Published online Jul 16, 2025. doi: 10.4253/wjge.v17.i7.107746
Revised: April 17, 2025
Accepted: May 21, 2025
Published online: July 16, 2025
Processing time: 103 Days and 4.3 Hours
Minimally invasive endoscopic resection techniques are the recommended first-line treatment strategy for the majority of large non-pedunculated colorectal polyps, with endoscopic mucosal resection (EMR) as a predominant resection modality due to its efficacy, efficiency, safety, and cost-effectiveness. A limitation of EMR is recurrence, which has historically occurred in 15%-20% of lesions. In the past 10 years, a number of effective mitigating strategies have been developed, including margin thermal ablation using snare-tip soft coagulation, argon plasma coagulation (APC), and hybrid-APC, alongside margin marking pre-resection. Moreover, techniques for effective recurrence management have also been deve
Core Tip: Endoscopic resection techniques are the recommended treatment strategy for most large non-pedunculated colorectal polyps, with endoscopic mucosal resection as a predominant resection modality. However, recurrence has historically occurred in 15%-20% of lesions. Margin thermal ablation and pre-resection margin marking can effec
- Citation: Pang S, Tavakoli P, Shahidi N. Prevention and treatment of recurrence after endoscopic resection of large non-pedunculated colorectal polyps. World J Gastrointest Endosc 2025; 17(7): 107746
- URL: https://www.wjgnet.com/1948-5190/full/v17/i7/107746.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i7.107746
Colorectal cancer is the second leading cause of cancer-related mortality worldwide[1]. During routine colonoscopy, large non-pedunculated colorectal polyps (LNPCPs) (≥ 20 mm) are identified as precursor lesions to colorectal adenocarcinoma and often require endoscopic or surgical resection. Endoscopic resection has emerged as the preferred first-line treatment for LNPCPs, offering comparable efficacy but superior safety to traditional surgical approaches[2-4]. The selection of an appropriate endoscopic resection technique is guided by a comprehensive clinical evaluation of lesion-specific characteristics, including predicted histopathology and the estimated risk of submucosal invasive cancer[5].
While numerous endoscopic resection techniques have been developed, endoscopic mucosal resection (EMR) is often the preferred technique for most LNPCPs[6-8]. EMR is safe and cost-effective and offers a less invasive approach with a lower risk of procedure-related complications than surgical resection[9-11]. However, recurrence following EMR remains a significant limitation, with reported rates of approximately 15%-20%[6,12,13]. This underlines the importance of optimizing recurrence mitigation and treatment as well as surveillance protocols to improve long-term clinical outcomes.
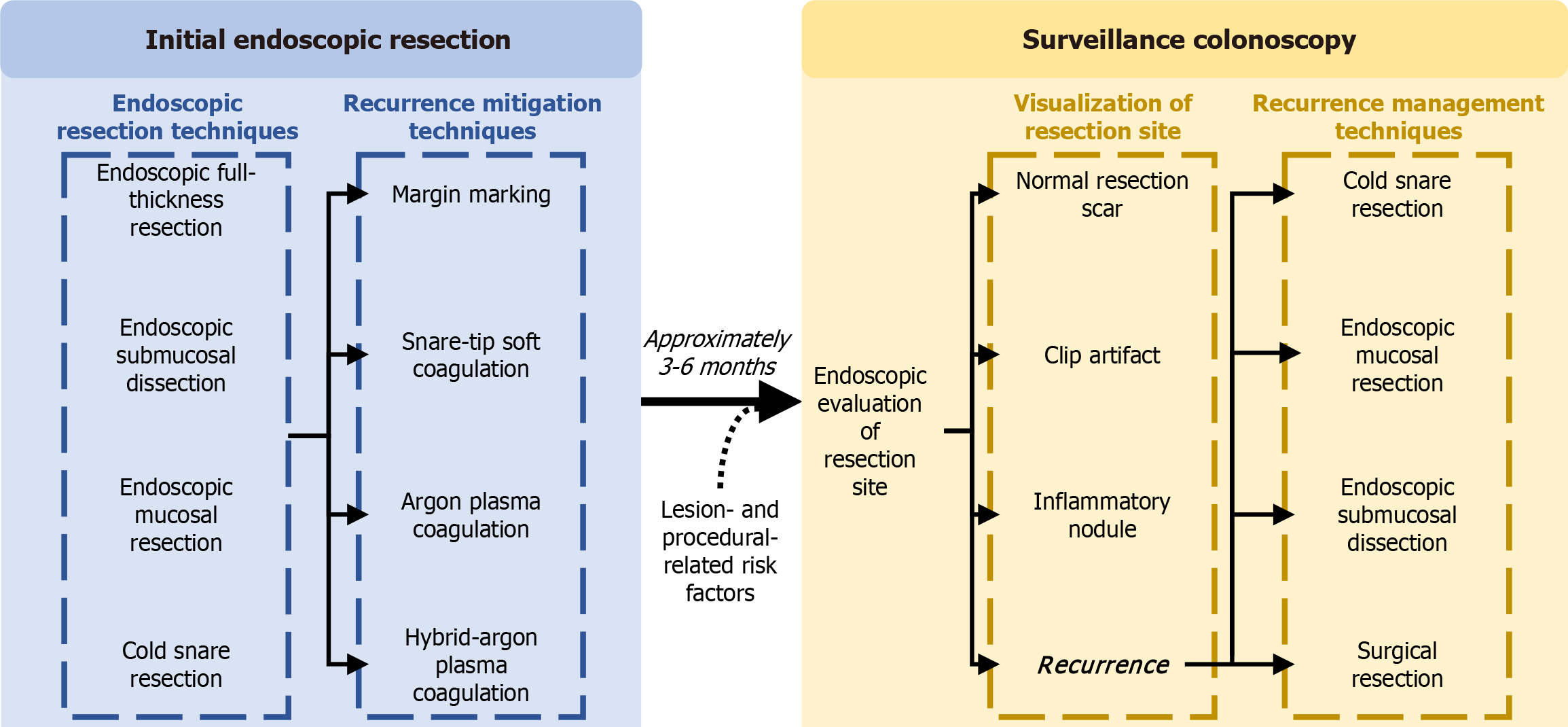
Recurrence currently poses a major drawback of EMR as it increases the likelihood of adverse outcomes[14,15]. Given its clinical significance, recurrence remains a primary outcome in most analyses when assessing the efficacy of endoscopic resection techniques. Ongoing innovations in mitigation modalities aim to reduce recurrence rates. Additionally, established techniques for managing recurrent lesions continue to evolve, enhancing treatment outcomes. This comprehensive review will examine the current evidence on the risks and etiologies of recurrence, explore strategies for recurrence mitigation, and evaluate the efficacy of endoscopic recurrence management (Figure 1).
Risk factors for recurrence following endoscopic resection of LNPCPs include a range of lesion-related and procedure-related factors. Understanding the risk factors associated with recurrence after endoscopic resection of LNPCPs enables endoscopists to identify patients at higher risk, guiding clinical decision-making.
Lesion size has consistently been demonstrated as a significant risk factor for recurrence, with larger lesions having a higher recurrence rate[16-18]. A prospective study evaluating 1000 LNPCPs identified that lesions > 40 mm were associated with a higher risk of recurrence during surveillance colonoscopy when compared to 20 mm lesions [odds ratio (OR) = 8.22, 95%CI: 3.90-17.3, P < 0.001]; however, recurrent lesions observed in this study were typically diminutive and endoscopically manageable[12]. Similarly, a retrospective study of 165 LNPCPs found that lesion size ≥ 30 mm was a risk factor for early recurrence at 3-6 months in a univariate analysis (P = 0.021) and lesion size ≥ 40 mm was a risk factor in both multivariate (P = 0.018) and univariate analyses (P = 0.006)[6]. Notably, early recurrence has different risk factors compared to late recurrence[19]. When examining recurrence at different surveillance intervals, recurrence was detected in 8.3% of cases at 3-6 months, 6.1% at 12 months, 6.4% at 24-36 months, and 13.5% at 36 months. For these intervals, lesions occupying ≥ 75% of the luminal circumference were an independent risk factor for recurrence detected at 3-6 months (OR = 5.6, P < 0.001), while lesions > 60 mm were a significant risk factor for late recurrence (OR = 6.3, P < 0.001), demonstrating the importance of scheduled surveillance in the years following endoscopic resection. The increased risk of recurrence associated with larger lesions may be due to the increased likelihood of incomplete endoscopic resection, resulting in microscopic residual tissue[20,21]. Likewise, the number of synchronous lesions requiring resection is also a risk factor for recurrent lesions as it increases the likelihood of incomplete removal of all lesions.
