Published online Jul 16, 2025. doi: 10.4253/wjge.v17.i7.107430
Revised: April 10, 2025
Accepted: June 7, 2025
Published online: July 16, 2025
Processing time: 108 Days and 8.9 Hours
Post-colonoscopic colorectal cancer (PCCRC), also known as interval CRC, is defined as CRC diagnosed more than six months after a colonoscopy in which no cancer was detected. It typically arises from missed lesions or incomplete resections and is now recognized as one of the most reliable quality indicators for assessing colonoscopy performance. With an incidence rate of 3.6% to 9.3%, PCCRC remains a significant concern, highlighting the limitations of colonoscopy in CRC screening—not only in terms of diagnostic accuracy but also in its preven
Core Tip: Post-colonoscopy colorectal cancer (PCCRC) remains the most reliable parameter for assessing colonoscopy quality, as it reflects its effectiveness in diagnosing and preventing colorectal cancer. More than 80% of cases are attributed to preventable factors, making their reduction a key challenge for endoscopy units. While technological advancements will enhance procedural quality, the most crucial step in reducing PCCRC is the continuous evaluation of endoscopy units to identify limitations and implement targeted improvement strategies.
- Citation: Delgado Galan M, Quintanilla Lazaro E, Rabago Torre LR. Postcolonoscopy colorectal cancer: What we need to know in the age of screening and magnifying endoscopy techniques. World J Gastrointest Endosc 2025; 17(7): 107430
- URL: https://www.wjgnet.com/1948-5190/full/v17/i7/107430.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i7.107430
Colorectal cancer (CRC) is the third most common cancer worldwide in both men and women and the second leading cause of cancer-related death[1]. The risk of CRC increases with age, occurring more frequently in individuals over 50 years old. Colonoscopy is the gold standard for CRC screening and diagnosis[2]. It allows for the detection and removal of early neoplastic lesions, significantly reducing CRC incidence and mortality.
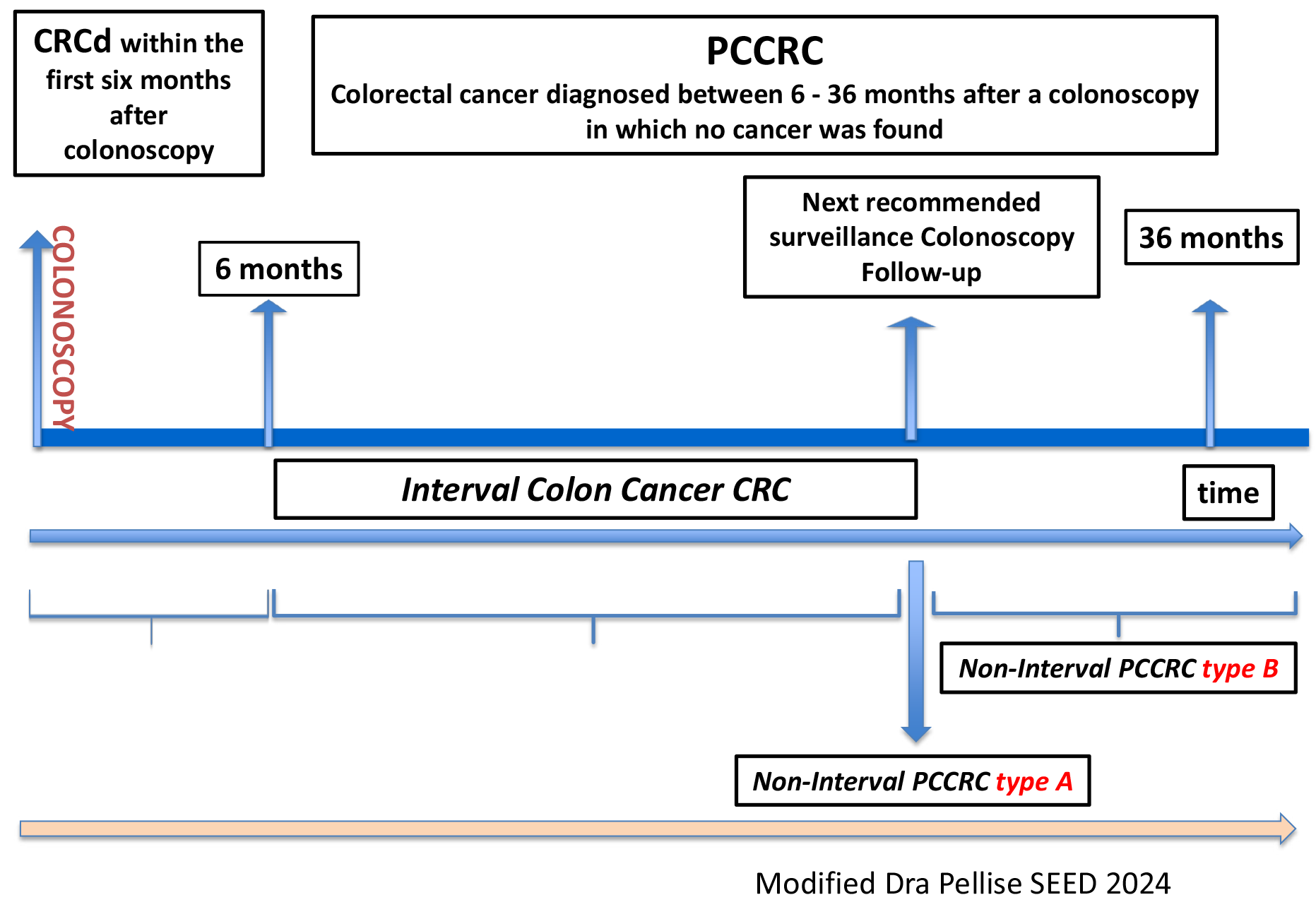
Patients must undergo repeat colonoscopies to detect and remove future neoplastic lesions. Therefore, in a CRC screening program, CRC can be diagnosed during the index colonoscopy of the colonoscopy or in the following 6 months of colonoscopy CRC detected (CRCd). However, even in well-structured follow-up programs, some cases of CRC are diagnosed shortly after six months following a prior colonoscopy -this is known as interval CRC.
According to the latest consensus document from the World Endoscopy Organization (WEO)[3] the preferred term for CRC diagnosed between 6 and 36 months after a colonoscopy in which no cancer was initially identified is post-colonoscopic CRC (PCCRC), previously referred to as interval colon cancer. The incidence of PCCRC varied from a low of 1.14 to a high of 2.24 cancers per 1000 person-years of follow-up[4]. PCCRC can be further classified into:
Interval CRC: When CRC is diagnosed before the next recommended surveillance colonoscopy.
