Published online Jun 16, 2025. doi: 10.4253/wjge.v17.i6.105904
Revised: March 21, 2025
Accepted: April 7, 2025
Published online: June 16, 2025
Processing time: 121 Days and 18.9 Hours
Neuroendocrine tumors (NETs) are an important type of neoplastic disease of the digestive tract. There is little data on NETs originating from the pancreato-hepatobiliary region of the digestive tract in Pakistan.
To evaluate different types of pancreato-hepatobiliary NETs (PHB-NET) diagnosed with endoscopic ultrasound (EUS) and to identify factors associated with high-grade NETs.
All patients diagnosed with PHB-NET through EUS-guided biopsy were included in the study. The site of origin, histology, and grade of PHB-NETs were noted and factors associated with high-grade lesions were analyzed. SPSS, version 20.0 was used for statistical analysis.
A total of 36 patients with PHB-NET were included. Males and females were equal in numbers, i.e., 18 (50%) each. The mean age was 48 ± 15.7 years with an age range of 17-70 years. The most common sites of origin of PHB-NET were: Pancreas 20 (55.6%), porta hepatis mass 8 (22.2%), perigastric mass 3 (8.3%) and others 5 (13.9%). The mean size of the PHB-NETs was 34.7 ± 22.5 mm. Among pancreatic NETs, the most commonly affected areas were body 9, tail 5, and head 5. Only 4 (11.1%) PHB-NETs were functioning, all of which were insulinomas originating from the body or tail of the pancreas. Two-thirds of PHB-NETs, 24 (66.6%), were benign (WHO grade I: 19; grade 2: 5) while one-third 12 (33.3%) were neuroendocrine cancers (NEC) (WHO grade III). Histological types were large cell 17 (47.2%), small cell 8 (22.2%), mixed 1 (2.8%), and undetermined 10 (27.8%). Factors associated with NECs were age > 40 years (P = 0.016), extra-pancreatic origin of the lesion (P = 0.014), and small cell histologic type (P < 0.001).
The most common site of PHB-NET detected through EUS was the pancreas. Although most were benign, about one-third were high-grade cancers. Insulinoma was the most common functioning tumor. NECs were associated with advanced age, extra-pancreatic origin, and small-cell histology.
Core Tip: Pancreato-hepatobiliary neuroendocrine tumors (PHB-NETs) are now mostly diagnosed via endoscopic ultrasound (EUS), with the pancreas being the most frequent site. While most PHB-NETs are benign, one-third are high-grade neuroendocrine cancers (NECs). Insulinomas are the predominant functioning neuroendocrine tumors. Key risk factors for NECs include age > 40 years, extra-pancreatic origin and small-cell histology. EUS-guided biopsy is essential for early detection, risk stratification, and treatment planning of PHB-NETs. Clinicians should maintain a high index of suspicion for NECs in older patients with non-pancreatic lesions and small-cell histology to optimize management strategies.
- Citation: Tasneem AA, Luck NH, Mubarak M. Pancreato-hepatobiliary neuroendocrine tumors diagnosed through endoscopic ultrasound: Clinical characteristics and factors associated with high-grade lesions. World J Gastrointest Endosc 2025; 17(6): 105904
- URL: https://www.wjgnet.com/1948-5190/full/v17/i6/105904.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i6.105904
Neuroendocrine tumors (NETs) form an important type of neoplastic disease of the digestive tract. They arise from the neuroendocrine cells and may affect any part of the digestive tract from the esophagus to the anus. However, the small intestine is the most commonly affected region[1]. These tumors can also arise from the pancreas, liver, and upper abdominal areas like peri-gastric, porta hepatis or retroperitoneal regions and can be classified as pancreato-hepatobiliary NETs (PHB-NETs). These can be sporadic or may be associated with hereditary syndromes like multiple endocrine neoplasia, von Hippel-Lindau syndrome, neurofibromatosis 1 and tuberous sclerosis[2]. These tumors can be functioning or non-functioning depending on the amount of hormones they produce. Histologically, these tumors can be divided into low/intermediate grade tumors that consist of well-differentiated tumors and high-grade ones that consist of poorly differentiated cancers[3]. Furthermore, these tumors may exhibit small-cell, large-cell, or mixed morphologies[4].
The NETs were previously considered to be largely benign lesions. However, recent research has shown that they can be very aggressive tumors that may be untreatable at the time of diagnosis[5]. The characteristics of NETs affecting the various organ systems of the body have been studied but data regarding PHB-NET is relatively scarce in the medical literature. This study aimed to study those PHB-NETs diagnosed through endoscopic ultrasound (EUS)-guided biopsy and to determine the risk factors associated with high-grade NETs.
This cross-sectional study was performed in the Department of Hepatogastroenterology, Sindh Institute of Urology and Transplantation, Karachi, Pakistan. All adult patients (≥ 17 years) of either gender who were found to have a neoplastic lesion in the pancreato-hepatobiliary (PHB) region on abdominal imaging and were subsequently diagnosed with NET of the PHB region on EUS-guided biopsy, from January 2019 to December 2024 were included. Patients with a past history of PHB-NETs were excluded. The demographic and clinical characteristics of these patients were noted from the case files. Besides, the morphology of the tumor, the organ involved, and the histologic grade of the tumor were recorded from the histopathology reports. Written informed consent was obtained from all patients for participation in this study. The study reporting followed the STROBE guidelines and was conducted following the principles of the Declaration of Helsinki.