Lesions with high-grade dysplasia (HGD) and/or villous features have an increased risk of recurrence[20,22,23]. HGD at the initial endoscopic resection is an independent risk factor for early recurrence at 3-6 months (OR = 3.89, 95%CI: 1.10-13.60, P = 0.034)[6]. Villous features were associated with recurrence in univariate analysis (OR = 2.09, 95%CI: 1.10-3.97, P = 0.023), but not in multivariate analysis[23]. In one prospective study of 237 patients, the recurrence rate of a tubulovillous lesion was nearly five times higher than that of a tubular lesion (OR = 4.75, 95%CI: 1.43-15.74, P = 0.011)[24].
Lastly, the location of the lesion is correlated with the risk of recurrence[25]. Two studies have demonstrated that polyps in the ascending colon have a greater risk for recurrent lesions than in the descending colon[26,27]. However, other studies could not replicate these results[24,28]. Additionally, differing accessibility to the lesion during resection can also play a factor in the risk of recurrence[29]. Complex accessibility is defined as polyps located in difficult-to-reach sites, such as the appendiceal orifice, ileocecal valve, or anorectal junction, and was associated with recurrence in univariate analysis (OR = 3.925, 95%CI: 1.782-8.642, P = 0.001). Accessibility is an important factor in completing endoscopic resection; difficult polyp access can lead to an increased risk of incomplete resection, resulting in residual tissue and recurrent lesions at follow-up.
As resection techniques evolve, studies continue to evaluate the safety, effectiveness, and recurrence rates of different techniques and modalities. In a cohort of 111 piecemeal resected lesions and 146 en bloc resected lesions, lesions that had undergone piecemeal resections were at a higher risk of recurrence (P < 0.001)[30]. However, piecemeal resections are often performed depending on the size, location, and circumferential involvement. Therefore, these results should be interpreted as two separate techniques with different recurrence risk factors rather than a direct comparison of the two techniques. Retrospective analyses have also found that piecemeal resection is a significant risk factor for recurrence in univariate analysis (OR = 3.741, 95%CI: 1.139-12.292, P = 0.01)[26]. However, no significant results were discovered in multivariate analysis, further demonstrating that piecemeal and en bloc techniques may have confounding factors to their selection and use and, therefore, have different associations with other risk factors and the overall risk of recurrence. When examining recurrence at successive surveillance intervals, piecemeal resection was a significant risk factor for late recurrence (OR = 4.4, P = 0.04)[19]. In a large prospective study with 1412 colorectal lesions, piecemeal resection was identified as a risk factor in univariate analysis (OR = 23.7, 95%CI: 8.72-57.8, P < 0.001) and an independent risk factor in multivariate analysis (OR = 24.3, 95%CI: 9.07-65.4, P < 0.001)[23]. In a recent retrospective study of 2557 lesions, these results were further supported, as recurrence rates of piecemeal resections were significantly higher than the rates of en bloc resections (2.8% vs 0.3%, P < 0.0001)[31]. However, it’s important to note the distinction in endoscopic resection methods in different countries. While en bloc resection is the preferred technique in Eastern countries, piecemeal EMR is more widely used in Western countries[32,33]. The difference in technique preference and potential difference in endoscopist training may play a role in these differing results.
Other resection techniques have also been compared for recurrence rates. Cold-snare resection (CSR) has been gaining popularity due to its safety profile since it does not incur any electrocautery-related complications. While this is a potential alternative, a randomized controlled trial of 177 LNPCPs demonstrated that CSR lacks efficacy as recurrence rates are higher in lesions removed with CSR (16/87, 18.4%) when compared to lesions removed with EMR [1/90, 1.1%; relative risk (RR) = 16.6, 95%CI: 2.24-122, P < 0.001][34]. Another randomized controlled trial involving 396 LNPCPs across 19 different centers revealed similar results; recurrence rates were significantly higher in lesions removed with CSR compared to EMR (23.7% vs 13.8%; OR = 1.94, 95%CI: 1.12-3.38, P = 0.020)[35]. Both trials demonstrate the superiority of EMR to CSR in reducing recurrence rates, making EMR a preferred approach in larger, recurrence-prone lesions.
The etiologies of recurrence following endoscopic resection of LNPCPs can stem from a combination of procedural factors. Most notably, procedural factors that impact the complete removal of a lesion can play a large role in recurrent or residual tissue. Understanding these underlying causes is crucial in identifying effective mitigation strategies and improving patient outcomes. Endoscopists can refine procedural techniques, enhance the effectiveness of resection, and implement interventional techniques to mitigate recurrence.
The leading theory for recurrence is incomplete removal of all polypoid tissue at index endoscopic resection[21,36]. Incomplete lesion removal could be due to technical difficulty or microscopic tissue unnoticed at the resection base or margin. Technical difficulty or procedural complexity, such as limited positioning of the colonoscope and the snare or access to the lesion, can lead to difficulty in completely removing the lesion. These difficulties can lead to undetected macroscopic residual tissue left on the resection defect, resulting in recurrence[37]. One retrospective study examined 41 patients and discovered microscopic residual tissue in visually normal margins for 8/41 lesions (19%) and residual tissue in visually normal base defects for 5/21 lesions (24%), confirming that microscopic lesion tissue does exist and may be a leading mechanism for recurrence[38]. A prospective study with 1427 patients identified that the incomplete resection rate was significantly higher for large polyps than for small polyps (17.3% vs 6.8%; RR = 2.1)[39]. A more recent study with 233 patients demonstrated that an incomplete resection can increase the risk for recurrence by a factor of 2.2 (95%CI: 1.5-3.4)[21]. In the same study, Pohl et al’s multivariable analysis showed incomplete resection was significantly asso
Effective strategies have emerged to improve resection outcomes and reduce recurrence during surveillance, such as margin thermal ablation, which includes snare-tip soft coagulation (STSC), argon plasma coagulation (APC), hybrid-APC (h-APC), and pre-resection margin marking. Studies on these mitigation techniques have continuously shown improved clinical outcomes during the surveillance colonoscopy[2,40-42].
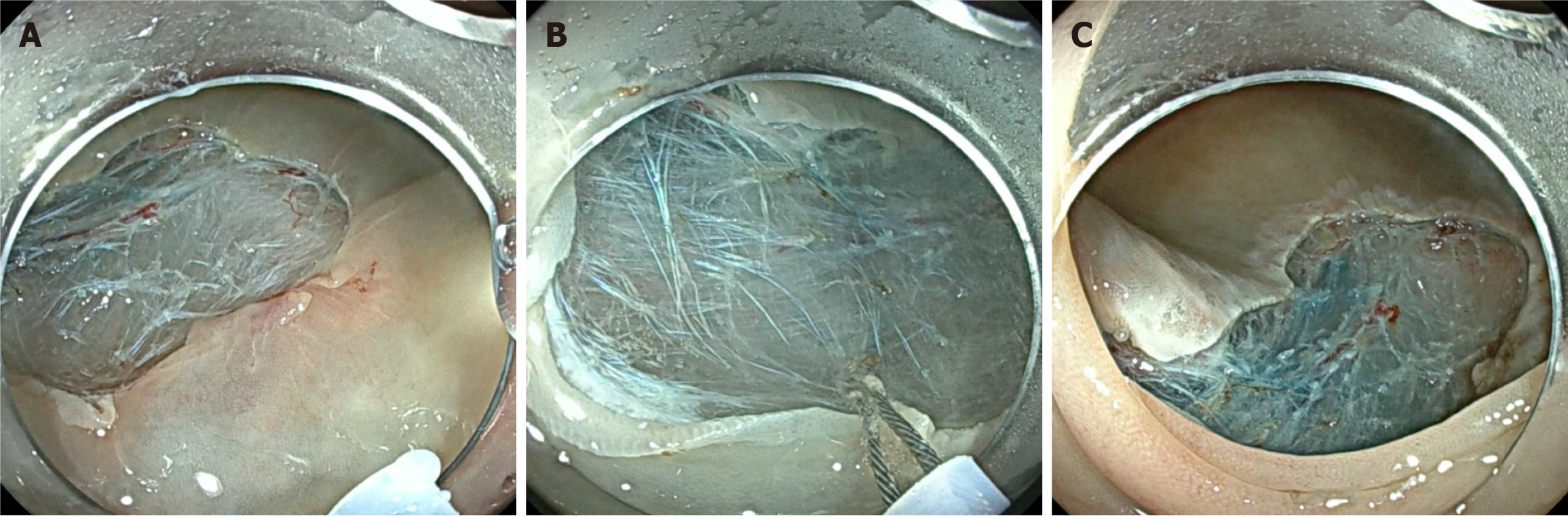
STSC is one method of margin thermal ablation, which utilizes soft-coagulation settings to cauterize potential microscopic residual tissue around the margin of the defect post-resection (Figure 2). Its popularity rose as multiple studies demonstrated its efficacy and safety as a recurrence reduction technique. In a randomized trial of 416 LNPCPs, STSC showed a significantly lower recurrence rate at first surveillance colonoscopy (5.2%) compared to the control group (21.0%; P < 0.001)[43]. One quality improvement project examined recurrence before and after STSC training of 120 LNPCPs to remove the variability of recurrence rates between different endoscopists[44]. Univariate analyses demonstrated STSC as a significant predictor of adenoma recurrence, with recurrence in the post-training group significantly lower than the pre-training group in both univariate (OR = 0.3, 95%CI: 0.11-0.80) and multivariate analyses (OR = 0.2, 95%CI: 0.12-0.92). In fact, lesions that have undergone a successful resection with STSC and have no recurrence at the first surveillance colonoscopy are unlikely to develop late recurrence in subsequent colonoscopies, demonstrating its efficacy in long-term surveillance[45].
