCP, which yielded negative results. This indicates that ERCP plays a crucial role in diagnosing malignant biliary strictures in the general population. However, owing to the low sensitivity of ERCP, clinicians are considering EUS-FNA to mitigate the limitations of ERCP. Furthermore, in some studies, combining ERCP with EUS has significantly increased the sensitivity and specificity of the combined approach[9].
Our study found that EUS-FNA had superior diagnostic efficacy compared with conventional diagnostic methods. Similar to our findings, a previous review by De Moura et al[30] found that EUS was superior to ERCP in the diagnosis of malignant biliary strictures. Another review reported similar results and highlighted the utility of EUS-FNA in diagnosing distal malignant biliary strictures[31]. While our review did not stratify our outcomes according to the anatomic location of the lesion, previous studies have highlighted EUS-FNA as a promising tool with positive results in the diagnosis of distal malignant biliary strictures[32]. However, more research should be conducted to determine if the anatomic location of the biliary stricture affects the diagnostic performance of EUS-FNA. It is imperative to note that both EUS-FNA and conventional methods, such as ERCP, have high specificity for malignant biliary strictures.
The current guidelines of the American Society of Gastrointestinal Endoscopy recommend ERCP as the initial intervention for patients with biliary strictures after liver transplantation[33]. However, the European Society of Gastrointestinal Endoscopy discourages routine biliary drainage in patients with biliary obstruction and instead recommends the placement of self-expandable metal stents for preoperative and palliative extrahepatic biliary obstruction[34]. The placement of these stents was mainly performed under ERCP guidance. However, when ERCP-guided stenting fails, EUS-guided drainage is preferred to percutaneous drainage[35]. Our review found no significant difference in the technical success of EUS-guided drainage after failed ERCP and transhepatic biliary drainage.
Our study also found that EUS-guided biliary stent placement was a promising and feasible method for managing biliary strictures. While we only summarized data from two studies, our findings indicate that EUS-guided biliary stenting exhibited a better clinical success rate than control interventions. However, as our review is based on a limited number of studies, further research is required to generate more empirical evidence on the utility of EUS-guided biliary stenting in managing biliary strictures.
In the current review, we employed a robust methodological approach to summarize the current evidence regarding the role of EUS in the diagnosis and management of biliary strictures. However, this review has some limitations. First, the study population was heterogeneous. The sample population included patients with malignant or other biliary strictures. The generalizability of the results may be limited because of the diversity in the patient population. Second, in the DTA meta-analysis, we could only determine the comparative diagnostic accuracy of EUS-FNA in the diagnosis of malignant biliary strictures. While malignant biliary strictures are of clinical significance due to their prognostic implications for the patient population, it is also essential to determine the accuracy of EUS-FNA in diagnosing other types of biliary strictures. Furthermore, we could only analyze the efficacy of EUS-guided biliary drainage and stent placement. However, only two of the included studies investigated the effectiveness of EUS-guided biliary stent placement. Therefore, this factor limits the sample size from which we draw our conclusions and findings. Lastly, the control interventions varied, including ERCP and percutaneous transhepatic biliary drainage, thereby affecting the outcome of our analysis. However, we could not conduct further subgroup analyses to determine whether different types of control interventions affected the comparative effectiveness of EUS-FNA. Here is a paragraph explicitly stating limitations due to heterogeneity in this article.
Other important limitations due to heterogeneity across the included studies. The study population was heterogeneous, including patients with both malignant and benign biliary strictures. This diversity in the patient population may limit the generalizability of the results. Additionally, there was heterogeneity in the control interventions used across studies, including both ERCP and percutaneous transhepatic biliary drainage. This variability in comparator inter
The article acknowledges potential publication bias as a limitation in the systematic review of EUS in managing biliary strictures. This bias may arise from the tendency to publish positive or significant results more frequently than negative or inconclusive findings, leading to an overrepresentation of favorable outcomes in the literature. The review's reliance on published studies could skew the perceived efficacy of EUS-guided interventions, as studies with less favorable results might remain unpublished or underreported. Consequently, this could affect the overall conclusions drawn regarding the effectiveness of EUS in diagnosing and managing biliary strictures, highlighting the need for more comprehensive research that includes unpublished data to provide a balanced perspective on the topic.
