INTRODUCTION
Initially used purely as a diagnostic tool, laparoscopy evolved rapidly in the 1960s-1980s into a therapeutic technique[1], with the first laparoscopic cholecystectomy in 1985[2] marking a turning point. Today, laparoscopic surgery is the preferred approach for many procedures due to the marked benefits afforded by minimally invasive surgeries[3]. Hence, there have been several innovations aimed at improving laparoscopic imaging for better visualisation of anatomy and in turn, safer surgeries.
Examples such as the progress to high-definition imaging and then 4K imaging has improved the real-time video quality in terms of resolution, which has led to better precision in dissection. Another example is the usage of intravenous indocyanine green (ICG) dye which binds to plasma proteins and can later be detected using infrared (IR) or near-infrared imaging systems for better intraoperative assessment of perfusion[4-6].
Such technological progress has improved surgical efficacy and patient safety over time. 4K ultra high definition systems have shortened operative time and reduced intra-operative blood loss in abdominal surgeries[7-9]. ICG has shown to improve surgical efficacy and minimise untoward errors[10]. In liver resections, it has shown to be useful in recognising hepatic boundaries and useful in preventing liver associated complications[11]. It has also shown to reduce events of anastomotic leaks in colorectal surgeries and reduce the post-operative length stay for these patients[12]. Keeping with the trend of imaging improvements leading to better surgical outcomes, we discuss the more recently added yellow enhancement (YE) mode.
YE mode has emerged as a novel tool that enhances the contrast between adipose tissue and surrounding structures in laparoscopic surgeries[13]. YE requires no additional interventions or drug administration to the patient for its function and it can be conveniently toggled on and off intraoperatively as required. YE’s benefits have yet to be fully established, given its fairly recent introduction, and surgeons have already started evaluating its utility in surgical practice[13].
Technology has been advancing rapidly over the past few decades with innovations as mentioned above, and will continue to develop as the search for stronger patient outcomes and benefits continues. With newer technologies showing improved patient outcomes, it is ideal for surgeons to embrace change and be adaptive to newer methods. Knowing when to use which technologies is the mark of a well-informed clinician who understands the benefits and limitations of the increasing number of resources available.
This review aims to introduce the basis of YE imaging, discuss the advantages of using YE in minimally invasive abdominal surgeries, illustrate the clinical situations where the usage of YE would be especially beneficial, and lastly, explore the potential of YE in surgical education.