Moreover, systematic reviews and meta-analyses demonstrate consistent results for STSC on recurrence rates across studies. When directly comparing lesions treated with EMR and STSC to lesions only treated with EMR, STSC had protective effects on recurrence rates (OR = 0.18, 95%CI: 0.13-0.26, P < 0.001)[46]. Kandel et al[47] examined the recurrence rate at the first follow-up between 534 lesions using STSC post-resection and 514 lesions with no adjuvant treatment. Focusing on LNPCPs, the recurrence rate in the STSC group was 0.06 (95%CI: 0.05-0.09) and 0.22 (95%CI: 0.18-0.26) in the non-STSC group. In another meta-analysis, STSC post-resection demonstrated a 73% protective effect on recurrence rates (OR = 0.27, 95%CI: 0.18-0.42, P < 0.001)[48]. Recently, another prospective cohort of 1872 LNPCPs has been published, further demonstrating that lesions treated with standardized margin thermal ablation had significantly lower recurrence rates compared to those with no margin thermal ablation applied (2.1% vs 13.5%, P < 0.001)[49].
APC serves as another method of margin thermal ablation. One retrospective study of 246 patients demonstrated a low recurrence rate after using APC (4.5%)[50]. It also showed a potential advantage to STSC as it is a contactless modality, decreasing the difficulty of ablating areas compared to STSC. In the same study, APC also demonstrated no increase in adverse events or excessive injury, which may be due to the distance created using an endoscope cap. In a randomized controlled trial with 21 lesions, fewer recurrences were reported in LNPCPs undergoing APC treatment post-resection compared to LNPCPs that received no adjuvant treatment (10% vs 63%, P = 0.02)[51].
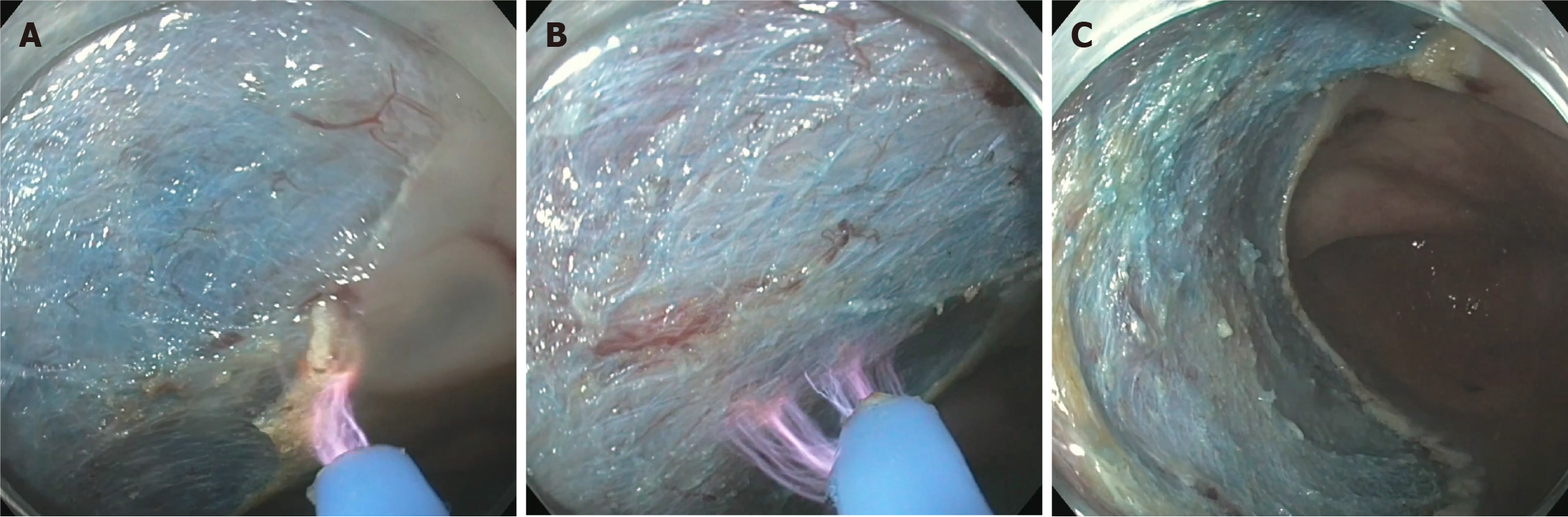
While both STSC and APC have shown effectiveness in reducing recurrence, h-APC has been gaining traction as a technique to ablate both the margin and base of the defect (Figure 3). In a pilot observational study with 44 LNPCPs, 7.5% of patients experienced post-polypectomy bleeding, which is comparable to resections receiving no adjuvant treatment, and 0% of patients had recurrence during their follow-up[41]. In a prospective multicentre study, h-APC had a high technical success rate, a low adverse event rate, and a low recurrence rate[52].
Multiple meta-analyses have demonstrated that margin thermal ablation techniques, specifically STSC and APC, reduce recurrence rates compared to resections with no adjuvant techniques[53-55]. One large meta-analysis included 34 prospective studies and demonstrated that margin thermal ablation with EMR resulted in a 3.3% recurrence rate, significantly lower than standard EMR with no mitigation treatment (15.2%)[14]. When comparing STSC and APC, one retrospective study of 101 patients observed no statistical difference in recurrence rates[56]. Furthermore, a recent meta-analysis analyzed five randomized controlled trials, emphasizing the non-inferiority in clinical outcomes between the two techniques[57]. However, STSC may be preferred over APC due to more supporting literature, lower recurrence rates, and more cost efficiency. One randomized controlled trial of 414 LNPCPs demonstrated that STSC and APC showed no significant difference in recurrence rate and adverse events; however, STSC was faster to apply, cost less, and reduced plastic waste as APC required an additional device[58].
More research is required to evaluate the comparative performance of STSC and h-APC. Based on the current literature, STSC has been consistently demonstrated as a safe, effective, and cost-friendly mitigation technique and should be considered the standard of care to prevent recurrent lesions[15,59].