Non-interval CRC: When CRC is diagnosed during the next surveillance colonoscopy (Type A), after the next surveillance colonoscopy (Type B), (Type C) occurs when CRC is diagnosed up to 10 years after an initial colonoscopy where no cancer was found, with no specific surveillance or follow-up interval determined, resulting from missed lesions or newly arising tumors, and the absence of an adequate surveillance program (Figure 1).
Interest in PCCRC as a quality marker for colonoscopy is increasing, and numerous studies are being conducted to determine its prevalence and causes. According to published studies, PCCRC accounts for approximately 3.6% to 9.3% of all CRC cases diagnosed (CCRd), with the majority classified as non-interval CRC Type C (51.5%)[5]. More than 80% of cases are attributed to preventable factors[6].
The second point of interest regarding PCCRC is its prognosis. Numerous publications, based on nationwide and population-based studies, suggest that PCCRC has a poorer prognosis compared to CRC diagnosed during the index colonoscopy. This is primarily due to the later stage at which PCCRC is diagnosed[7-9].
However, there is not complete agreement on this, as some authors have found that interval CRCs are associated with earlier-stage cancer and a lower risk of death[10].
The presence of PCCRC highlights the limitations of colonoscopy in detecting, preventing, and treating CRC, making it the most significant quality indicator in colonoscopic practice.
Current evidence shows that most PCCRC cases result from lesions that were not detected during the initial colonoscopy (52%-63%)[4,5].
Incomplete resection: Another possibility is that PCCRC may also arise from lesions that were incompletely resected during the initial colonoscopy. Studies suggest that around 20% of PCCRC cases result from this issue[4].
The problem of incomplete resection has gained increasing recognition and was directly assessed in the CARE study. In this study, biopsies were taken from the edges of lesions after resection. Using this method, incomplete resection was found in 10% of removed lesions, with higher rates observed in larger polyps (17% in 10-20 mm polyps vs. 6.8% in polyps < 10 mm), in serrated adenomas compared to conventional adenomas (31% vs 7.2%), and in piecemeal-resected polyps (20.4% vs 8.4%)[11].
De novo lesions: A much less common cause of PCCRC is the development of tumors from newly emerging lesions. The most likely explanation is that these tumors follow a different genetic pathway in the adenoma-to-adenocarcinoma transition, leading to faster tumor growth.
To standardize terminology and facilitate research and study development, the WEO recommends classifying PCCRC into five categories based on their most probable cause[3]: (1) Possible undetected lesion, adequate colonoscopy; (2) Possible undetected lesion, inadequate previous colonoscopy; (3) Detected lesion, not resected; (4) Probable incomplete resection of a previously detected lesion; and (5) Probable newly developed CRC.
