Published online Jul 16, 2025. doi: 10.4253/wjge.v17.i7.106208
Revised: April 6, 2025
Accepted: May 20, 2025
Published online: July 16, 2025
Processing time: 140 Days and 4 Hours
Collagenous sprue is a serious intestinal malabsorption disorder characterized by dull and flattened intestinal villi with subepithelial collagen deposition. At pre
The authors present a case of a 34-year-old female patient who presented with severe watery diarrhea with significant weight loss. She also experienced blurred binocular vision. The intestinal lesions were located mainly in the small intestine, which presented flat and dull intestinal villi with subepithelial collagen deposi
Collagenous stomatitis diarrhea is a rare intestinal malabsorption disease that needs to be diagnosed in combination with special clinical manifestations and uni
Core Tip: Collagenous sprue is a rare and unrecognized cause of diarrhea and weight loss and affects mainly the duodenum and small bowel. The clinical picture often resembles that of celiac sprue, the main differential diagnosis, albeit refractory to a gluten-free diet. The histological features are fundamentally characterized by the deposition of collagen beneath the basement membrane of the gut mucosa. Treatment should be initiated as soon as the diagnosis is established to prevent the progression of fibrosis. We describe the case of a 34-year-old woman with a collagenous sprue, her diagnostic workup, histopathological examination, and response to treatment. This case can inform future clinicians to aid in timely and correct diagnosis.
- Citation: Chen CN, Fan XY, Yang CM, Zhang XH, Liu J. Refractory chronic diarrhea complicated with optic neuritis in a young woman: A case report. World J Gastrointest Endosc 2025; 17(7): 106208
- URL: https://www.wjgnet.com/1948-5190/full/v17/i7/106208.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i7.106208
Collagenous sprue is a rare and severe malabsorptive disorder that is histologically characterized by small intestinal villous atrophy and thick subepithelial collagen band deposition with a thickness > 5 μm[1]. The etiology is unclear, and immune-mediated mechanisms, infection, inflammation, drugs and the environment may be involved. Diarrhea, malabsorption and weight loss are typical symptoms. Some patients may have other autoimmune or immune disorders, such as IgA nephropathy, peripheral neuropathy, autoimmune hepatitis, autoimmune arthritis and dermatitis herpeti
However, there are no reports on collagenous sprue complicated with optic neuritis. The treatment of collagenous sprue is undefined, and most patients do not respond to a gluten-free diet (GFD). Glucocorticoids and thiopurine/thio
A 34-year-old female presented to the Shandong Provincial Hospital Affiliated to Shandong First Medical University due to chronic watery diarrhea lasting for 6 mo.
Over the past 6 mo, she experienced intermittent watery diarrhea > 10 times a day, associated with fatigue and 10 kg weight loss. Shortly after the onset of diarrhea symptoms, she also suffered blurred binocular vision.
The patient underwent a cesarean section 12 years ago. The COVID-19 vaccine was obtained on May 27, 2021. Six months prior, she began to have yellow, watery stools > 10 times a day, with a total amount of > 1 L/d, accompanied by fatigue, no abdominal pain, no fever, night sweats, and no oral or vulvar ulcers. She was treated in a local clinic for infection and symptomatic diarrhea, and there was no improvement for > 10 d.
After 2 wk, the patient presented symptoms of blurred vision and decreased light sensation. The results of the fundus examination in ophthalmology were as follows: bilateral papilledema; orbital enhanced magnetic resonance imaging showed that the bilateral optic nerves were thickened and strengthened; and bilateral optic neuritis was considered. She was admitted to the Department of Ophthalmology, Peking Union Medical College Hospital on July 16. The results of the demyelination tests were all normal, and the patient was diagnosed with optic neuritis. Then, 1 g methylprednisolone was given for 3 d, which was gradually reduced to oral administration, and yellow soft stool was collected during hospitalization, once or twice a day. She was discharged on July 28 after the ocular condition stabilized. When discharged, the dose of prednisone was 110 mg/d, which was reduced by two tablets per week to 10 mg. The drug was discontinued after 2 wk, during which the patient’s stool was yellow and soft, two or three times a day.
In September 2019, the patient developed yellow, watery stools seven or eight times per day. There were no abnormalities in routine blood tests, routine stool tests or bacterial culture in local hospitals. Gastroscopy revealed chronic nonatrophic gastritis. Colonoscopy revealed no abnormalities. Abdominal enhanced computer tomography revealed cholecystitis and small stones in the left kidney. After symptomatic treatment, diarrhea symptoms were relieved, and the patient was subsequently discharged. After discharge, the symptoms of diarrhea recurred, and a Chinese medicine decoction was prescribed orally at the local clinic. Two weeks prior, the diarrhea symptoms worsened, and the stools were yellow, watery, > 10 times a day, with a total volume > 1 L/d. Nausea, vomiting, and fatigue gradually increased, and the patient was subsequently admitted to our hospital.