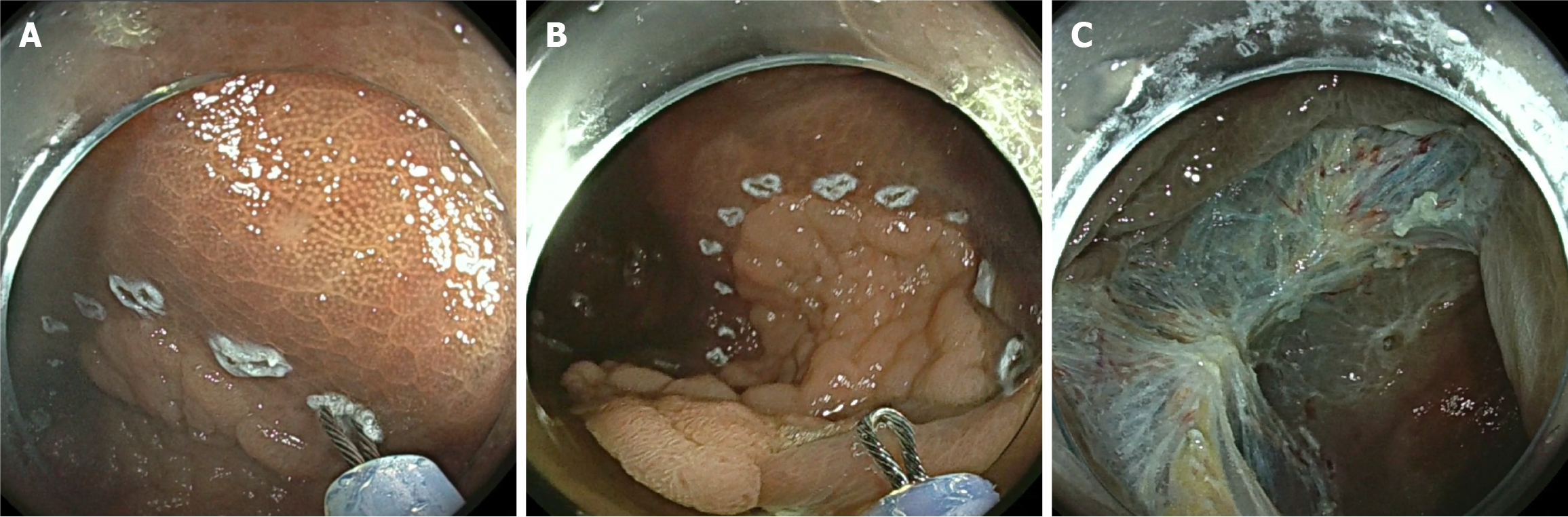
While margin thermal ablation techniques are applied after complete resection, margin marking is applied before the resection and was first used for endoscopic submucosal dissection (ESD). For pre-resection margin marking, endoscopists use the snare tip to apply cautery marks approximately 3 mm around the polyp margin (Figure 4). One study of 210 LNPCPs demonstrated that LNPCPs treated with margin marking had a recurrence rate of 8% compared to the 20% recurrence rate of the historical control group (P < 0.001)[42]. Besides polyp size, margin marking was the strongest predictor of recurrence in a multivariate analysis (OR = 0.20, 95%CI: 0.13-0.64, P = 0.003). This study also found that margin marking was not significantly associated with an increased rate of adverse events, demonstrating the safety profile of this novel technique. It has been hypothesized that margin marking may reduce the risk of residual lesion tissue near the margin as this technique better delineates the edges of the lesion[46,60]. Yang et al’s study demonstrated that this technique is relatively simple and is as cost-effective as STSC since it only requires a snare tip and no additional devices[42]. However, there has been no literature to date to demonstrate the superiority of this technique compared to margin thermal ablation techniques in terms of the rates of recurrence and adverse events. Further exploration into comparing the technical and clinical success of these techniques will allow for a better understanding and perhaps standardization of recurrence mitigation.
Margin thermal ablation and margin marking are adjuvant treatments to mitigate recurrence, either before or after the EMR is completed. Alternatively, another method to reduce mitigation may be to perform an entirely different resection technique from EMR, namely ESD. ESD has been found to provide a more reliable complete resection, lower rates of recurrence, and a potential cure for superficial cancer[10]. However, it has its drawbacks. ESDs are generally more time-consuming, technical, and require specialized training by the endoscopist. While ESD is a safer alternative to surgery, it still poses a substantial risk for adverse events[17].
Focusing on recurrence rates between ESD and EMR, one systematic review with 1850 included lesions demonstrated that EMR had significantly higher rates of recurrence compared to ESD (OR = 5.88, 95%CI: 2.15-16.07, P = 0.037), though EMR had fewer complications (OR = 0.40, 95%CI: 0.23-0.71, P < 0.001)[61]. These results remained consistent with previous studies, highlighting the importance of weighing the risks and benefits of each resection technique based on lesion characteristics[9]. One randomized controlled trial of 318 LNPCPs demonstrated that recurrence was significantly lower in ESD cases (0.6% vs 5.1%; RR = 0.12, 95%CI: 0.01-0.96)[62]. However, adverse events occurred more frequently after ESD cases (35.6% vs 24.5%; RR = 1.4, 95%CI: 1.0-2.0), highlighting the critical considerations for switching techniques. To optimize the benefits and minimize the risks of each technique, studies have explored the use of a selective algorithm to determine which technique is appropriate based on lesion characteristics[3,63,64]. ESD is recommended for potential submucosal invasive superficial cancers since an en bloc resection with negative margins is favoured and is potentially curative. EMR is recommended for most LNPCPs with visually benign optics due to its lower complication rate. While EMR may lead to recurrence at follow-up colonoscopies, strategies for recurrence management at follow-up have been evolving, and emerging evidence suggests improved recurrence treatment without requiring surgery.
With the rise of innovative recurrence mitigation strategies, recurrence rates after EMR for LNPCPs have decreased over the last several years[65]. However, recurrence at surveillance colonoscopies may still occur with the use of mitigation strategies during the resection, burdening patients by increasing the need for procedures, risk of procedural complications, and psychological distress or worriedness. Effective management strategies for recurrence provide endoscopists with the appropriate tools to handle and treat recurrent or residual tissue at surveillance to minimize future recurrence and the need for complex intervention. Several strategies, such as surveillance protocols and endoscopic treatment, can minimize complex recurrence cases and reduce the need to use complex procedures, specifically surgical intervention.
Post-resection surveillance colonoscopies for benign lesions are crucial in ensuring the absence of recurrence and synchronous/metachronous lesions, as these may lead to the development of cancer. Undergoing just one surveillance colonoscopy showed a significant reduction in post-polypectomy colorectal cancer [hazards ratio = 0.57, 95%CI: 0.40-0.80, P = 0.0029)[66]. One study found that 0.981 (95%CI: 0.966-0.99) of patients who underwent a surveillance colonoscopy at 4 months and 16 months had no recurrence and required no surgical intervention[12]. However, an increase in surveillance requirements puts a large burden on health authorities[67]. Therefore, patients at higher risk of recurrence, such as those who underwent piecemeal resections, had LNPCPs removed, or had histologically confirmed HGD, should be targeted for strict adherence to surveillance recommendations.
Current guidelines from multiple associations, such as the United States Multi-Society Task Force (USMSTF), the European Society of Gastrointestinal Endoscopy (ESGE), and the Japan Gastroenterological Endoscopy Society have suggested the first surveillance colonoscopy to occur at 3-6 months post-resection for all LNPCPs removed via EMR to detect early recurrence[68]. ESGE has also recommended a second surveillance colonoscopy be performed 12 months after the first surveillance to detect late recurrence[69]. Following this, the USMSTF also suggests a third surveillance three years after the second surveillance[70]. These guidelines are due to the higher recurrence rate detected following piecemeal resections. Some studies support these guidelines, indicating that surveillance colonoscopies should be completed within three years after baseline due to the risk of recurrence and cancer; however, there is a lack of studies comparing specific surveillance intervals to confirm the most appropriate time for surveillance[71]. Even with set guidelines, global adherence to these guidelines is low; over 50% of surveillance colonoscopies were performed either too early or too late[72]. Follow-ups performed too early may result in increased procedure volumes, while those performed too late may result in the discovery of potentially progressed recurrent lesions. Future research into appropriate follow-up timeframes can refine surveillance intervals for better adherence and reduced recurrence rates.
While adhering to surveillance recommendations is important in the timely detection of recurrence, post-resection scar evaluation and the ability to treat recurrence are equally important. Firstly, it is crucial to appreciate the potential optical features of a post-resection scar. A normal resection scar is characterized by a pale area with a disruption of the normal colonic pit/microvascular pattern, and fold convergence is commonly seen. On careful evaluation, a non-neoplastic pit pattern can be appreciated in comparison to the neoplastic pit pattern seen with recurrence. Other potential non-neoplastic findings include inflammatory nodules and clip artifacts[5,73]. A prospective study of 183 post-EMR scars demonstrated a high diagnostic accuracy (94%) in detecting recurrence by using a standardized imaging protocol[74]. Accurate identification of recurrence, as opposed to other optical features such as clip artifacts and inflammatory nodules, allows endoscopists to provide appropriate and proactive treatments at the time of recurrence detection. Additionally, biopsies taken at the resection site during surveillance colonoscopies can give histopathologic confirmation of recurrence, which reduces the possibility of missing microscopic recurrence[75]. Adopting routine biopsy sampling into the standard of practice at surveillance can reduce the margin of error when visually assessing for recurrence.
If recurrence is detected during surveillance, there are several techniques appropriate for the endoscopic treatment of recurrent lesions[76]. Most notably, piecemeal EMR is sufficient for treating recurrent lesions. It’s important to appreciate the difficulties in resecting recurrent lesions, such as submucosal fibrosis, or the potential requirement for cold-forceps avulsion with adjuvant STSC when non-lifting is encountered. One prospective study demonstrated a high success rate of recurrence treatment using piecemeal EMR, cold-forceps avulsion with adjuvant STSC, or a combination of the two techniques (149/161; 92.5%)[77].