A systematic review of six studies using tandem colonoscopies found a missed polyp rate of 22%. When considering lesion size, the miss rate was 2% for lesions > 10 mm, 13% for lesions between 5-10mm, and 26% for lesions < 5 mm[13].
Lesion morphology also impacts detectability, as sessile or flat polyps have a fivefold higher risk of being missed compared to pedunculated polyps[14].
Adequate bowel cleansing is crucial for colonoscopy to remain the most effective test for diagnosing CRC[15].
Bowel preparation is considered adequate when it achieves a Boston Bowel Preparation Scale score of 6 or more on the Boston scale, with each segment scoring greater than 1, which allows for the detection of polyps larger than 5 mm[16]. In their study on the causes of PCCRC, Anderson et al[6] identified that 19% of colonoscopies had inadequate preparation, and in 45% of these cases, there was no documented recommendation for a repeat colonoscopy, alternative evaluation, or further follow-up[6].
The use of validated bowel cleansing scales is crucial and should be documented in colonoscopy reports. In cases of inadequate preparation, the endoscopist must specify whether a repeat examination is necessary, when it should be performed, or if additional tests are required.
Split dose: A meta-analysis comparing split-dose administration vs single-dose administration showed that split dosing resulted in higher rates of adequate preparation, greater patient satisfaction, and increased willingness to undergo repeat[17]. Numerous studies have been conducted to improve bowel cleansing, and scientific societies have developed clinical practice guidelines with recommendations to achieve optimal preparation.
Missing lesion: Inadequate bowel preparation is associated with a higher rate of missed lesions, prolonged procedure times and more challenging procedures, and incomplete examinations.
Incomplete resections: Additionally, it increases the risk of incomplete resections due to poor lesion margin delineation[14,16].
Cecal intubation rate: Cecal intubation rate (CIR) is a key quality parameter in all endoscopy guidelines, and its photo documentation is mandatory. Without it, it is impossible to determine whether the examination was complete.
Given the capabilities of modern endoscopy equipment, the inclusion of this parameter in all reports is essential.
Failure to intubate the cecum increases the risk of PCCRC due to undetected lesions. Baxter et al[18] demonstrated that patients who underwent colonoscopy by endoscopists with a CIR > 95% had a 30% lower probability of being diagnosed with proximal PCCRC compared to those examined by endoscopists with a CIR < 80%[18]. The main causes of failed cecal intubation include inadequate bowel preparation (22%), stenosis (22%), diverticulosis (22%), loop formation (11%), and patient discomfort (11%)[6].
Withdrawal time: Adenoma detection rate (ADR) is the primary quality parameter in colonoscopy and is directly linked to a reduced PCCRC incidence.
Although the exact mechanism by which withdrawal time impacts PCCRC risk remains unclear, it serves as a surrogate marker for thorough mucosal examination.
A shorter withdrawal time is independently associated with a lower ADR and a higher PCCRC risk. While there is no definitive cutoff to define an adequate withdrawal time for screening colonoscopies, Shaukat et al[19] suggest that the incidence of PCCRC remains very low and stable when the average withdrawal time is ≥ 8 minutes.
ADR: ADR is considered the primary quality parameter in colonoscopy, and its increase is directly linked to a reduced PCCRC incidence. It is defined as the proportion of patients undergoing colonoscopy in whom one or more adenomas are detected. This is an objective and difficult-to-falsify parameter that reflects the primary goal of colonoscopy while indirectly indicating other quality markers such as bowel preparation, CIR, and withdrawal time.
Studies show that endoscopists with an ADR < 20% have a 10-fold higher likelihood of PCCRC compared to those with an ADR > 20%[20].
Additionally, there is an inverse association between ADR and PCCRC risk, with the latter decreasing by 3% for every 1% increase in ADR[21].
Technological developments aimed at enhancing lesion visibility and exposing blind spots behind colonic folds are essential for improving ADR, reducing missed adenomas, and ultimately lowering PCCRC incidence.
The use of chromoendoscopy (CE) recommended for enhancing the detection, characterization, and delineation of early neoplastic lesions, ensuring complete resection of neoplastic tissue and improving the diagnostic yield of colonoscopy[22]. The application of stains in CE can be performed using two techniques:
Applied once a lesion has been detected with white-light endoscopy to enhance visualization.
Applied over large mucosal areas to increase lesion detection. However, pancolonic CE has not been shown to significantly increase lesion detection compared to conventional endoscopy and is not recommended for the general population[23].
Over the last 15 years, significant advancements in endoscopic technology have enhanced colonic mucosa visualization, leading to improvements in the ADR.