The patient denied any family history of malignant tumors.
Vital signs were as follows: Body temperature, 36.9 °C; blood pressure, 97/78 mmHg; heart rate, 105 beats/min; respira
The abnormal laboratory test results were as follows: Hemoglobin 86 mg/L, serum potassium 2.79 mmol/L, sodium 125.2 mmol/L, calcium 2.0 mmol/L, serum albumin 30.2 g/L, globulin 15.9 g/L, immunoglobulin G 4.23 g/L, and C-reactive protein 21.86 mg/L. Other laboratory tests, including celiac disease-related antibodies, basic autoimmune antibodies, food intolerance tests, tuberculosis-specific enzyme-linked immunospot assay, Clostridium difficile toxin test and neuromyelitis optica spectrum disorder-related antibodies were all negative.
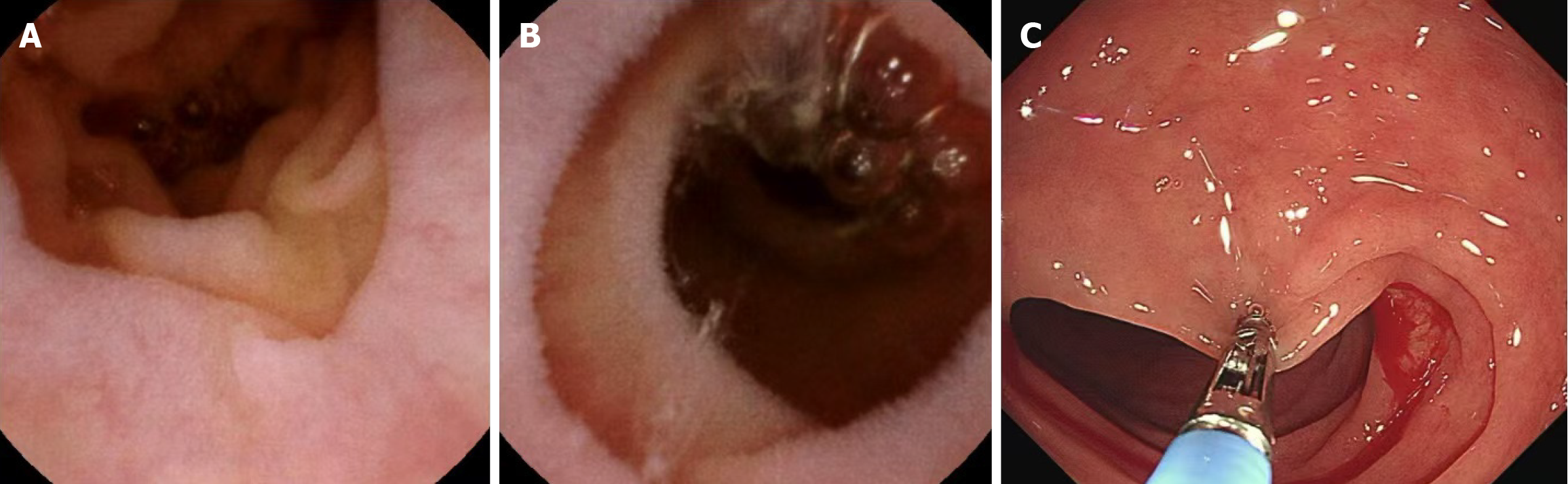
Endoscopy examinations: The patient had initial gastroenteroscopy at the local hospital. There were no duodenal or colonoscopic abnormalities. No biopsy was done. Capsule endoscopy revealed extensive atrophy of the villi of the small intestine, especially in the duodenum and proximal jejunum (Figure 1). Biopsy samples were taken from the descending segment of the duodenum via gastroscopy for definitive diagnosis (Figure 1).