Alternatively, ESD is also effective for treating recurrence[78]. However, the presence of submucosal fibrosis increases the technical complexity of ESD. Other studies have considered CSR to treat recurrence[79]. CSR has great advantages, including shorter procedure time, reduced bleeding risk, and lower costs. Using CSR to treat diminutive and visually benign recurrent lesions may be a safe and effective alternative to traditional EMR and ESD[80,81]. However, studies have demonstrated significantly higher recurrence rates with CSR, making it a suboptimal choice in treating recurrent lesions as it can lead to further recurrence[34,35]. Other techniques, such as thermal ablation for small recurrent lesions, are also less opted for. In fact, using APC as a primary resection tool may lead to increased recurrence rates at the next surveillance[12]. This suggests that while thermal ablation may assist resection techniques (i.e., EMR and ESD) in reducing recurrence rates by ablating microscopic residual tissue, using them as the primary treatment for macroscopically visible lesions is not recommended. Based on current literature, EMR remains the optimal choice in recurrence treatment.
To further advance our understanding of mitigation and management strategies, more prospective and multicentre trials are needed to create a full picture of the effective techniques across different patient populations and practice settings. Current standards of practice differ across countries as they depend on the patient population and endoscopists’ training[31,32]. Having large-scale, prospective trials can potentially enhance the generalizability of findings to guide global standards of practice. Multicentre trials can also facilitate direct comparisons of recurrence mitigation techniques. The safety and efficacy of these techniques can be evaluated, with subgroup differences of recurrence risk aiding in surveillance protocols. Lastly, larger multicentre trials will provide robust data to accurately inform clinical guidelines and shape the standard of care while supporting personalized surveillance management based on specific risk factors.
As technology advances, tools such as artificial intelligence (AI) and machine-learning methods may be implemented into endoscopic procedures to assist endoscopists during resection or the planning for surveillance colonoscopies. Different guidelines have already begun to examine the use of AI in the endoscopic evaluation of LNPCPs and assistance in resection techniques[82]. Addressing recurrence, one study using a retrospective cohort evaluated the use of a prediction model to predict recurrence after EMR[27]. Using previously identified risk factors such as lesion size, histopathology, lesion location, and resection technique, this model can moderately predict recurrence better than previously established prediction tools. Other studies may use prediction models to create personalized surveillance schedules. One study used retrospective data from 1694 patients to determine potential risk factors for recurrence and develop a machine-learning model to predict a patient’s risk for recurrence[83]. This model can potentially facilitate endoscopists to determine appropriate and accurate surveillance protocols for each patient based on their risk factors. While there are benefits and challenges in implementing new technology, future research can focus on validating predictive capabilities and benchmarking outcomes to ensure best clinical practice.
As recurrence is detected through long-term surveillance colonoscopies, it is crucial to ensure patients are scheduled for surveillance visits and follow their surveillance protocol promptly. A large retrospective cohort study of 33011 patients evaluated the incidence of colorectal cancer after endoscopic resection over 10 years[84]. However, 50.1% of low-risk patients, 39.5% of intermediate-risk patients, and 33.5% of high-risk patients did not have a recorded surveillance visit, demonstrating a prominent challenge in collecting long-term data. Loss to follow-up can be due to multiple factors, including scheduling difficulties, relocation, or patient non-compliance. Without long-term surveillance, conclusions regarding recurrence rates and risk trajectories are more difficult to reach. By addressing this limitation using stan
Another limitation in examining recurrence rates and evaluating mitigation and management techniques is con
By evaluating recurrence mitigation and management techniques, endoscopists can better understand the most effective techniques, optimizing technical and clinical outcomes and standardizing clinical practice. Future research in recurrence rates, techniques, and even risk factors for recurrence can shape and revise the current guidelines for surveillance colonoscopies. Surveillance protocols are crucial in reducing colorectal cancer as recurrent lesions can be caught and removed before dysplastic or malignant development. However, risk factors can play a large role in surveillance protocols; low-risk patients may not require stricter protocols, while high-risk patients may need a more rigorous approach, highlighting the need for adaptable and personalized surveillance timelines. Standardizing these guidelines while allowing for flexibility based on a patient’s risk profile can optimize patient outcomes and reduce procedural burdens on the healthcare system.
Reducing recurrence rates not only benefits patient outcomes but may also benefit environmental outcomes. Endoscopic procedures significantly impact a healthcare centre’s carbon footprint[89]. One study demonstrated that reducing recurrence rates and subsequent recurrence treatment procedures could favour piecemeal EMR, as it has a lower carbon footprint than ESD[90]. This novel finding further solidifies the need for standardized recurrence mitigation techniques to prevent recurrence, ultimately working towards an environmentally responsible future.
Further research in this field will continue to optimize recurrence mitigation and management strategies. While addressing specific limitations such as difficult long-term data collection and confounding risk factors, large prospective multi-institutional trials can validate effective mitigation and treatment strategies across different patient populations and local practice settings. This will allow for a better evaluation of robust data to develop and refine a clearer standardization of endoscopic practice. Additionally, observational studies and randomized controlled trials may support the use of risk-stratified surveillance and personalized follow-up schedules.
Recurrence following endoscopic resection of LNPCPs remains a significant clinical challenge, with multiple risk factors contributing to its occurrence. Understanding these risk factors allows for improved procedural and surveillance planning. Mitigation strategies such as margin thermal ablation and margin marking are effectively reduce recurrence rates by ensuring complete resection and minimizing the risk of residual lesion tissue. Furthermore, management strategies for recurrence, such as surveillance protocols and endoscopic treatment options, play a crucial role in addressing the recurrent tissue without requiring surgery. From integrating safe and effective adjuvant techniques during endoscopic resection to maintaining a strict surveillance schedule, future research will improve patient outcomes and reduce the burden of recurrence.
| 1. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1827] [Reference Citation Analysis (5)] |
| 2. | Cronin O, Bourke MJ. Endoscopic Management of Large Non-Pedunculated Colorectal Polyps. Cancers (Basel). 2023;15:3805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 3. | Jiang SX, Shahidi N. Large non-pedunculated colorectal polyp management: The elephant in the room. World J Gastroenterol. 2024;30:3126-3131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Mathews AA, Draganov PV, Yang D. Endoscopic management of colorectal polyps: From benign to malignant polyps. World J Gastrointest Endosc. 2021;13:356-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (8)] |
| 5. | Shahidi N, Bourke MJ. How to Manage the Large Nonpedunculated Colorectal Polyp. Gastroenterology. 2021;160:2239-2243.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Chaoui I, Demedts I, Roelandt P, Willekens H, Bisschops R. Endoscopic mucosal resection of colorectal polyps: results, adverse events and two-year outcome. Acta Gastroenterol Belg. 2022;85:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 7. | Kaltenbach T, Anderson JC, Burke CA, Dominitz JA, Gupta S, Lieberman D, Robertson DJ, Shaukat A, Syngal S, Rex DK. Endoscopic Removal of Colorectal Lesions: Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2020;115:435-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 8. | Thiruvengadam SS, Fung BM, Barakat MT, Tabibian JH. Endoscopic Mucosal Resection: Best Practices for Gastrointestinal Endoscopists. Gastroenterol Hepatol (N Y). 2022;18:133-144. [PubMed] |