WHAT IS YE MODE?
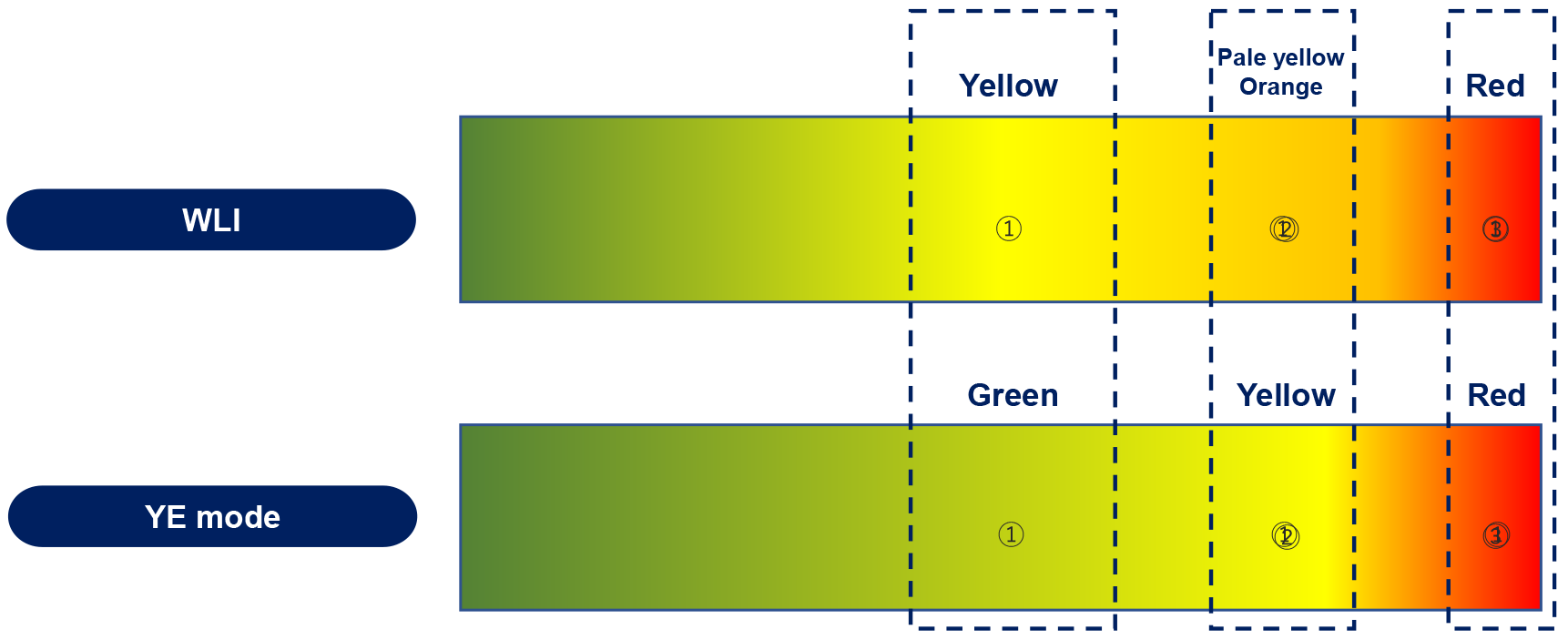
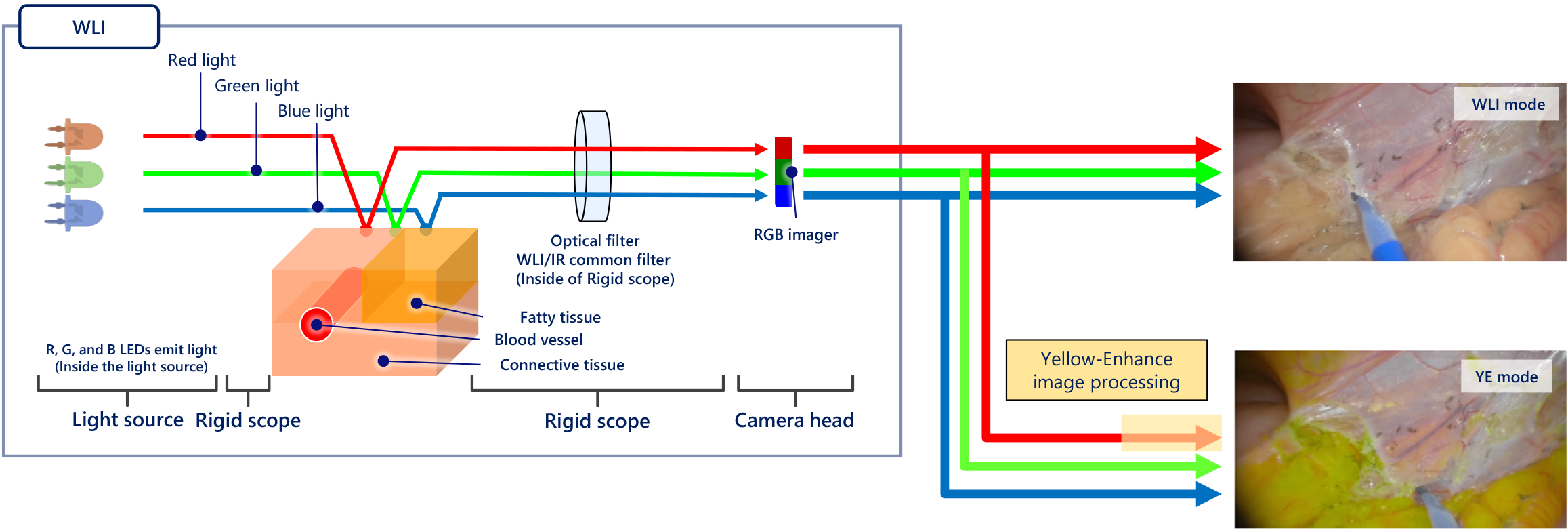
YE is a novel addition to laparoscopic imaging that aims to aid surgeons in improving intraoperative identification of structures during minimally invasive abdominal surgeries. It increases the contrast between colours of structures by making pale yellow-orange structures appear more distinctly yellow (Figure 1). An internal image processor is used to perform colour conversions, changing the pale yellow-orange colour of adipose tissue to a starker fluorescent yellow while retaining the appearance of all other colours (Figure 2)[14]. This colour conversion highlights the differentiation between structures of different shades of yellow and structures of other colours. Such enhancement of the appearance of yellow allows improved ease in the identification of planes between two adipose-heavy tissues. This is particularly useful in abdominal or gastrointestinal surgeries, where abdominal, mesenteric and retroperitoneal fat surrounds critical structures. This may be counterintuitive; instead of highlighting the structures of interest directly, the system highlights the areas around them by changing the colour of everything surrounding the structure itself. Despite this, this enhancement of the colour yellow improves the definition of these interfaces.
Figure 1 Changes in colour between white light mode and yellow enhancement mode done by yellow enhancement's image processor[14].
Image obtained from Olympus corporation. YE: Yellow enhancement; WLI: White light imaging. Citation: Singh H, Suherman R, Koh FH. Yellow enhancement imaging facilitates identification of surgical planes and key structures during challenging high anterior resection in a patient with obesity. Ann Coloproctol 2025. Copyright© The Authors 2025. Published by The Korean Society of Coloproctology Publishers. The authors have obtained the permission (Supplementary material).
Figure 2 The process by which yellow enhancement converts white light images into yellow enhanced images[14].
Citation: Singh H, Suherman R, Koh FH. Yellow enhancement imaging facilitates identification of surgical planes and key structures during challenging high anterior resection in a patient with obesity. Ann Coloproctol 2025. Copyright© The Authors 2025. Published by The Korean Society of Coloproctology Publishers. The authors have obtained the permission (Supplementary material).
A brief example of how highlighting the fat tissue allows for safer abdominal surgeries is seen in Figure 2, where the YE mode allows for clearer identification of the surgical plane of dissection, which is the interface between the yellow fat on the colonic serosa and the white of the posterolateral peritoneal wall.
PRACTICAL USE OF YE
We discuss how the usage of YE allows for better identification of: Surgical planes of dissection; nerves, arteries and veins; lymph nodes in lymphadenectomy; ureters.