High-definition endoscopes with over 1 million pixels provide superior image quality. Despite this, their use has only led to a modest 3.5% increase in ADR compared to conventional endoscopes[24].
Different new devices have been designed to be attached to the endoscope in order to increase the lesion detection rate. In 2018, Williet et al[25] published the results of a meta-analysis on Endocuff, demonstrating a higher ADR in procedures using Endocuff compared to standard colonoscopy. This difference was particularly significant among endoscopists with low-to-moderate ADR[25,26].
In an effort to improve lesion detection and characterization by achieving an endoscopic-histological correlation that facilitates more precise therapeutic decision-making, commercial companies have developed various tools that build upon the advantages of dye-based CE.
Magnification enables image enlargement up to 150 times[27], allowing for detailed analysis of the crypt pattern of colon lesions. The Kudo classification categorizes lesions based on their crypt pattern, aiding in the differentiation between neoplastic and non-neoplastic lesions, with high intra- and inter-observer agreement among expert endoscopists[28,29]. A meta-analysis reported a sensitivity of 89% and a specificity of 85.7% for distinguishing neoplastic from non-neoplastic lesions using magnification[30].
Thus, accurate characterization of lesions enables the selection of the most appropriate endoscopic treatment, ensuring complete lesion removal and enhancing the therapeutic efficacy of colonoscopy.
Virtual CE refers to endoscopic imaging technology that provides a much more detailed view of the mucosal surface and blood vessels. This image enhancement can be achieved through: Narrowing the wavelength of emitted light, as seen in Narrow Band Imaging (NBI).
Post-processing image enhancement systems, such as Flexible Spectral Imaging Color Enhancement (FICE) or i-scan, which improve visualization of mucosal surfaces and vascular structure[28,31].
Studies comparing NBI with high-definition colonoscopy found no significant differences in polyp or ADR. However, NBI has proven to be useful in the characterization and classification of polyps[31].
Similarly, i-scan and FICE have demonstrated their effectiveness in differentiating adenomas from non-neoplastic lesions, with no significant difference in ADR compared to high-definition white-light endoscopy[31].
Additionally, studies comparing NBI with i-scan and NBI with FICE have found no significant differences in lesion characterization or polyp detection[31].
Recently, Olympus introduced Texture and Color Enhanced Imaging (TXI), a technology designed to improve mucosal surface contrast and blood vessel visibility. TXI has demonstrated a significant increase in ADR compared to white-light imaging.
In 2023, a prospective randomized clinical trial compared the use of Endocuff + TXI vs TXI alone, showing that the combination significantly improved ADR compared to TXI alone[32].
Additionally, TXI has shown superior ADR compared to white-light endoscopy in patients undergoing CE[33].
Artificial intelligence (AI)-powered systems are being developed to assist in reducing endoscopist errors and conse
A systematic review by Hassan et al[34], which included five randomized controlled trials involving 4354 patients, demonstrated a significantly higher ADR in the CADe-assisted group compared to the control group. This ADR improvement was consistent across: Small polyps (< 5 mm), medium polyps (6–9 mm), and large polyps (> 10 mm); Both proximal and distal colon locations; Flat and pedunculated polyps; Sessile serrated polyps, although not for advanced adenomas.
A later systematic review with a much larger cohort (18232 patients) confirmed that CADe-assisted colonoscopy significantly increases ADR but does not improve advanced adenoma detection. Additionally, CADe was associated with a higher rate of unnecessary resections of non-neoplastic polyps, increasing the risk of post-procedure complications[10,35].
Several patient-dependent risk factors for PCCRC have been identified. These can be classified into biological factors and factors affecting colonoscopy quality.
Age: PCCRC is more common in individuals over 65 years old[10].
Sex: Some studies suggest a higher incidence of PCCRC in females.
Personal or family history of CRC: First-degree relatives of CRC patients and individuals with inflammatory bowel disease (IBD) have an increased risk of PCCR[10,36].
History of adenoma removal: Patients who had adenomas removed in their initial colonoscopy are at greater risk[10,37,38].
Diverticular disease: This condition has been associated with a higher risk of PCCRC[39].
Multiple comorbidities: Patients with significant underlying health conditions may also be at increased risk.
This distinction is important, as each of these factors may require different management strategies. Identifying patient-dependent risk factors enables the establishment of risk groups that could benefit from more targeted and rigorous surveillance protocols.
A study by Anderson et al[6] reported that 43% of PCCRC cases occur in patients with known colonic pathology or an elevated CRC risk. They introduced the concept of a “hot colon” or “unstable colon”, which includes patients with: (1) Previous CRC; (2) Multiple large polyps or piecemeal resections; (3) IBD; and (4) Hereditary cancer syndromes.