| 9. | De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, Bhandari P, Boeri F, Conio M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 2016;104:138-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Heitman SJ, Bourke MJ. Endoscopic submucosal dissection and EMR for large colorectal polyps: "the perfect is the enemy of good". Gastrointest Endosc. 2017;86:87-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Rex DK. What can colonoscopists do now to move management of large benign laterally spreading lesions in the colorectum from surgery to EMR? Gastrointest Endosc. 2020;91:132-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K, Bourke MJ. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 13. | Kemper G, Turan AS, Schoon EJ, Schrauwen RWM, Epping LSM, Gerges C, Beyna T, Neuhaus H, Gündug U, Siersema PD, van Geenen EJM; ENDOCARE Study Group. Endoscopic techniques to reduce recurrence rates after colorectal EMR: systematic review and meta-analysis. Surg Endosc. 2021;35:5422-5429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Rotermund C, Djinbachian R, Taghiakbari M, Enderle MD, Eickhoff A, von Renteln D. Recurrence rates after endoscopic resection of large colorectal polyps: A systematic review and meta-analysis. World J Gastroenterol. 2022;28:4007-4018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (2)] |
| 15. | Sidhu M, Shahidi N, Gupta S, Desomer L, Vosko S, Arnout van Hattem W, Hourigan LF, Lee EYT, Moss A, Raftopoulos S, Heitman SJ, Williams SJ, Zanati S, Tate DJ, Burgess N, Bourke MJ. Outcomes of Thermal Ablation of the Mucosal Defect Margin After Endoscopic Mucosal Resection: A Prospective, International, Multicenter Trial of 1000 Large Nonpedunculated Colorectal Polyps. Gastroenterology. 2021;161:163-170.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 16. | Michielan A, Merola E, Vieceli F, Rogger TM, Crispino F, Sartori C, Decarli NL, de Pretis G, de Pretis N. Recurrence rates after piecemeal endoscopic mucosal resection of large colorectal laterally spreading tumors. Ann Gastroenterol. 2023;36:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 17. | Seidel J, Färber E, Baumbach R, Cordruwisch W, Böhmler U, Feyerabend B, Faiss S. Complication and local recurrence rate after endoscopic resection of large high-risk colorectal adenomas of ≥3 cm in size. Int J Colorectal Dis. 2016;31:603-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Zhan T, Hielscher T, Hahn F, Hauf C, Betge J, Ebert MP, Belle S. Risk Factors for Local Recurrence of Large, Flat Colorectal Polyps after Endoscopic Mucosal Resection. Digestion. 2016;93:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 19. | Emmanuel A, Lapa C, Ghosh A, Gulati S, Burt M, Hayee B, Haji A. Risk factors for early and late adenoma recurrence after advanced colorectal endoscopic resection at an expert Western center. Gastrointest Endosc. 2019;90:127-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Tate DJ, Desomer L, Klein A, Brown G, Hourigan LF, Lee EY, Moss A, Ormonde D, Raftopoulos S, Singh R, Williams SJ, Zanati S, Byth K, Bourke MJ. Adenoma recurrence after piecemeal colonic EMR is predictable: the Sydney EMR recurrence tool. Gastrointest Endosc. 2017;85:647-656.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Pohl H, Anderson JC, Aguilera-Fish A, Calderwood AH, Mackenzie TA, Robertson DJ. Recurrence of Colorectal Neoplastic Polyps After Incomplete Resection. Ann Intern Med. 2021;174:1377-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Chang JJ, Chien CH, Chen SW, Chen LW, Liu CJ, Yen CL. Long term outcomes of colon polyps with high grade dysplasia following endoscopic resection. BMC Gastroenterol. 2020;20:376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Komeda Y, Watanabe T, Sakurai T, Kono M, Okamoto K, Nagai T, Takenaka M, Hagiwara S, Matsui S, Nishida N, Tsuji N, Kashida H, Kudo M. Risk factors for local recurrence and appropriate surveillance interval after endoscopic resection. World J Gastroenterol. 2019;25:1502-1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 24. | Sohrabi M, Obeidinia M, Adelani MR, Hassanzadeh P, Shirani A, Pirniakan R, Abbasi F, Sami M, Babaki AH, Ajdarkosh H, Zamani F. The Recurrence Rate of Colorectal Polyps among Patients with Average Risk of Colorectal Cancer. Asian Pac J Cancer Prev. 2024;25:2823-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Hao Y, Wang Y, Qi M, He X, Zhu Y, Hong J. Risk Factors for Recurrent Colorectal Polyps. Gut Liver. 2020;14:399-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Briedigkeit A, Sultanie O, Sido B, Dumoulin FL. Endoscopic mucosal resection of colorectal adenomas > 20 mm: Risk factors for recurrence. World J Gastrointest Endosc. 2016;8:276-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Gomez Cifuentes JD, Berger S, Caskey K, Jove A, Sealock R, Hair C, Velez M, Jarbrink-Sehgal M, Thrift AP, da Costa WL Jr, Gyanprakash K. New Model to Predict Recurrence After Endoscopic Mucosal Resection of Non-pedunculated Colonic Polyps ≥ 20 mm. Dig Dis Sci. 2023;68:3935-3942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 28. | Facciorusso A, Di Maso M, Serviddio G, Vendemiale G, Spada C, Costamagna G, Muscatiello N. Factors Associated With Recurrence of Advanced Colorectal Adenoma After Endoscopic Resection. Clin Gastroenterol Hepatol. 2016;14:1148-1154.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Mandic O, Jovanovic I, Cvetkovic M, Maksimovic J, Radonjic T, Popovic M, Nikolic N, Brankovic M. Factors Predicting Malignant Occurrence and Polyp Recurrence after the Endoscopic Resection of Large Colorectal Polyps: A Single Center Experience. Medicina (Kaunas). 2022;58:1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Santos-Antunes J, Pioche M, Ramos-Zabala F, Cecinato P, Gallego F, Barreiro P, Mascarenhas A, Sferrazza S, Berr F, Wagner A, Lemmers A, Ferreira MF, Albéniz E, Uchima H, Küttner-Magalhães R, Fernandes C, Morais R, Gupta S, Martinho-Dias D, Faria-Ramos I, Marques M, Bourke MJ, Macedo G. Risk of Residual Neoplasia after a Local-Risk Resection of Colorectal Lesions by Endoscopic Submucosal Dissection: A Multinational Study. J Clin Med. 2023;12:5356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Ishikawa T, Okimoto K, Matsumura T, Ogasawara S, Fukuda Y, Kitsukawa Y, Yokoyama Y, Kanayama K, Akizue N, Iino Y, Ohta Y, Ishigami H, Taida T, Tsuchiya S, Saito K, Kamezaki H, Kobayashi A, Kikuchi Y, Tada M, Shiko Y, Ozawa Y, Kato J, Yamaguchi T, Kato N. Risk factors of unintentional piecemeal resection in endoscopic mucosal resection for colorectal polyps ≥ 10 mm. Sci Rep. 2024;14:493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Alfarone L, Maselli R, Hassan C, Spaggiari P, Spadaccini M, Capogreco A, Massimi D, De Sire R, Mastrorocco E, Repici A. Endoscopic submucosal dissection for proximal colonic lesions: An effective therapeutic option. Endosc Int Open. 2025;13:a24431609. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 33. | Ansari J, Bapaye H, Shah J, Raina H, Gandhi A, Bapaye J, B R A, Pagadapelli AA, Bapaye A. Clinical audit of endoscopic sub-mucosal dissection performed for complex lateral spreading colorectal tumors from a region non-endemic for colorectal cancer. Indian J Gastroenterol. 2024;43:1002-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | O'Sullivan T, Cronin O, van Hattem WA, Mandarino FV, Gauci JL, Kerrison C, Whitfield A, Gupta S, Lee E, Williams SJ, Burgess N, Bourke MJ. Cold versus hot snare endoscopic mucosal resection for large (≥15 mm) flat non-pedunculated colorectal polyps: a randomised controlled trial. Gut. 2024;73:1823-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (1)] |