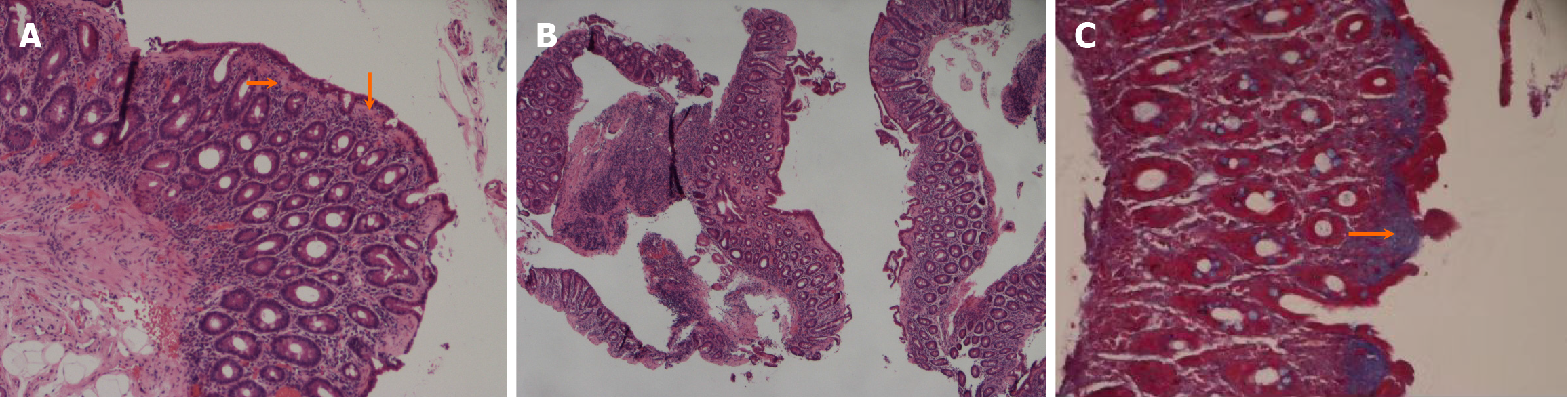
Pathologic findings: Part of the mucosal epithelium of the descending duodenum was exfoliated, the subepithelial collagen band was thickened, the mucosal villi were significantly atrophied, and the whole mucosa was infiltrated by plasma cells. Local mucosal muscle degeneration was suspected (Figure 2).
The pathological results revealed marked blunting of the villous architecture with a diffusely thickened subepithelial eosinophil hyaline band and plasma cell infiltration in the whole mucosa (Figure 2). Masson’s trichrome stain confirmed the presence of subepithelial collagen deposition with a thickness > 10 μm (Figure 2). These findings confirmed the diagnosis of collagenous sprue.
The patient first received a GFD for 7 d with no relief of diarrhea symptoms. She subsequently received glucocorticoid treatment (methylprednisolone, 60 mg once daily). Her diarrhea symptoms improved in the initial stage of treatment but recurred after hormone reduction. Her ocular symptoms were not completely relieved.
After treatment, the patient’s stool frequency was reduced to four or five times a day, and the dose of the methylprednisolone was reduced to 44 mg; after which the patient was discharged with medication. Half a year after discharge, the amount of methylprednisolone tablets was gradually reduced by one tablet per week, and then the drug was stopped after it was reduced to 8 mg. After drug discontinuation, the diarrhea symptoms did not reappear, the stools were yellow and soft two or three times a day, weight increased to approximately 2.5 kg, and the binocular vision did not recover.
Collagenous oral diarrhea, also known as collagenous enteritis, is a rare disease. Absorption disorders of the small intestine are rare. The disease was first reported in 1947 by Schein[5] and described as an idiopathic absorption disorder with small intestinal subepithelial eosinophilic deposition. In 1970, Weinstein et al[6] officially named it collagenous sto
The etiology of collagen-induced stomatitis is still unclear, and most current opinions suggest that it is an immune-mediated disease of the small intestine. The immunopathogenesis of collagenous sprue remains unclear, but immune dysregulation and T-cell-triggered inflammation may play a role[7]. Other studies suggest that collagen production may be affected by a variety of factors, such as diet and drugs (the antihypertensive angiotensin II receptor antagonist olmesartan)[8]. Regarding the mechanism of collagen deposition, it is believed that, owing to the overexpression of collagen-producing genes, such as tissue inhibitor of metalloproteinase-1, the degradation rate of collagen fibers remains unchanged. As a result, many collagen fibers accumulate under the intestinal mucosa, and the absorption of water in the intestinal mucosa and the obstruction of electrolyte exchange inside and outside the intestinal cavity occur. Autoimmune enteropathy can trigger a systemic inflammatory response, which may have an impact on the nervous system. For instance, inflammatory factors entering the bloodstream may cross the blood–brain barrier, leading to neuroinflammation. Additionally, the imbalance of the gut microbiota may also affect the nervous system through the gut–brain axis.