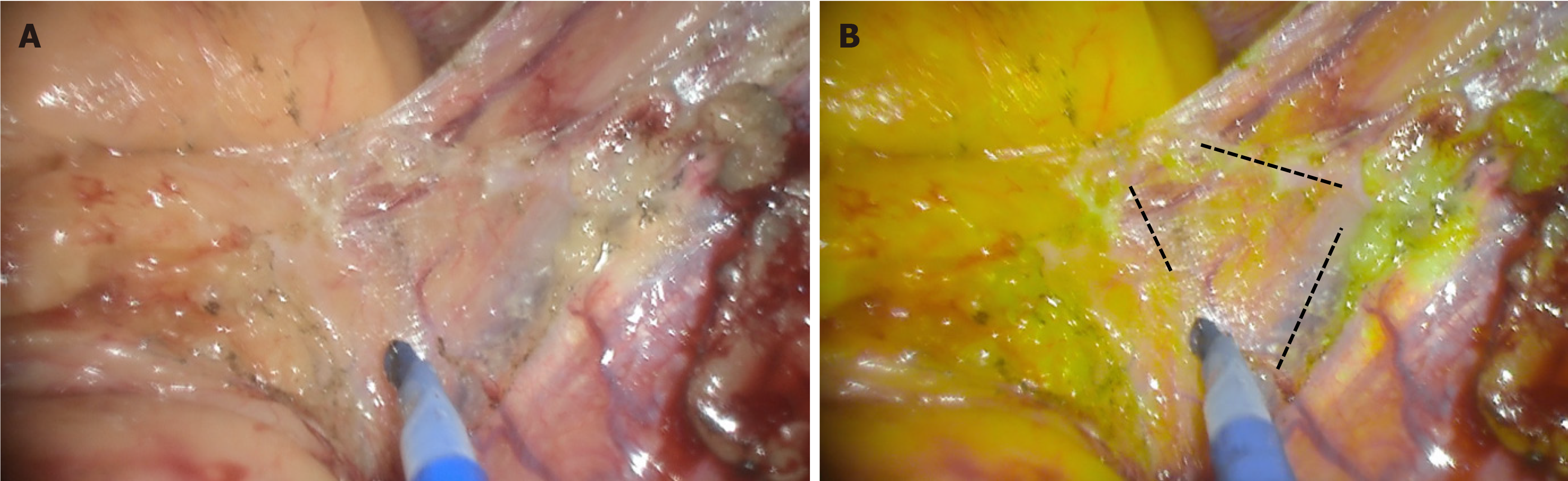
Firstly, on the surgical planes of dissection. YE has benefit in identifying the avascular plane of dissection between the visceral and parietal peritoneum. By colour converting the mesenteric adipose tissue, the true avascular plane becomes more evident as the plane of dissection. This is demonstrated in Figure 3, where the white plane of dissection only becomes clear when the YE mode is turned on. Though it is possible to identify the plane without YE, there is a clearer identification of the transition zone with it, which allows for precise dissections. The enhancement also allows for dissections closer to the bowel wall, as identifying a plane near the bowel wall is made easier. This in turn minimises the risk of injury to other structures, particularly blood vessels. This is especially beneficial in patients with obesity as there is a thickened and short mesentery with greater adipose tissue content which may result in distortion of surgical planes[15].
Figure 3 Surgical planes with white light (left) and yellow enhancement (right)[14].
Avascular segments are easier to identify with YE and allow for safer dissections as indicated by the dotted lines. A: Left; B: Right. Citation: Singh H, Suherman R, Koh FH. Yellow enhancement imaging facilitates identification of surgical planes and key structures during challenging high anterior resection in a patient with obesity. Ann Coloproctol 2025. Copyright© The Authors 2025. Published by The Korean Society of Coloproctology Publishers. The authors have obtained the permission (Supplementary material).
Specific examples where YE finds benefits include the initiation of dissection of right hemicolectomy in the inferior-to-superior appro and medial-to-lateral dissections in Anterior Resections. Both dissections involve identification of the plane between the mesentery and the retroperitoneal fat or structures.
In the right hemicolectomy, the dissection involves mobilising the right colon and its mesentery from retroperitoneal structures, which requires the identification of key landmarks such as the duodenum, Gerota’s fascia and ureter[16]. YE may find benefit in alleviating the challenging nature of complete mesocolic excisions and D3-lymphadenectomy in surgical management of right colon cancer by easier identifications of the medial edge of the superior mesenteric vein covered by lymph adipose tissue[16].
In anterior resections, the medial-to-lateral approach starts the dissection at the root of the inferior mesenteric artery (IMA) and moves laterally to identify and preserve key retroperitoneal structures. We find that these structures may be better identified using YE, especially in patients with obesity, where increased mesenteric fat may complicate the identification of anatomical landmarks and the dissection of vascular structures, thus leading to longer operating times[17,18].
Furthermore, there may be benefits in adhesion debridement. Since adhesions are also avascular, the concept is identical to that of surgical plane dissection. The boundaries of the adhesions become clearer with YE, and even obscured adhesions can be identified and debrided with this.