Special attention should be given to these patients to determine optimal management strategies, ensure strict adherence to surveillance protocols, and explore potential methods for preventing PCCRC in this high-risk population.
Certain conditions can make colonoscopy technically challenging, emphasizing the need for experienced endoscopists in these cases to reduce the risk of missed lesions.
Recent findings indicate that PCCRC differs from sporadic CRC (CCRd) in certain aspects.
Proximal colon location: PCCRC is more frequently found in the proximal colon, 55% of PCCRCs occur in the right colon, 19.5% in the left colon, 20% at the rectosigmoid junction. This could be due to the predilection of sessile serrated adenomas for the right colon[14], and the fact that the proximal colon is harder to prepare and examine properly. Samadder et al[10] also found that proximal colon cancer (49.5-55.4) was more frequent than distal colon cancer (19.5-26.4). Singh et al[40] reported that the interval CRC is 2.4 times more likely to occur in the proximal colon.
Earlier stage diagnosis: PCCRC is often diagnosed at earlier stages[10]. However, data on PCCRC prognosis is limited and conflicting. Some studies suggest that PCCRC may have a better prognosis than sporadic CRC, while others do not support this conclusion.
Genetic features: PCCRC more frequently exhibits a methylator phenotype and microsatellite instability compared to CRCd.
This correlates with proximal location, diagnosis at earlier stages, and likely a faster carcinogenesis than CRCd, displaying a behavior more similar to Lynch syndrome tumors[10,40].
Mortality and aggressiveness: This remains an unresolved issue, as there is no clear consensus. However, based on the largest studies, PCCRC appears to have a poorer prognosis compared to CRC diagnosed during the index colonoscopy, primarily due to its later stage at diagnosis[7-9].
Nevertheless, some authors have reported that interval CRCs are associated with earlier-stage cancer and a lower risk of death[10].
Several contextual factors have also been identified as contributors to increased PCCRC risk. In Anderson et al[6] 17% of PCCRC cases were attributed to administrative issues (e.g., delayed or lost appointment requests). Additionally, in 27% of cases, the endoscopist's decision-making process influenced tumor development.
Therefore, it is crucial that the colonoscopy report clearly states the follow-up plan, especially if there is any reasonable doubt about the procedure or if the case deviates from standard surveillance guidelines.
PCCRC is diagnosed more than six months after a colonoscopy in which no cancer was detected. In 2018, the WEO published a consensus document aimed at facilitating and standardizing the classification of PCCRC based on its most probable cause[3].
This classification facilitates the study of PCCRC incidence across different endoscopy units, helping identify areas for improvement in routine clinical practice. By enhancing colonoscopy quality, these improvements can help reduce the incidence of PCCRC.
According to current evidence, the most common cause of PCCRC is lesions that were not detected during the initial colonoscopy[4,5]. These include interval CRC and non-interval CRC types A, B, and C, with type C being the most significant.
Significant technological advancements in modern endoscopy such as high-definition imaging, magnification, CE, external devices for enhanced mucosal examination, and AI systems[22-31] have led to an increased ADR and improved lesion characterization and delineation. This allows for the selection of the most appropriate treatment at any given moment and ensures complete lesion resection. However, there is currently no reliable information about their true impact on clinical practice, and their role in reducing the incidence of PCCRC remains uncertain. Further implementation of additional measures will be necessary to accurately assess the real value of advancing technological tools.
Therefore, endoscopists must utilize the best available technology at their disposal to optimize ADR and achieve complete resections. By doing so, they will enhance colonoscopy quality, directly contributing to a reduction in PCCRC incidence.
However, when removing advanced adenomas, even with the most advanced tools available, it could be beneficial to perform a second-look colonoscopy six months later to confirm the absence of recurrence before resuming the scheduled surveillance program. This approach warrants further evaluation through appropriate studies.
Proper bowel preparation is particularly crucial for detecting missed lesions. Current evidence supports the use of a split-dose bowel preparation regimen, which has been shown to enhance colon cleanliness and is better tolerated by patients[17].
Clear and easy-to-understand preparation protocols should be provided to patients, with adequate time allocated to explaining the procedure to ensure proper preparation. Only with adequate bowel preparation, which enables thorough examination of the colonic mucosa, can colonoscopy be an effective tool for the diagnosis and prevention of CRC.
In addition, several endoscopist-dependent factors play a key role in the development of PCCRC, as they can compromise the quality of colonoscopy. These include withdrawal time, CIR, and ADR. Not all endoscopists are equally suited to perform every procedure; it is crucial to acknowledge individual limitations, and complex polyps—whether due to size, location, or suspicion of dysplasia or malignancy—should be handled by specialists within the unit who are specifically trained for such cases. Endoscopists must be aware of their strengths and limitations, which requires periodic self-assessment of their clinical practice to identify and address any deficiencies. Only through this process can they recognize their limitations and implement improvement measures.