| 35. | Steinbrück I, Ebigbo A, Kuellmer A, Schmidt A, Kouladouros K, Brand M, Koenen T, Rempel V, Wannhoff A, Faiss S, Pech O, Möschler O, Dumoulin FL, Kirstein MM, von Hahn T, Allescher HD, Gölder SK, Götz M, Hollerbach S, Lewerenz B, Meining A, Messmann H, Rösch T, Allgaier HP. Cold Versus Hot Snare Endoscopic Resection of Large Nonpedunculated Colorectal Polyps: Randomized Controlled German CHRONICLE Trial. Gastroenterology. 2024;167:764-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 36. | Tate DJ, Vosko S, Bar-Yishay I, Desomer L, Shahidi N, Sidhu M, McLeod D, Bourke MJ. Incomplete mucosal layer excision during EMR: a potential source of recurrent adenoma (with video). Gastrointest Endosc. 2024;100:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Djinbachian R, Pohl H, Rex DK, Levenick JM, Pleskow DK, Wallace MB, Khashab M, Singh A, Melson J, Yang D, Gavrić A, von Renteln D. Thermal ablation after endoscopic mucosal resection of large colorectal polyps: not only the margins, but also the base? Gut. 2023;73:12-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Emmanuel A, Williams S, Gulati S, Ortenzi M, Gunasingam N, Burt M, Ratcliff S, Hayee B, Haji A. Incidence of microscopic residual adenoma after complete wide-field endoscopic resection of large colorectal lesions: evidence for a mechanism of recurrence. Gastrointest Endosc. 2021;94:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 40. | Abu Arisha M, Scapa E, Wishahi E, Korytny A, Gorelik Y, Mazzawi F, Khader M, Muaalem R, Bana S, Awadie H, Bourke MJ, Klein A. Impact of margin ablation after EMR of large nonpedunculated colonic polyps in routine clinical practice. Gastrointest Endosc. 2023;97:559-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 41. | Motz VL, Lester C, Moyer MT, Maranki JL, Levenick JM. Hybrid argon plasma coagulation-assisted endoscopic mucosal resection for large sessile colon polyps to reduce local recurrence: a prospective pilot study. Endoscopy. 2022;54:580-584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Yang D, Draganov PV, King W, Liu N, Sarheed A, Bhat A, Jiang P, Ladna M, Ruiz NC, Wilson J, Gorrepati VS, Pohl H. Margin marking before colorectal endoscopic mucosal resection and its impact on neoplasia recurrence (with video). Gastrointest Endosc. 2022;95:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Klein A, Tate DJ, Jayasekeran V, Hourigan L, Singh R, Brown G, Bahin FF, Burgess N, Williams SJ, Lee E, Sidhu M, Byth K, Bourke MJ. Thermal Ablation of Mucosal Defect Margins Reduces Adenoma Recurrence After Colonic Endoscopic Mucosal Resection. Gastroenterology. 2019;156:604-613.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 44. | Kandel P, Werlang ME, Ahn IR, Woodward TA, Raimondo M, Bouras EP, Wallace MB, Gómez V. Prophylactic Snare Tip Soft Coagulation and Its Impact on Adenoma Recurrence After Colonic Endoscopic Mucosal Resection. Dig Dis Sci. 2019;64:3300-3306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | O'Sullivan T, Mandarino FV, Gauci JL, Whitfield AM, Kerrison C, Elhindi J, Neto do Nascimento C, Gupta S, Cronin O, Sakiris A, Prieto Aparicio JF, Arndtz S, Brown G, Raftopoulos S, Tate D, Lee EY, Williams SJ, Burgess N, Bourke MJ. Impact of margin thermal ablation after endoscopic mucosal resection of large (≥20 mm) non-pedunculated colonic polyps on long-term recurrence. Gut. 2024;74:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 46. | Radadiya D, Desai M, Patel H, Srinivasan S, Chandrasekar VT, Hassan C, Repici A, Rex D, Sharma P. Analyzing methods for reducing recurrence rates after EMR of large nonpedunculated colorectal polyps: an indirect pairwise comparison. Gastrointest Endosc. 2024;99:326-336.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Kandel P, Hussain M, Yadav D, Dhungana SK, Brahmbhatt B, Raimondo M, Lukens FJ, Bachuwa G, Wallace MB. Post-EMR for colorectal polyps, thermal ablation of defects reduces adenoma recurrence: A meta-analysis. Endosc Int Open. 2022;10:E1399-E1405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 48. | Chandan S, Facciorusso A, Ramai D, Deliwala S, Mohan BP, Kassab LL, Draganov PV, Othman MO, Kochhar GS. Snare tip soft coagulation (STSC) after endoscopic mucosal resection (EMR) of large (> 20 mm) non pedunculated colorectal polyps: a systematic review and meta-analysis. Endosc Int Open. 2022;10:E74-E81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Gauci JL, Mandarino FV, Kerrison C, Whitfield AM, O'Sullivan T, Gupta S, Lam B, Perananthan V, Cronin O, Lee EY, Williams SJ, Burgess N, Bourke MJ. Margin thermal ablation eliminates size as a risk factor for recurrence after piecemeal endoscopic mucosal resection of large non-pedunculated colorectal polyps. Gut. 2025;74:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Raju GS, Lum P, Abu-Sbeih H, Ross WA, Thirumurthi S, Miller E, Lynch P, Lee J, Bhutani MS, Shafi M, Weston B, Rashid A, Wang Y, Chang GJ, Carlson R 3rd, Hagan K, Davila M, Stroehlein J. Cap-fitted endoscopic mucosal resection of ≥ 20 mm colon flat lesions followed by argon plasma coagulation results in a low adenoma recurrence rate. Endosc Int Open. 2020;8:E115-E121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Brooker JC, Saunders BP, Shah SG, Thapar CJ, Suzuki N, Williams CB. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc. 2002;55:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 52. | Motchum L, Levenick JM, Djinbachian R, Moyer MT, Bouchard S, Taghiakbari M, Repici A, Deslandres É, von Renteln D. EMR combined with hybrid argon plasma coagulation to prevent recurrence of large nonpedunculated colorectal polyps (with videos). Gastrointest Endosc. 2022;96:840-848.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Haghbin H, Zakirkhodjaev N, Fatima R, Kamal F, Aziz M. Efficacy and Safety of Thermal Ablation after Endoscopic Mucosal Resection: A Systematic Review and Network Meta-Analysis. J Clin Med. 2024;13:1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 54. | Meulen LWT, Bogie RMM, Winkens B, Masclee AAM, Moons LMG. Thermal ablation of mucosal defect margins to prevent local recurrence of large colorectal polyps: a systematic review and meta-analysis. Endosc Int Open. 2022;10:E1127-E1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 55. | Santo P, Meine GC, Holanda EU, Barbosa EC, Baraldo S, Nau AL, Henry Moore KM. Thermal ablation of margins for recurrence prevention after endoscopic mucosal resection: a systematic review and meta-analysis. Surg Endosc. 2025;39:741-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Katsinelos P, Lazaraki G, Chatzimavroudis G, Anastasiadis S, Georgakis N, Xanthis A, Gatopoulou A, Anastasiadou K, Kountouras J. A retrospective comparative study of argon plasma versus polypectome snare tip coagulation: effect on recurrence rate after resection of large laterally spreading type lesions. Ann Gastroenterol. 2019;32:178-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 57. | Smith E, Wu Y, Wang Y, Dahiya DS, Chandan S, Maida M, Spadaccini M, Facciorusso A, Shaukat A, Ramai D, Miranda C. Soft Coagulation Versus Argon Plasma Coagulation After Large Non-pedunculated Colorectal Polyp Resection: A Meta-analysis. J Clin Gastroenterol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 58. | Rex DK, Haber GB, Khashab M, Rastogi A, Hasan MK, DiMaio CJ, Kumta NA, Nagula S, Gordon S, Al-Kawas F, Waye JD, Razjouyan H, Dye CE, Moyer MT, Shultz J, Lahr RE, Yuen PYS, Dixon R, Boyd L, Pohl H. Snare Tip Soft Coagulation vs Argon Plasma Coagulation vs No Margin Treatment After Large Nonpedunculated Colorectal Polyp Resection: a Randomized Trial. Clin Gastroenterol Hepatol. 2024;22:552-561.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 59. | Jiang SX, Zarrin A, Walia A, Galorport C, Xiong W, Enns R, Lam E, Shahidi N. A133 Margin Thermal Ablation with Snare-Tip Soft Coagulation Effectively Mitigates Recurrence after Endoscopic Mucosal Resection of Large Non-Pedunculated Colorectal Polyps. J Can Assoc Gastroenterol. 2024;7:101-102. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 60. | Han L, Li J, Liang C, Chu Y, Wang Y, Lv L, Liu D, Tan Y. Risk factors for positive resection margins after endoscopic resection for gastrointestinal neuroendocrine tumors. Surg Endosc. 2024;38:2041-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 61. | Wang N, Shu L, Liu S, Yang L, Bai T, Shi Z, Liu X. Comparing endoscopic mucosal resection with endoscopic submucosal dissection in colorectal adenoma and tumors: Meta-analysis and system review. PLoS One. 2023;18:e0291916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 62. | Jacques J, Schaefer M, Wallenhorst T, Rösch T, Lépilliez V, Chaussade S, Rivory J, Legros R, Chevaux JB, Leblanc S, Rostain F, Barret M, Albouys J, Belle A, Labrunie A, Preux PM, Lepetit H, Dahan M, Ponchon T, Crépin S, Marais L, Magne J, Pioche M. Endoscopic En Bloc Versus Piecemeal Resection of Large Nonpedunculated Colonic Adenomas : A Randomized Comparative Trial. Ann Intern Med. 2024;177:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 63. | Bang JY, Bourke MJ. Selection of EMR and ESD for Laterally Spreading Lesions of the Colon. Curr Treat Options Gastroenterol. 2018;16:376-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | Shahidi N, Vosko S, Gupta S, Whitfield A, Cronin O, O'Sullivan T, van Hattem WA, Sidhu M, Tate DJ, Lee EYT, Burgess N, Williams SJ, Bourke MJ. A Rectum-Specific Selective Resection Algorithm Optimizes Oncologic Outcomes for Large Nonpedunculated Rectal Polyps. Clin Gastroenterol Hepatol. 