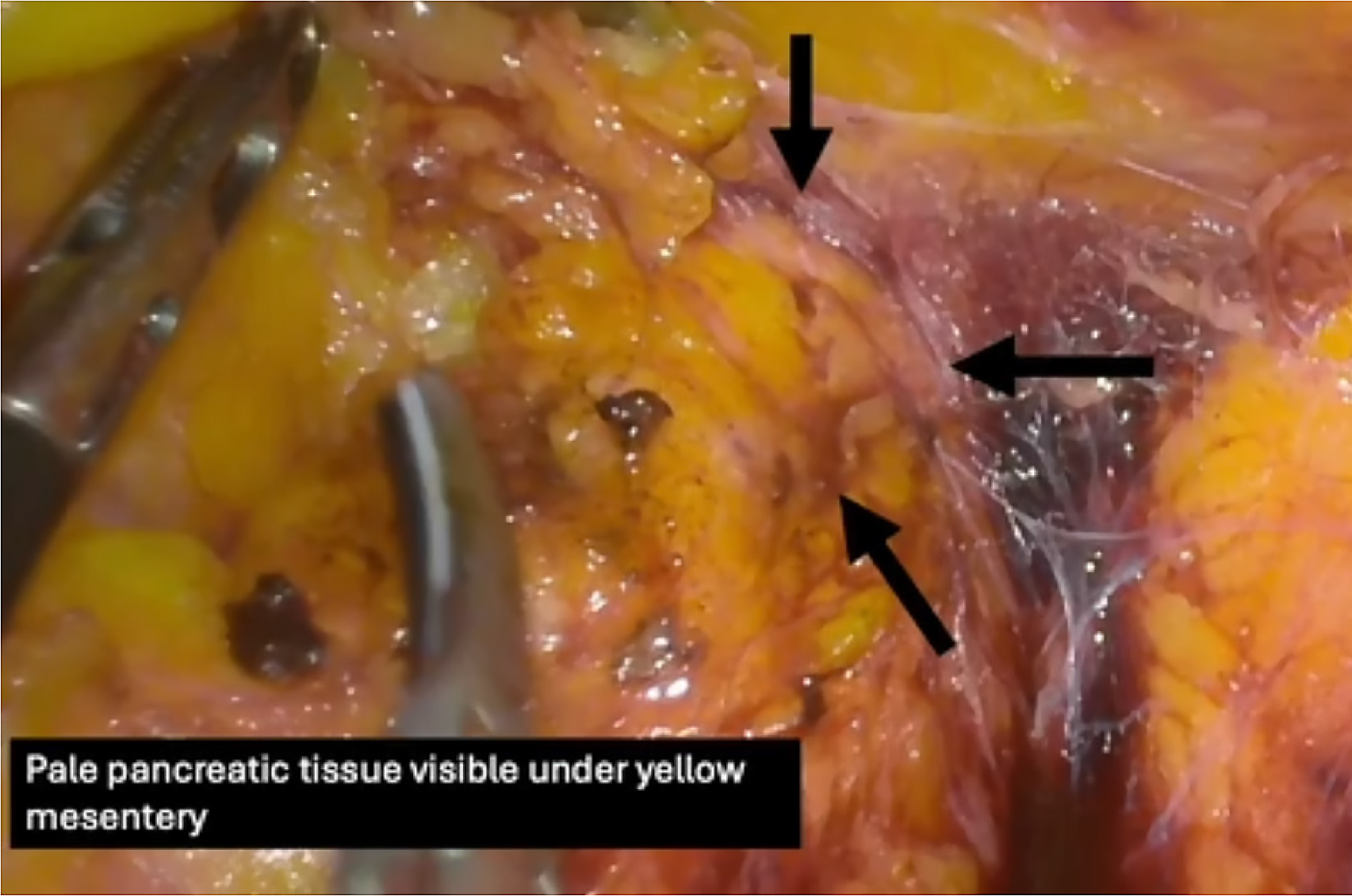
As discussed, the identification of retroperitoneal structures is important in several operations. We see YE’s usage in identifying structures surrounded by visceral adiposities. An example of this is the pancreas. The paler pancreatic tissue can be distinguished from the peripancreatic fat as shown in Figure 4[13]. Upon identification of the pancreatic parenchyma, the dissections around the region can be safer, minimising pancreatic injury. Other examples include identification of adrenals in retroperitoneal laparoscopic approaches for abdominal surgeries and differentiation of prostate and seminal vesicles from the mesorectal and perivisceral fat during total mesorectal excision (TME).
Figure 4 Identification of Pancreatic tissue underneath peripancreatic and mesenteric fat tissue[13].
Citation: Suherman RC, Singh H, Aw DKL, Chong CXZ, Ng JL, Sivarajah S, Tan WJ, Foo FJ, Ladlad J, Khoo N, Tan CHM, Koh FH. The role of yellow enhancement in laparoscopy. Br J Surg 2024; 111. Copyright© The Authors 2024. Published by Oxford University Press on behalf of BJS Foundation Ltd. The authors have obtained the permission (Supplementary material).
With larger amounts of adipose tissue under the Gerota’s fascia, surrounding the adrenals, identification of the adrenals and their vasculature is difficult. Variant adrenal venous anatomy is not uncommon and can also be surrounded by adiposities, making its identification harder[19]. The adrenals are highly vascular structures and damage to these can lead to severe blood loss[20]. Having intraoperative guidance with this identification using YE can reduce risk of damage to the vessels and/or the adrenals, which in turn reduces the risk of severe blood loss and need for transfusions. YE can enhance the adiposities around the vessels and the adrenals and make them easier to identify.