Patient-dependent risk factors have also been identified, including biological factors such as age over 65, female sex, a personal history of IBD or previous polypectomy, and a family history of CRC. Additionally, certain conditions that affect colonoscopy quality, such as diverticular disease or significant comorbidities, increase the risk of developing PCCRC. Recognizing these high-risk patients allows for the implementation of strict and targeted surveillance programs using the best available diagnostic and therapeutic tools to prevent PCCRC. There are no specific guidelines in the literature regarding shortened colonoscopy surveillance for this particular group. However, it seems reasonable to follow a similar approach to post-treatment surveillance for colon cancer, with a second colonoscopy one year after the index procedure to ensure no missed lesions or incomplete resections.
Therefore, all endoscopy units should conduct regular audits, ideally annually, to identify and review PCCRC cases within their services, as well as assess key endoscopist performance indicators (e.g., CIR, ADR). Furthermore, protocols should be implemented to identify high-risk patient groups and adjust personalized surveillance intervals without delays. Whenever possible, procedures should be performed by the most experienced endoscopists using all necessary techniques to ensure optimal lesion detection.
A distinct scenario, not classified as PCCRC but rather as CRC recurrence after endoscopic treatment, occurs when a seemingly benign polyp is found to contain pT1 carcinoma after resection, yet a decision is made not to pursue additional surgical treatment. Although not strictly classified as PCCRC, this situation represents a recurrence of neoplastic disease detected after a screening colonoscopy, where polyps initially believed to be cured by resection later show evidence of recurrence.
In these situations, determining whether additional surgery is necessary or if endoscopic follow-up alone is sufficient requires a thorough risk assessment. This includes evaluating the polypectomy technique—particularly for signs of piecemeal resection or incomplete removal—as well as assessing histopathological factors associated with a high risk of recurrence. These factors include lymphovascular invasion, tumor budding, submucosal invasion depth, resection margin status, adenocarcinoma differentiation grade, integrity of the muscularis mucosae, and the presence of poorly differentiated clusters.
Several contextual factors have also been defined that may increase the risk of PCCRC. In Anderson et al[6], 17% of PCCRC cases were attributed to administrative problems (delays in appointments, loss of appointment requests, etc.), and in 27% of cases, the decision-making process of the endoscopist influenced the development of the tumor. Therefore, it is of vital importance that the endoscopist includes the recommended follow-up plan in the colonoscopy report, especially when there is reasonable doubt or if the case falls outside standard follow-up protocols.
PCCRC is a significant concern, accounting for 3.4%–9.3% of all CRC cases. The incidence of PCCRC reflects the limitations of colonoscopy in CRC diagnosis and prevention, making it the most critical quality parameter in clinical practice. Current studies indicate that the majority of PCCRC cases stem from preventable factors, with undetected lesions during the initial colonoscopy being the primary cause, followed by unexpected recurrence of previously resected polyps. Clinical factors such as advanced age, a family history of CRC or genetically related cancers (e.g., Lynch syndrome), high comorbidity, diverticular disease, and a history of polypectomy have been associated with an increased risk PCCRC. Additionally, endoscopist-related factors, including a low ADR, low CIR, incomplete resection evaluation, and suboptimal follow-up strategies for resected pT1 polyps, are linked to a higher PCCRC risk. Biological factors, such as microsatellite instability and the methylator phenotype, also contribute to the development of PCCRC. Identifying high-risk patients enables personalized surveillance, including adjustments to surveillance timing. These procedures should never be delayed, should be allocated additional time, and should be performed by the most experienced endos
| 1. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1830] [Reference Citation Analysis (5)] |
| 2. | Bisschops R, East JE, Hassan C, Hazewinkel Y, Kamiński MF, Neumann H, Pellisé M, Antonelli G, Bustamante Balen M, Coron E, Cortas G, Iacucci M, Yuichi M, Longcroft-Wheaton G, Mouzyka S, Pilonis N, Puig I, van Hooft JE, Dekker E. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2019. Endoscopy. 2019;51:1155-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (1)] |