The diagnostic criteria for cytomegalovirus diarrhea include the following: typical clinical features such as severe malabsorption and weight loss; ineffective GFD; and typical histological changes in the small intestinal mucosa, including villi atrophy and obtuse, subepithelial collagen deposition, irregularly thickened collagen fiber bands of > 10 m, ca
When treating this disease, dietary restriction should be the first step even though patients often do not respond partially or completely to a GFD, and parenteral nutrition has been proposed as an adjunct therapy for patients with malnutrition. In addition, the combination of other drugs, such as steroids (corticosteroids) and immunosuppressants, may be a useful choice. Some case series have reported the success of treatment with a single oral glucocorticoid or combination of oral glucocorticoids (budesonide at 9 mg/d for 8 wk, followed by a gradual reduction in dose) and thiopurine/tioguanine (tioguanine at 10 mg once daily)[3,4]. Additionally, corticosteroids have been proven to be successful, and when necessary, escalation to infliximab has also shown promise[3,10]. Tacrolimus, by potently inhibiting the activity of T cells through acting as a calcineurin inhibitor, has demonstrated its effectiveness in treating refractory severe collagenous sprue[11]. Glucocorticoids were effective for our patient, but there seemed to be glucocorticoid dependence, and she currently does not use immunosuppressants.
Currently, there is a lack of long-term follow-up data on the treatment of this disease. The case first reported in the literature had a poor prognosis, including progressive worsening of malabsorption and diarrhea, which ultimately led to death[12]. However, recent reports of a large amount of biopsy data show that the collagen deposition has completely subsided, indicating that the lesions may be reversed in the long term after corticosteroid treatment[4,13], suggesting a good overall prognosis after appropriate treatment.
Chronic diarrhea caused by rare gastrointestinal disorders like collagenous sprue is diagnostically challenging due to overlapping clinical/endoscopic features with celiac disease. In seronegative patients with villous atrophy and mucosal pallor/scalloping on endoscopy, duodenal biopsies remain critical for diagnosis. Gastroenterologists should suspect collagenous sprue and collaborate closely with pathologists to confirm subepithelial collagen deposition via immunohistochemistry. Standardized biopsy protocols and clinical awareness may enable early intervention, arresting fibrosis and improving outcomes in this underdiagnosed condition.
| 1. | He H, Mei S, Sun L. A Rare Case of Chronic Diarrhea and Weight Loss. Clin Gastroenterol Hepatol. 2016;14:e111-e112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 2. | Vakiani E, Arguelles-Grande C, Mansukhani MM, Lewis SK, Rotterdam H, Green PH, Bhagat G. Collagenous sprue is not always associated with dismal outcomes: a clinicopathological study of 19 patients. Mod Pathol. 2010;23:12-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | van Gils T, van de Donk T, Bouma G, van Delft F, Neefjes-Borst EA, Mulder CJ. The first cases of collagenous sprue successfully treated with thioguanine. BMJ Open Gastroenterol. 2016;3:e000099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Lan N, Shen B, Yuan L, Liu X. Comparison of clinical features, treatment, and outcomes of collagenous sprue, celiac disease, and collagenous colitis. J Gastroenterol Hepatol. 2017;32:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Schein J. Syndrome on non tropical sprue with hitherto undescribed lesions of the intestine. Gastroenterology. 1947;8:438-460. [PubMed] |
| 6. | Weinstein WM, Saunders DR, Tytgat GN, Rubin CE. Collagenous sprue--an unrecognized type of malabsorption. N Engl J Med. 1970;283:1297-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 113] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | van Wanrooij RLJ, Bontkes HJ, Neefjes-Borst EA, Mulder CJ, Bouma G. Immune-mediated enteropathies: From bench to bedside. J Autoimmun. 2021;118:102609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Kulai T, Arnason T, MacIntosh D, Igoe J. Duodenal Villous Atrophy in a TTG-Negative Patient Taking Olmesartan: A Case Report and Review of the Literature. Can J Gastroenterol Hepatol. 2016;2016:6091571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Busto-Bea V, Crespo-Pérez L, García-Miralles N, Ruiz-del-Árbol-Olmos L, Cano-Ruiz A. Collagenous sprue: don´t forget connective tissue in chronic diarrhea evaluation. Rev Esp Enferm Dig. 2013;105:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Al-Bawardy B, Ramos GP, Wu T, Rubio-Tapia A, Murray JA. Sa1409 Collagenous Sprue: 15 Year Experience of a Large Tertiary Center. Gastroenterology. 2016;150:S307-S308. [DOI] [Full Text] |
| 11. | Lange K, Stallhofer J, Gaßler N, Ripoll C, Stallmach A. Successful Long-Term Treatment of Collagenous Sprue With Tacrolimus in a 25-Year-Old With Severe Intestinal Failure. Inflamm Bowel Dis. 2025;31:298-299. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Doe WF, Evans D, Hobbs JR, Booth CC. Coeliac disease, vasculitis, and cryoglobulinaemia. Gut. 1972;13:112-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Schreiber FS, Eidt S, Hidding M, Schmidt-Walczuch J, Werning C. Collagenous duodenitis and collagenous colitis: a short clinical course as evidenced by sequential endoscopic and histologic findings. Endoscopy. 2001;33:555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/