YE may be used in distinguishing the prostate and seminal vesicles against the surrounding adiposities around the Denonvilliers' fascia. The lateral edge of the fascia harbours abundant adipose tissue alongside several tiny neurovascular bundles[21] which can be better distinguished intraoperatively via YE.
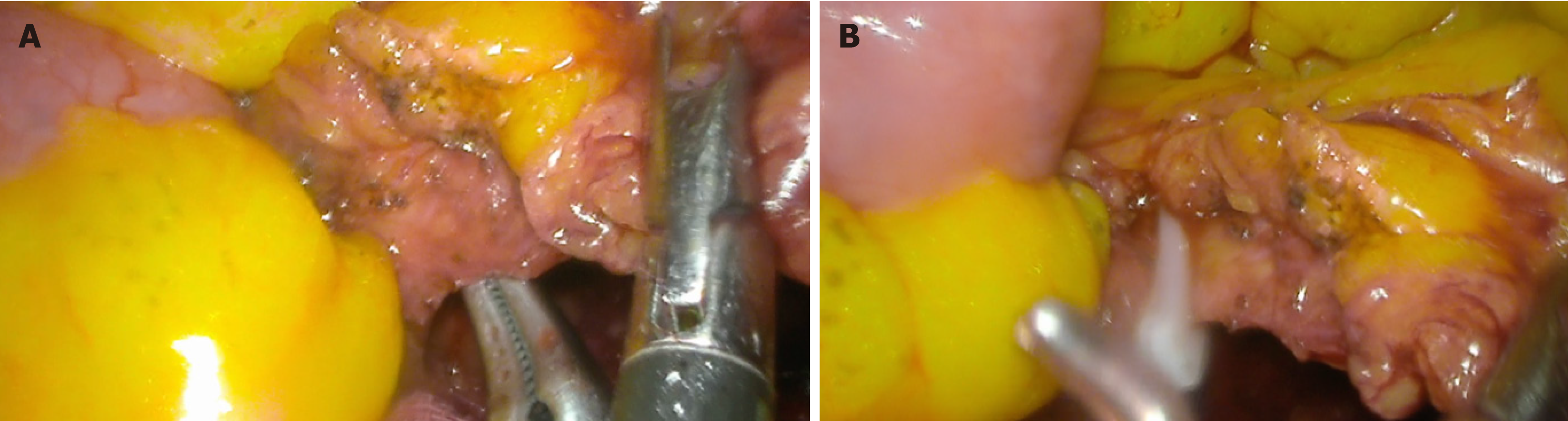
Secondly, on identification of nerves, arteries and veins. Shown earlier in Figure 3, the vessels also appear clearer upon enhancement of the fat, as the contrast between the mesenteric fat and the vessels is stark. Early identification of non-adipose tissue from adiposities intraoperatively can prevent accidental injury to structures and reduce the risk of avoidable bleeding and/or nerve damage. Tracing and clipping of major arteries, such as the superior or inferior mesenteric, the renal or splenic arteries, etc. is part of the procedure for several surgeries, and it is important to ensure this is done safely. Besides this, avoiding these major vessels in some surgeries is also imperative. Figure 5 shows an example of YE’s usage in tracing and clipping of the IMA. The upper border of the IMA is covered with adiposities that had to be dissected carefully. During this dissection, damage to the IMA is avoided by early identification of the pale pink vessel wall as it stands out amongst the sea of yellow adiposity.
Figure 5 Usage of yellow enhancement in tracing and clipping of the inferior mesenteric artery in a laparoscopic anterior resection[14].
A: Demonstration of the skeletonised inferior mesenteric artery amidst a lack of space from encroaching bowel in an obese patient with short colonic mesentery; B: Application of a hemostatic clip on the inferior mesenteric artery. Citation: Singh H, Suherman R, Koh FH. Yellow enhancement imaging facilitates identification of surgical planes and key structures during challenging high anterior resection in a patient with obesity. Ann Coloproctol 2025. Copyright© The Authors 2025. Published by The Korean Society of Coloproctology Publishers. The authors have obtained the permission (Supplementary material).
Not just for major vessels, but the minor vessels shown in Figure 3 can also be avoided and bleeding can be kept to a minimum during the operations via the usage of YE. Smaller vessels are identified early and sealed/clipped prophylactically which in turn reduces blood loss and any need for transfusions, which may have negative oncological impacts[22,23].
Vessels are key landmarks for several colorectal operations and their early identification is greatly beneficial. In a medial to lateral approach for right hemicolectomy, the intersection of the ileocolic vessel pedicle and the superior mesenteric vein is the optimal starting point[24]. For extended right hemicolectomy, the middle colic artery must be carefully skeletonised and ligated at the origin[25]. For left hemicolectomy, it would be the left colic artery and so on. With these vessels being key landmarks, earlier identification and easier tracing and skeletonization via YE would be greatly beneficial ensuring patient safety and reducing operative duration.
Furthermore, identification of nerves is also made clearer with YE as demonstrated in Figure 6, where we can see a hypogastric nerve being identified and, in this case, sacrificed. This nerve identification is useful when identifying the inferior mesenteric plexus and bladder branches to minimise unintentional injury. Preservation of the pelvic autonomic nerves in anterior resections aids in retaining genitourinary function in most patients[26]. It is important to pay great attention to fascial planes and autonomic nerve plexuses to preserve patients’ postoperative sexual, urinary and bowel functions[27]. Damage to the hypogastric nerve plexus commonly happens during ligation of the IMA, dissection of the retrorectal space and division of Denonvilliers' fascia during colorectal, urological and gynaecological procedures[28]. These dissections may benefit from using YE to preserve the autonomic nerves.