Our study results show that EUS-FNA is superior to ERCP and other conventional diagnostic modalities for the diagnosis of malignant biliary strictures. Furthermore, EUS-guided therapies have shown promising results in the management of biliary strictures. However, the current evidence of their superior efficacy is inconsistent and is based on a limited number of studies. Therefore, we recommend that further studies should be conducted to determine the feasibility and effectiveness of a wider range of EUS-guided therapies for managing biliary strictures.
| 1. | Mekky MA, Abbas WA. Endoscopic ultrasound in gastroenterology: from diagnosis to therapeutic implications. World J Gastroenterol. 2014;20:7801-7807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Tehami N, Kaushal K, Maher B. The contribution of EUS to the management of endoscopic and surgical complications. Best Pract Res Clin Gastroenterol. 2024;69:101914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Tang RSY. Endoscopic evaluation of indeterminate biliary strictures: Cholangioscopy, endoscopic ultrasound, or both? Dig Endosc. 2024;36:778-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Rodrigues T, Boike JR. Biliary Strictures: Etiologies and Medical Management. Semin Intervent Radiol. 2021;38:255-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Tummala P, Munigala S, Eloubeidi MA, Agarwal B. Patients with obstructive jaundice and biliary stricture ± mass lesion on imaging: prevalence of malignancy and potential role of EUS-FNA. J Clin Gastroenterol. 2013;47:532-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 6. | Keane MG, Devlin J, Harrison P, Masadeh M, Arain MA, Joshi D. Diagnosis and management of benign biliary strictures post liver transplantation in adults. Transplant Rev (Orlando). 2021;35:100593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (37)] |
| 7. | Khan MA, Akbar A, Baron TH, Khan S, Kocak M, Alastal Y, Hammad T, Lee WM, Sofi A, Artifon EL, Nawras A, Ismail MK. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:684-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 8. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 50939] [Article Influence: 10187.8] [Reference Citation Analysis (2)] |
| 9. | Novis M, Ardengh JC, Libera ED, Nakao FS, Ornellas LC, Santo GC, Venco F, Ferrari AP. [Prospective comparative study of ERCP brush cytology and EUS-FNA for the differential diagnosis of biliary strictures]. Rev Col Bras Cir. 2010;37:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Fritscher-Ravens A, Broering DC, Knoefel WT, Rogiers X, Swain P, Thonke F, Bobrowski C, Topalidis T, Soehendra N. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2004;99:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Ohshima Y, Yasuda I, Kawakami H, Kuwatani M, Mukai T, Iwashita T, Doi S, Nakashima M, Hirose Y, Asaka M, Moriwaki H. EUS-FNA for suspected malignant biliary strictures after negative endoscopic transpapillary brush cytology and forceps biopsy. J Gastroenterol. 2011;46:921-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Weilert F, Bhat YM, Binmoeller KF, Kane S, Jaffee IM, Shaw RE, Cameron R, Hashimoto Y, Shah JN. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014;80:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | DeWitt J, Misra VL, Leblanc JK, McHenry L, Sherman S. EUS-guided FNA of proximal biliary strictures after negative ERCP brush cytology results. Gastrointest Endosc. 2006;64:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 152] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Rösch T, Hofrichter K, Frimberger E, Meining A, Born P, Weigert N, Allescher HD, Classen M, Barbur M, Schenck U, Werner M. ERCP or EUS for tissue diagnosis of biliary strictures? A prospective comparative study. Gastrointest Endosc. 2004;60:390-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 215] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Lee YN, Moon JH, Choi HJ, Kim HK, Lee HW, Lee TH, Choi MH, Cha SW, Cho YD, Park SH. Tissue acquisition for diagnosis of biliary strictures using peroral cholangioscopy or endoscopic ultrasound-guided fine-needle aspiration. Endoscopy. 2019;51:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Yeo SJ, Cho CM, Jung MK, Seo AN, Bae HI. Comparison of the Diagnostic Performances of Same-session Endoscopic Ultrasound- and Endoscopic Retrograde Cholangiopancreatography-guided Tissue Sampling for Suspected Biliary Strictures at Different Primary Tumor Sites. Korean J Gastroenterol. 2019;73:213-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Eloubeidi MA, Chen VK, Jhala NC, Eltoum IE, Jhala D, Chhieng DC, Syed SA, Vickers SM, Mel Wilcox C. Endoscopic ultrasound-guided fine needle aspiration biopsy of suspected cholangiocarcinoma. Clin Gastroenterol Hepatol. 