| 3. | Rutter MD, Beintaris I, Valori R, Chiu HM, Corley DA, Cuatrecasas M, Dekker E, Forsberg A, Gore-Booth J, Haug U, Kaminski MF, Matsuda T, Meijer GA, Morris E, Plumb AA, Rabeneck L, Robertson DJ, Schoen RE, Singh H, Tinmouth J, Young GP, Sanduleanu S. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology. 2018;155:909-925.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 278] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 4. | Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, Cross AJ, Zauber AG, Church TR, Lance P, Greenberg ER, Martínez ME. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 5. | Gimeno-García AZ, Hernández-Pérez A, Benítez F, Segura N, Nicolás-Pérez D, Quintero E, Hernández-Álvarez N, Betancor I, Salido E, Hernández-Guerra M. Postcolonoscopy colorectal cancer: Prevalence, categorization and root-cause analysis based on the World Endoscopic Organization system. Gastroenterol Hepatol. 2024;47:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 6. | Anderson R, Burr NE, Valori R. Causes of Post-Colonoscopy Colorectal Cancers Based on World Endoscopy Organization System of Analysis. Gastroenterology. 2020;158:1287-1299.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 7. | Baile-Maxía S, Mangas-Sanjuan C, Sala-Miquel N, Barquero C, Belda G, García-Del-Castillo G, García-Herola A, Penalva JC, Picó MD, Poveda MJ, de-Vera F, Zapater P, Jover R. Incidence, characteristics, and predictive factors of post-colonoscopy colorectal cancer. United European Gastroenterol J. 2024;12:309-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (1)] |
| 8. | Forsberg A, Widman L, Bottai M, Ekbom A, Hultcrantz R. Postcolonoscopy Colorectal Cancer in Sweden From 2003 to 2012: Survival, Tumor Characteristics, and Risk Factors. Clin Gastroenterol Hepatol. 2020;18:2724-2733.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 9. | Macken E, Van Dongen S, De Brabander I, Francque S, Driessen A, Van Hal G. Post-colonoscopy colorectal cancer in Belgium: characteristics and influencing factors. Endosc Int Open. 2019;7:E717-E727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Samadder NJ, Curtin K, Tuohy TM, Pappas L, Boucher K, Provenzale D, Rowe KG, Mineau GP, Smith K, Pimentel R, Kirchhoff AC, Burt RW. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 11. | Pohl H, Srivastava A, Bensen SP, Anderson P, Rothstein RI, Gordon SR, Levy LC, Toor A, Mackenzie TA, Rosch T, Robertson DJ. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74-80.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 12. | Pellisé M. Actualización en CCR postcolonoscopia e importancia de tasa de deteccion de adenomas. Modified Dra Pellisé SEED. 2024;. |
| 13. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 931] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 14. | Adler J, Robertson DJ. Interval Colorectal Cancer After Colonoscopy: Exploring Explanations and Solutions. Am J Gastroenterol. 2015;110:1657-64; quiz 1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | Jacobson BC, Anderson JC, Burke CA, Dominitz JA, Gross SA, May FP, Patel SG, Shaukat A, Robertson DJ. Optimizing Bowel Preparation Quality for Colonoscopy: Consensus Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2025;120:738-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Kastenberg D, Bertiger G, Brogadir S. Bowel preparation quality scales for colonoscopy. World J Gastroenterol. 2018;24:2833-2843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (10)] |
| 17. | Kilgore TW, Abdinoor AA, Szary NM, Schowengerdt SW, Yust JB, Choudhary A, Matteson ML, Puli SR, Marshall JB, Bechtold ML. Bowel preparation with split-dose polyethylene glycol before colonoscopy: a meta-analysis of randomized controlled trials. Gastrointest Endosc. 2011;73:1240-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 410] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 19. | Shaukat A, Rector TS, Church TR, Lederle FA, Kim AS, Rank JM, Allen JI. Longer Withdrawal Time Is Associated With a Reduced Incidence of Interval Cancer After Screening Colonoscopy. Gastroenterology. 2015;149:952-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 190] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 20. | Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan R, Feeney M, Haslam N; British Society of Gastroenterology, the Joint Advisory Group on GI Endoscopy, the Association of Coloproctology of Great Britain and Ireland. UK key performance indicators and quality assurance standards for colonoscopy. Gut. 2016;65:1923-1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 21. | Corley DA, Levin TR, Doubeni CA. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Eisen GM. Chromoendoscopy of the colon. Gastrointest Endosc Clin N Am. 2004;14:453-460, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Brooker JC, Saunders BP, Shah SG, Thapar CJ, Thomas HJ, Atkin WS, Cardwell CR, Williams CB. Total colonic dye-spray increases the detection of diminutive adenomas during routine colonoscopy: a randomized controlled trial. Gastrointest Endosc. 