Figure 6 Hypogastric nerve being identified and, in this case, sacrificed[14].
Citation: Singh H, Suherman R, Koh FH. Yellow enhancement imaging facilitates identification of surgical planes and key structures during challenging high anterior resection in a patient with obesity. Ann Coloproctol 2025. Copyright© The Authors 2025. Published by The Korean Society of Coloproctology Publishers. The authors have obtained the permission (Supplementary material).
Thirdly, on the benefits of YE in lymphadenectomy. The dissections at the fat package away from the vessels is also useful in lymphadenectomy in hepatopancreatobiliary and upper gastrointestinal oncological surgeries where lymph node stations are along the arterial supplies. This may increase lymph node yields in dissections[29] and keep any bleeding risk to a minimum. In gallbladder cancer surgeries, the technique described for lymphadenectomy involves dissections along the posterior aspect of the duodenum, followed by the head of pancreas and superiorly to the retroportal area[30]. For colorectal surgeries, briefly touched on earlier, complete mesocolic excisions and D3-lymphadenectomy[16] and TME may benefit from YE as these regions are difficult to dissect along due to high adipose tissue content. For laparoscopic gastrectomy, high pre-operative visceral fat area has shown to predict intraoperative adverse events during lymphadenectomy[31], particularly in the infrapyloric and suprapancreatic regions. Having a tool to specifically distinguish adipose tissue would be beneficial for such regions to minimise intraoperative adverse events. However, specific studies on YE’s benefit in lymph node identification for lymphadenectomies are currently limited.
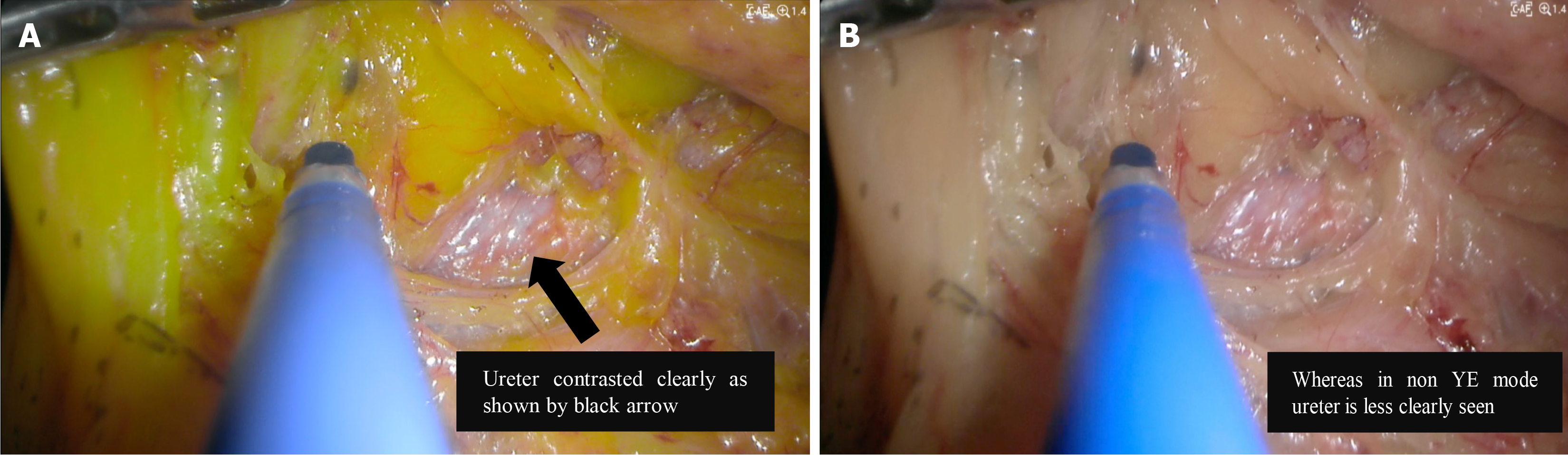
Lastly, on identification of ureters intraoperatively. The ureters must be identified to ensure no damage has occurred especially in colorectal and gynaecological surgeries[32,33]. In trans abdominal laparoscopic surgeries, some suggest ureteral catheter placements for better identification of the ureters and in turn lower risk of iatrogenic injury to them[34,35]. In gynaecological surgeries, higher adipose tissue content has led to more difficult identification of the left ureter[36]. For colorectal surgeries, it was discussed earlier on how adiposities make distinguishing retroperitoneal structures, including the ureter, in Right Hemicolectomies and Anterior Resections more difficult. We therefore see the advantage of YE in assisting in this identification of the ureters as the contrast with the adiposities around make them much easier to identify, as demonstrated in Figure 7[13]. In many cases, significant adipose tissue dissection needs to be done to reach the ureters and identifying the ureter quickly during this dissection minimises the risk of injury as well as reduces operative time.
Figure 7 Identification of ureters with and without yellow enhancement[13].