2004;2:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Lee YN, Moon JH, Choi HJ, Kim HK, Choi SY, Choi MH, Lee TH, Lee TH, Cha SW, Park SH. Diagnostic approach using ERCP-guided transpapillary forceps biopsy or EUS-guided fine-needle aspiration biopsy according to the nature of stricture segment for patients with suspected malignant biliary stricture. Cancer Med. 2017;6:582-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Bang JY, Navaneethan U, Hasan M, Hawes R, Varadarajulu S. Stent placement by EUS or ERCP for primary biliary decompression in pancreatic cancer: a randomized trial (with videos). Gastrointest Endosc. 2018;88:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 20. | Paik WH, Lee TH, Park DH, Choi JH, Kim SO, Jang S, Kim DU, Shim JH, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH. EUS-Guided Biliary Drainage Versus ERCP for the Primary Palliation of Malignant Biliary Obstruction: A Multicenter Randomized Clinical Trial. Am J Gastroenterol. 2018;113:987-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (1)] |
| 21. | Sharaiha RZ, Kumta NA, Desai AP, DeFilippis EM, Gabr M, Sarkisian AM, Salgado S, Millman J, Benvenuto A, Cohen M, Tyberg A, Gaidhane M, Kahaleh M. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc. 2016;30:5500-5505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Bapaye A, Dubale N, Aher A. Comparison of endosonography-guided vs. percutaneous biliary stenting when papilla is inaccessible for ERCP. United European Gastroenterol J. 2013;1:285-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Huang P, Zhang H, Zhang XF, Lv W, Lou S. Comparison of Endoscopic Ultrasonography Guided Biliary Drainage and Percutaneous Transhepatic Biliary Drainage in the Management of Malignant Obstructive Jaundice After Failed ERCP. Surg Laparosc Endosc Percutan Tech. 2017;27:e127-e131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Lee TH, Choi JH, Park do H, Song TJ, Kim DU, Paik WH, Hwangbo Y, Lee SS, Seo DW, Lee SK, Kim MH. Similar Efficacies of Endoscopic Ultrasound-guided Transmural and Percutaneous Drainage for Malignant Distal Biliary Obstruction. Clin Gastroenterol Hepatol. 2016;14:1011-1019.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 25. | Khashab MA, Valeshabad AK, Afghani E, Singh VK, Kumbhari V, Messallam A, Saxena P, El Zein M, Lennon AM, Canto MI, Kalloo AN. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 27. | Bill JG, Darcy M, Fujii-Lau LL, Mullady DK, Gaddam S, Murad FM, Early DS, Edmundowicz SA, Kushnir VM. A comparison between endoscopic ultrasound-guided rendezvous and percutaneous biliary drainage after failed ERCP for malignant distal biliary obstruction. Endosc Int Open. 2016;4:E980-E985. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Paranandi B, Oppong KW. Biliary strictures: endoscopic assessment and management. Frontline Gastroenterol. 2017;8:133-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Liao W, Wang Q, Jiang Q, Wu X, Yang Y, Yang A. Enhancing diagnostic strategies for biliary strictures: an evolving landscape. Hepatobiliary Surg Nutr. 2024;13:885-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | De Moura DTH, Moura EGH, Bernardo WM, De Moura ETH, Baraca FI, Kondo A, Matuguma SE, Almeida Artifon EL. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: Systematic review and meta-analysis. Endosc Ultrasound. 2018;7:10-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 94] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 31. | Sadeghi A, Mohamadnejad M, Islami F, Keshtkar A, Biglari M, Malekzadeh R, Eloubeidi MA. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83:290-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Chiang A, Theriault M, Salim M, James PD. The incremental benefit of EUS for the identification of malignancy in indeterminate extrahepatic biliary strictures: A systematic review and meta-analysis. Endosc Ultrasound. 2019;8:310-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 33. | Kohli DR, Amateau SK, Desai M, Chinnakotla S, Harrison ME, Chalhoub JM, Coelho-Prabhu N, Elhanafi SE, Forbes N, Fujii-Lau LL, Kwon RS, Machicado JD, Marya NB, Pawa S, Ruan W, Sheth SG, Thiruvengadam NR, Thosani NC, Qumseya BJ; (ASGE Standards of Practice Committee Chair). American Society for Gastrointestinal Endoscopy guideline on management of post-liver transplant biliary strictures: summary and recommendations. Gastrointest Endosc. 2023;97:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 34. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 534] [Article Influence: 66.8] [Reference Citation Analysis (0)] |
| 35. | Fernandez Y Viesca M, Arvanitakis M. Early Diagnosis And Management Of Malignant Distal Biliary Obstruction: A Review On Current Recommendations And Guidelines. Clin Exp Gastroenterol. 2019;12:415-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/