2023;21:72-80.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 65. | Gomez Cifuentes JD, Berger S, Caskey K, Jove A, Sealock RJ, Hair C, Velez M, Jarbrink-Sehgal M, Thrift AP, da Costa W, Gyanprakash K. Evolution of endoscopic mucosal resection (EMR) technique and the reduced recurrence of large colonic polyps from 2012 to 2020. Scand J Gastroenterol. 2023;58:435-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 66. | Atkin W, Wooldrage K, Brenner A, Martin J, Shah U, Perera S, Lucas F, Brown JP, Kralj-Hans I, Greliak P, Pack K, Wood J, Thomson A, Veitch A, Duffy SW, Cross AJ. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18:823-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 67. | Baile-Maxía S, Jover R. Surveillance after colorectal polyp resection. Best Pract Res Clin Gastroenterol. 2023;66:101848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Jung YS. Summary and comparison of recently updated post-polypectomy surveillance guidelines. Intest Res. 2023;21:443-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 69. | Hassan C, Antonelli G, Dumonceau JM, Regula J, Bretthauer M, Chaussade S, Dekker E, Ferlitsch M, Gimeno-Garcia A, Jover R, Kalager M, Pellisé M, Pox C, Ricciardiello L, Rutter M, Helsingen LM, Bleijenberg A, Senore C, van Hooft JE, Dinis-Ribeiro M, Quintero E. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2020. Endoscopy. 2020;52:687-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 357] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 70. | Gupta S, Lieberman D, Anderson JC, Burke CA, Dominitz JA, Kaltenbach T, Robertson DJ, Shaukat A, Syngal S, Rex DK. Recommendations for Follow-Up After Colonoscopy and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;158:1131-1153.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 71. | Jover R, Dekker E. Surveillance after colorectal polyp removal. Best Pract Res Clin Gastroenterol. 2016;30:937-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 72. | Djinbachian R, Dubé AJ, Durand M, Camara LR, Panzini B, Bouchard S, von Renteln D. Adherence to post-polypectomy surveillance guidelines: a systematic review and meta-analysis. Endoscopy. 2019;51:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 73. | Shahidi N, Gupta S, Whitfield A, Vosko S, McKay O, Cronin O, Zahid S, Burgess NG, Bourke MJ. Simple optical evaluation criteria reliably identify the post-endoscopic mucosal resection scar for benign large non-pedunculated colorectal polyps without tattoo placement. Endoscopy. 2022;54:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 74. | Desomer L, Tutticci N, Tate DJ, Williams SJ, McLeod D, Bourke MJ. A standardized imaging protocol is accurate in detecting recurrence after EMR. Gastrointest Endosc. 2017;85:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 75. | Knabe M, Pohl J, Gerges C, Ell C, Neuhaus H, Schumacher B. Standardized long-term follow-up after endoscopic resection of large, nonpedunculated colorectal lesions: a prospective two-center study. Am J Gastroenterol. 2014;109:183-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 76. | Trindade AJ, Kumta NA, Bhutani MS, Chandrasekhara V, Jirapinyo P, Krishnan K, Melson J, Pannala R, Parsi MA, Schulman AR, Trikudanathan G, Watson RR, Maple JT, Lichtenstein DR. Devices and techniques for endoscopic treatment of residual and fibrotic colorectal polyps (with videos). Gastrointest Endosc. 2020;92:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 77. | Tate DJ, Desomer L, Argenziano ME, Mahajan N, Sidhu M, Vosko S, Shahidi N, Lee E, Williams SJ, Burgess NG, Bourke MJ. Treatment of adenoma recurrence after endoscopic mucosal resection. Gut. 2023;72:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 78. | Kuroki Y, Hoteya S, Mitani T, Yamashita S, Kikuchi D, Fujimoto A, Matsui A, Nakamura M, Nishida N, Iizuka T, Yahagi N. Endoscopic submucosal dissection for residual/locally recurrent lesions after endoscopic therapy for colorectal tumors. J Gastroenterol Hepatol. 2010;25:1747-1753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 79. | Ishibashi F, Suzuki S, Nagai M, Mochida K, Morishita T. Colorectal cold snare polypectomy: Current standard technique and future perspectives. Dig Endosc. 2023;35:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 80. | Yoshida N, Inoue K, Tomita Y, Hashimoto H, Sugino S, Hirose R, Dohi O, Naito Y, Morinaga Y, Kishimoto M, Inada Y, Murakami T, Itoh Y. Cold snare polypectomy for large sessile serrated lesions is safe but follow-up is needed: a single-centre retrospective study. United European Gastroenterol J. 2021;9:370-377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 81. | Yoshida N, Hashimoto H, Inoue K, Kobayashi R, Tomita Y, Sugino S, Hirose R, Dohi O, Morinaga Y, Inada Y, Murakami T, Itoh Y. Repeat Cold Snare Polypectomy Can Be Performed for Recurrent Benign Lesions After Cold Snare Polypectomy. Dig Dis Sci. 2022;67:3192-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 82. | Messmann H, Bisschops R, Antonelli G, Libânio D, Sinonquel P, Abdelrahim M, Ahmad OF, Areia M, Bergman JJGHM, Bhandari P, Boskoski I, Dekker E, Domagk D, Ebigbo A, Eelbode T, Eliakim R, Häfner M, Haidry RJ, Jover R, Kaminski MF, Kuvaev R, Mori Y, Palazzo M, Repici A, Rondonotti E, Rutter MD, Saito Y, Sharma P, Spada C, Spadaccini M, Veitch A, Gralnek IM, Hassan C, Dinis-Ribeiro M. Expected value of artificial intelligence in gastrointestinal endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2022;54:1211-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 104] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 83. | Shi YH, Liu JL, Cheng CC, Li WL, Sun H, Zhou XL, Wei H, Fei SJ. Construction and validation of machine learning-based predictive model for colorectal polyp recurrence one year after endoscopic mucosal resection. World J Gastroenterol. 2025;31:102387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (2)] |
| 84. | Cross AJ, Robbins EC, Pack K, Stenson I, Kirby PL, Patel B, Rutter MD, Veitch AM, Saunders BP, Duffy SW, Wooldrage K. Long-term colorectal cancer incidence after adenoma removal and the effects of surveillance on incidence: a multicentre, retrospective, cohort study. Gut. 2020;69:1645-1658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 85. | Sharma P, Burke CA, Johnson DA, Cash BD. The importance of colonoscopy bowel preparation for the detection of colorectal lesions and colorectal cancer prevention. Endosc Int Open. 2020;8:E673-E683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 86. | Shahini E, Sinagra E, Vitello A, Ranaldo R, Contaldo A, Facciorusso A, Maida M. Factors affecting the quality of bowel preparation for colonoscopy in hard-to-prepare patients: Evidence from the literature. World J Gastroenterol. 2023;29:1685-1707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (11)] |
| 87. | Desai M, Rex DK, Bohm ME, Davitkov P, DeWitt JM, Fischer M, Faulx G, Heath R, Imler TD, James-Stevenson TN, Kahi CJ, Kessler WR, Kohli DR, McHenry L, Rai T, Rogers NA, Sagi SV, Sathyamurthy A, Vennalaganti P, Sundaram S, Patel H, Higbee A, Kennedy K, Lahr R, Stojadinovikj G, Campbell C, Dasari C, Parasa S, Faulx A, Sharma P. Impact of withdrawal time on adenoma detection rate: results from a prospective multicenter trial. Gastrointest Endosc. 2023;97:537-543.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 88. | Zhao S, Yang X, Wang S, Meng Q, Wang R, Bo L, Chang X, Pan P, Xia T, Yang F, Yao J, Zheng J, Sheng J, Zhao X, Tang S, Wang Y, Wang Y, Gong A, Chen W, Shen J, Zhu X, Wang S, Yan C, Yang Y, Zhu Y, Ma RJ, Wang R, Ma Y, Li Z, Bai Y. Impact of 9-Minute Withdrawal Time on the Adenoma Detection Rate: A Multicenter Randomized Controlled Trial. Clin Gastroenterol Hepatol. 2022;20:e168-e181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 89. | Maida M, Vitello A, Shahini E, Vassallo R, Sinagra E, Pallio S, Melita G, Ramai D, Spadaccini M, Hassan C, Facciorusso A. Green endoscopy, one step toward a sustainable future: Literature review. Endosc Int Open. 2024;12:E968-E980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 90. | Grau R, Cottinet PJ, Le MQ, Schaefer M, Wallenhorst T, Rösch T, Lépilliez V, Chaussade S, Rivory J, Legros R, Chevaux JB, Leblanc S, Lafeuille P, Rostain F, Rodriguez de Santiago E, Pohl H, Baddeley R, Grinberg D, Buiron C, Cunha Neves JA, Barret M, Albouys J, Belle A, Lepetit H, Dahan M, Jacquette F, Masgnaux LJ, Marais L, Ponchon T, Jacques J, Pioche M. Endoscopic En Bloc Vs Piecemeal Resection of Large Colonic Adenomas: Carbon Footprint Post Hoc Analysis of a Randomized Trial. Clin Gastroenterol Hepatol. 2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/