A: With yellow enhancement; B: Without yellow enhancement. Citation: Suherman RC, Singh H, Aw DKL, Chong CXZ, Ng JL, Sivarajah S, Tan WJ, Foo FJ, Ladlad J, Khoo N, Tan CHM, Koh FH. The role of yellow enhancement in laparoscopy. Br J Surg 2024; 111. Copyright© The Authors 2024. Published by Oxford University Press on behalf of BJS Foundation Ltd. The authors have obtained the permission (Supplementary material).
With clearer identification of anatomical structures and planes of dissection, YE may show increased surgical precision, surgical efficiency and patient safety. Earlier identification of vasculature and easier tracing and skeletonization of those vessels leads to increased surgical efficiency where tracing vessels to their roots is required. It also improves patient safety and patient outcomes as unintentional damage to vessels, autonomic nerves or other retroperitoneal structures such as the ureters or pancreas is avoided.
THE POTENTIAL CLINICAL INDICATIONS FOR AND ADVANTAGES OF YE
Since YE can be turned on and off intraoperatively, the surgeon can decide whether there is a need to use it throughout the surgery or only some select parts of the surgery where they find the structures they wish to identify are more obscured. Areas in which YE may be beneficial for identification of surgical planes, neurovascular structures, parenchyma and nerves have been discussed above. However, not all patients or dissections involving identification of those regions would need YE. Specific situations we find may benefit most from the use of YE include: Regions with larger amounts of adipose tissue-such as in mesorectal dissection and peripancreatic dissection; patients with obesity that may have thickened and shortened mesentery; Intraoperative identification of anomalies-aberrant anatomy, adhesions.
Firstly, we find that YE may have benefits in mesorectal dissections or excisions. A good understanding and identification of the fascial planes around this region is necessary as proper dissections along these planes enable a “bloodless” operation[37]. This holds true for TME, abdominoperineal resections and anterior resections[37]. The mesorectum tapers distally, particularly in the lower rectum, which makes consistent plane identification difficult[37,38]. We find that the easier identification of surgical planes with the usage of YE would show benefits in consistently dissecting along the correct planes. A big concern with mesorectal dissections is the amount of adiposity surrounding it, with the mesorectal fat area being correlated with more difficult surgery and longer operating times[39]. We see that being able to distinguish the parenchyma and surgical planes from the adiposity in the region is important for safer surgeries and for potentially reducing operating time. Dissections in this region may be even more difficult for patients with obesity which is discussed below.
The pancreas is another region where surgeries are difficult due to larger amounts of adipose tissue. Navigating around peripancreatic fat without causing injury to the pancreas is very important in laparoscopic abdominal surgeries. Intraoperative injuries to the pancreas may result in post-operative acute pancreatitis[39] or even pancreatic fistula formation. High visceral fat area[40] and high body mass index[41] are known risk factors for pancreatic fistula formation. The risk factors mentioned make it difficult to identify the contour of the pancreas and to distinguish pancreatic tissue from the surrounding fat tissue[40,42,43]. Hence, we believe in using YE to identify the pancreatic parenchyma, avoiding any injury to the pancreas. Thus, YE can potentially reduce the rates of post-operative pancreatic complications such as fistula formation and acute inflammation, but there is insufficient data and usage yet.
YE also shows promise in laparoscopic abdominal surgeries for patients with obesity. Patients with obesity have additional challenges during laparoscopic surgeries. There is reduced space for manoeuvrability and bowel retraction, leading to impaired visualisation[44]. There is an increased risk of nerve injury due to compression or traction[44]. The thick and excessive omentum and mesentery make access to the deeper areas of the pelvis limited, alongside access to the splenic and hepatic flexures[15]. This leads to a higher incidence of bleeding and a distortion of surgical planes. In gynaecological surgeries, it was found that the course of the inferior epigastric vessels is more difficult to identify in overweight patients[36]. With views being obscured and vessels being much harder to identify, a longer operation duration is expected in obese patients which comes with its own anaesthesia related risks[45]. With a larger amount of adipose tissue and limited retraction of bowels, there is not much buffer for the dissection line and the identification of this plane is very important. With YE, this dissection line is much easier to identify as the white plane to cut amongst the contrasted fluorescent yellow appearing mesenteric fat. The vessels also stand out with the enhancement and can be avoided in dissections. With quicker identification of structures and planes, we expect operation times to be reduced as well. However, whether this reduction in time would be significant or not is yet to be explored.
YE’s potential in usage for intraoperative identification of anomalies is currently limited. Regarding adhesions, the concept is like the identification of avascular planes. The adhesions appear white or pale and stand out on a background of yellow adipose tissue, making them easy to identify intraoperatively. Hence, YE’s use in adhesion debridement is not unexpected. A possible use of YE in anomaly identification might be aberrant anatomies, specifically on aberrant vasculature. Patients with intestinal malrotation have aberrant vasculature which in turn makes abdominal surgeries very difficult. Balachandran et al[46] found that only 44.7% of cases of patients with intestinal malrotation with colon cancer worldwide were managed laparoscopically out of concerns regarding vasculature anatomy[46]. Aberrant vasculature has led to complications involving severe bleeding before[47], and we find that YE would be a great help in avoiding such complications. Even if the vessel variations have been mapped out pre-operatively via imaging, we found that having YE intraoperatively as a tool to identify and correlate with the pre-operative imaging is beneficial. We hope that with more use over time, YE would help boost the confidence of surgeons looking to operate on patients with anatomical variations and aberrant vessels laparoscopically.