2002;56:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Subramanian V, Mannath J, Hawkey CJ, Ragunath K. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Williet N, Tournier Q, Vernet C, Dumas O, Rinaldi L, Roblin X, Phelip JM, Pioche M. Effect of Endocuff-assisted colonoscopy on adenoma detection rate: meta-analysis of randomized controlled trials. Endoscopy. 2018;50:846-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 26. | Triantafyllou K, Gkolfakis P, Tziatzios G, Papanikolaou IS, Fuccio L, Hassan C. Effect of Endocuff use on colonoscopy outcomes: A systematic review and meta-analysis. World J Gastroenterol. 2019;25:1158-1170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Sánchez-Montes C, García-Rodríguez A, Córdova H, Pellisé M, Fernández-Esparrach G. Advanced endoscopy technologies to improve the detection and characterisation of colorrectal polyps. Gastroenterol Hepatol. 2020;43:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Pamudurthy V, Lodhia N, Konda VJA. Advances in endoscopy for colorectal polyp detection and classification. Proc (Bayl Univ Med Cent). 2020;33:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Huang Q, Fukami N, Kashida H, Takeuchi T, Kogure E, Kurahashi T, Stahl E, Kudo Y, Kimata H, Kudo SE. Interobserver and intra-observer consistency in the endoscopic assessment of colonic pit patterns. Gastrointest Endosc. 2004;60:520-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Li M, Ali SM, Umm-a-OmarahGilani S, Liu J, Li YQ, Zuo XL. Kudo's pit pattern classification for colorectal neoplasms: a meta-analysis. World J Gastroenterol. 2014;20:12649-12656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | ASGE Technology Committee; Manfredi MA, Abu Dayyeh BK, Bhat YM, Chauhan SS, Gottlieb KT, Hwang JH, Komanduri S, Konda V, Lo SK, Maple JT, Murad FM, Siddiqui UD, Wallace MB, Banerjee S. Electronic chromoendoscopy. Gastrointest Endosc. 2015;81:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Pattarajierapan S, Tipmanee P, Supasiri T, Wisedopas N, Khomvilai S. Texture and color enhancement imaging (TXI) plus endocuff vision versus TXI alone for colorectal adenoma detection: a randomized controlled trial. Surg Endosc. 2023;37:8340-8348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 33. | Toyoshima O, Nishizawa T, Hiramatsu T, Matsuno T, Yoshida S, Mizutani H, Ebinuma H, Matsuda T, Saito Y, Fujishiro M. Colorectal adenoma detection rate using texture and color enhancement imaging versus white light imaging with chromoendoscopy: a propensity score matching study. J Gastroenterol Hepatol. 2024;39:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 34. | Hassan C, Spadaccini M, Iannone A, Maselli R, Jovani M, Chandrasekar VT, Antonelli G, Yu H, Areia M, Dinis-Ribeiro M, Bhandari P, Sharma P, Rex DK, Rösch T, Wallace M, Repici A. Performance of artificial intelligence in colonoscopy for adenoma and polyp detection: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93:77-85.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 350] [Article Influence: 70.0] [Reference Citation Analysis (5)] |
| 35. | Hassan C, Spadaccini M, Mori Y, Foroutan F, Facciorusso A, Gkolfakis P, Tziatzios G, Triantafyllou K, Antonelli G, Khalaf K, Rizkala T, Vandvik PO, Fugazza A, Rondonotti E, Glissen-Brown JR, Kamba S, Maida M, Correale L, Bhandari P, Jover R, Sharma P, Rex DK, Repici A. Real-Time Computer-Aided Detection of Colorectal Neoplasia During Colonoscopy: A Systematic Review and Meta-analysis. Ann Intern Med. 2023;176:1209-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 36. | Da Cunha T, Vaziri H. Interval Colorectal Cancer in Inflammatory Bowel Disease: A Review. J Clin Gastroenterol. 2024;58:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Polychronidis G, He MM, Vithayathil M, Knudsen MD, Wang K, Song M. Risk of colorectal neoplasia after removal of conventional adenomas and serrated polyps: a comprehensive evaluation of risk factors and surveillance use. Gut. 2024;73:1675-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 38. | Muñoz García-Borruel M, Hervás Molina AJ, Rodríguez Perálvarez ML, Moreno Rincón E, Pérez Medrano I, Serrano Ruiz FJ, Casáis Juanena LL, Pleguezuelo Navarro M, Naranjo Rodríguez A, Villar Pastor C. Post-colonoscopy colorectal cancer: Characteristics and predictive factors. Med Clin (Barc). 2018;150:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 39. | Troelsen FS, Sørensen HT, Erichsen R. Risk of a post-colonoscopy colorectal cancer in patients with diverticular disease: a population-based cohort study. Endoscopy. 2024;56:471-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 40. | Singh S, Singh PP, Murad MH, Singh H, Samadder NJ. Prevalence, risk factors, and outcomes of interval colorectal cancers: a systematic review and meta-analysis. Am J Gastroenterol. 2014;109:1375-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/