With increased usage of YE over time, we expect more clinical indications and advantages of the technology to surface. We find that the usage of YE may lead to improved surgical outcomes with lesser complications, reduced intraoperative injuries, reduced operative time and aid in dissections around areas with higher adipose tissue-i.e. mesorectal dissections and peripancreatic dissections.
To a smaller extent, we find some potential in reducing surgeon fatigue with YE, as constant checking for surgical planes or crucial structures is made significantly easier. Since the surgeon does not need to strain their eyes to source the structures, they might find comfort in using YE.
THE POTENTIAL OF YE IN SURGICAL EDUCATION
The enhancement of surgical planes would be good to demonstrate to novice surgeons on what they should be looking out for during dissections. Seasoned surgeons can use YE to educate residents or students on what makes certain surgeries difficult and why identifying planes of dissections is imperative for patient safety. Examples of where this might be useful include mesorectal surgeries where sharp dissection along the embryonic plane is necessary[48] and where the mesorectum tapers distally and causes problems in consistent plane identification[37,38]. YE may also be used in demonstrating anatomical variations as it enables junior residents or students to visualise the surrounding structures better and therefore better appreciate the variations.
Globally, surgical training is long and tiring which length of post-graduate training ranging from 4 to 10 years. This training can be tiring with work hours per week going up to 88 hours and some countries having no limit[49]. During this training, surgeons It is understood that at the beginning of surgical training, the learning curve is difficult to mount with surgical times being long initially before having a rapid drop after some experience. It was found that surgical times rapidly dropped after the first 20 Laparoscopic cholecystectomies before plateauing at approximately 60 cases[50]. Similar findings of 60 cases to plateau in timing was noticed on a different study looking at appendectomies[51]. Some of the problems surgical trainees have reported facing include the identification of planes and senior surgeons have discussed trainees being in incorrect planes and needing to guide them rather frequently[52]. Even graduate trainees entering surgical subspeciality training in North America were found to have trouble with surgical plane identification with 26% of program directors stating that they found their trainees to be having trouble with it[53].
With YE, we find that some of these difficulties may be alleviated as it is a helpful tool in teaching the identification of the surgical planes. We hope that with the usage of YE, the learning curve is alleviated. We understand that it is not a complete solution as surgical training involves much more than surgical plane and structure identification. Nonetheless, YE would be of great help in resolving some of the issues newer residents and surgeons may have and can be a great teaching tool. With a portion of the learning curve becoming easier to mount, we can hope for reduced surgical times at a faster rate and indirectly, earlier competency with fewer working hours.
Hence, YE may help junior surgeons or junior residents with their confidence in surgical planes and allow them to know with greater certainty that their dissections are safe. This is especially for situations where the fat tissue content is high, and they may be unsure of their dissections without YE clarifying the images. YE also helps residents and surgeons working long hours that are fatigued to be able to still carry out safe surgeries and aims to reduce the fatigue and learning curve for junior surgeons. There is also potential in integrating YE into curriculums to better teach surgical plane of dissection.
The image output that YE provides is very different from the white light imaging that seasoned surgeons are used to. Hence, there might be pushback on the usage of YE due to its unusual appearance. However, pushbacks on newer technologies are usually due to reasons like additional workload for the surgeons or additional trainings required for technology usage without proportional gain by using the technology[54], which are absent in the case of YE. Since YE can be toggled on and off intraoperatively, the surgeons that find it to be too out of the ordinary for them are able to turn it off at will. To truly reap the benefits of any new technologies, a positive attitude and an open mind are necessary. We hope attempts to use YE would be made by such surgeons, and we hope they find the abovementioned benefits to its usage.
Another possible disadvantage to YE is the possibility of visual fatigue as the colours may be too bright and contrasted. Prolonged exposure to warm fluorescent lighting may be linked with visual fatigue[55,56]. Therefore, while starting out with YE, it might be suitable to only turn it on during stages of operations where dissection around fat is expected to be more extensive and toggled off when dissection is complete or at a minimal-i.e. during anastomosis creation, during intracorporeal stitching, measuring bowel lengths, etc. to reduce visual fatigue. Indications for when to turn it on or situations when turning it on may be useful have been discussed in this review.
A limitation for this review however, is that there are currently only a few studies that discuss the usage of YE in laparoscopic surgeries. Most of the discussion is centered around potential benefits and areas of usage that have yet to be fully established. We found several gaps and/or areas in which complications may arise in current surgical methodologies and we discussed how YE may bridge the gaps and reduce its complications. While YE’s full capabilities may not have been tested, surgeons have already been using it for their operations and we see great potential in this technology for producing safer patient outcomes and reduced operative durations.
CONCLUSION
YE mode is a novel light mode in laparoscopic surgery that enhances the identification of surgical planes, neurovascular structures, and critical abdominal structures, that has the potential of improving surgical efficiency, safety and education. It is particularly beneficial in cases with high adipose tissue content.
ACKNOWLEDGEMENTS
The authors acknowledge the support provided by Sengkang General Hospital, Singapore and Singhealth which facilitated this research.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report’s classification
Scientific Quality: Grade B, Grade B, Grade C
Novelty: Grade B, Grade B, Grade B
Creativity or Innovation: Grade B, Grade B, Grade B
Scientific Significance: Grade B, Grade B, Grade B
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
P-Reviewer: Shukla A; Wu CE S-Editor: Liu H L-Editor: A P-Editor: Zhang L