The PHB region approached through EUS was classified as pancreatic or extra-pancreatic. Pancreatic regions consisted of the head (including the uncinate process), neck, body, or tail of the pancreas. The extra-pancreatic region included porta hepatis, peri-gastric region, retroperitoneal region, liver, peri-ampullary region, and mediastinal lymph nodes. The areas visualized with EUS and the different structural lesions identified in these regions are shown in Table 1.
| Region | Structures biopsied | |
| 1 | Porta hepatis | Lymph nodal masses along inferior surface of liver |
| Solid masses, distinct from liver along inferior surface of liver | ||
| 2 | Peri-gastric | Lymph nodal masses in areas anterior and lateral to stomach |
| Solid peri-pancreatic or peri-splenic masses | ||
| 3 | Retroperitoneal | Lymph nodal masses posterior to stomach (excluding the pancreas), behind upper peritoneal cavity |
| Para-aortic or para-caval masses | ||
| 4 | Hepatic | Lesions in left lobe of liver |
| 5 | Periampullary | Deep periampullary masses (not visible on luminal examination) |
| 6 | Mediastinal | Subcarinal, right or left hilar LNs (metastatic from subsequently biopsy proven malignant neuroendocrine PHB primary) |
Linear array endosonoscopes used were Pentax EG38-J10UT, Pentax EG-3870UTK, and Olympus TGF-UC180J. For tissue acquisition, the needles used were AcquireTM fine needle biopsy (FNB device) (Boston Scientific; 22G and 25G). The facility of rapid on-site evaluation was not available. Therefore, macroscopic on-site evaluation was performed, whereby, a biopsy specimen consisting of at least 2 continuous tissue cores of lengths at least one inch each was considered satisfactory. The biopsy samples were fixed in 10% neutral buffered formalin and submitted to the Histopathology laboratory for further processing for light microscopy and immunohistochemistry (IHC). Cell blocks were made of the remaining tiny fragments and were processed along with larger cores for pathological evaluation. The biopsy samples were examined by two experienced histopathologists, first independently and then jointly to arrive at a consensus diagnosis to reduce the interobserver variability.
The diagnosis of NETs was made on a combination of histologic examination (for size, shape, and arrangement of cells), IHC staining (for chromogranin A, synaptophysin, CD 56 and Ki-67 index for tumor proliferation), and tumor characteristics [NETs (well-differentiated) and neuroendocrine cancers (NEC) (poorly differentiated)]. The classification was performed according to the WHO 2022 Epithelial Neuroendocrine Neoplasms Classification[6], as shown in Table 2.
| Neuroendocrine neoplasm | Classification | Diagnostic criteria |
| Well-differentiated neuroendocrine tumors (NET) | NET, grade 1 | < 2 mitoses/2 mm2 and/or Ki67 < 3% |
| NET, grade 2 | 2-20 mitoses/2 mm2 and/or Ki67 3-20% | |
| NET, grade 3 | > 20 mitoses/2 mm2 and/or Ki67 > 20% | |
| Poorly differentiated neuroendocrine carcinoma (NEC) | Small cell NEC | > 20 mitoses/2 mm2 and/or Ki67 > 20% (often > 70%), and small cell cytomorphology |
| Large cell NEC | > 20 mitoses/2 mm2 and/or Ki67 > 20% (often > 70%), and large cell cytomorphology |
The retrieved data of the patients was recorded in the pre-structured proforma. It was then entered into the SPSS, version 26.0 (IBM Corp, Armonk, NY, United States). The frequency of various types of NETs was computed. Values were expressed as mean ± SD for continuous variables depending on the distribution or as percentages for categorical variables. Statistical analysis was performed on categorical data (age < 40 vs > 40, male vs female, pancreatic or extra-pancreatic origin of lesion, size of lesion < 20 mm vs > 20 mm, functional vs non-functional tumor, small cell vs non-small cell histology) for which χ2 test or Fisher’s exact test was employed to identify factors associated with the malignant/high-grade NETs. A P value of < 0.05 was considered statistically significant.
A total of 36 patients with PHB-NETs were included, in which both genders were equal in number i.e., 18 (50%). The mean age was 48 ± 15.7 years with an age range of 17 to 70 years and the median age was 48.4 with an interquartile range of 38 to 63.5 years. Three-fourths of the patients were of age more than 40 years, i.e., 27 (75.0%) while only 9 patients were younger (Table 3). The mean size of the NETs was 34.7 ± 22.5 mm. The commonest histological type was large cell type accounting for 17 (47.2%) cases followed by small cell 8 (22.2%), mixed 1 (2.8%), and undetermined 10 (27.8%) types (Table 3).
| No. of patients | Percentage (%) | ||
| Age | < 40 years | 9 | 25.0 |
| > 40 years | 27 | 75.0 | |
| Gender | Males | 18 | 50.0 |
| Females | 18 | 50.0 | |
| PHB regions affected | Pancreas | 20 | 55.6 |
| Others | 16 | 44.4 | |
| Grade of NETs | WHO grade I (Ki 67: < 2%) | 19 | 52.8 |
| WHO grade II (Ki 67: 2-20%) | 5 | 13.9 | |
| WHO grade III (Ki 67: > 20%) | 12 | 33.3 | |
| Histologic type | Large cell | 17 | 47.2 |
| Small cell | 8 | 22.2 | |
| Mixed | 1 | 2.8 | |
| Undetermined | 10 | 27.8 | |
| Functionality | Non-functioning | 32 | 88.9 |
| Functioning | 4 | 11.1 | |
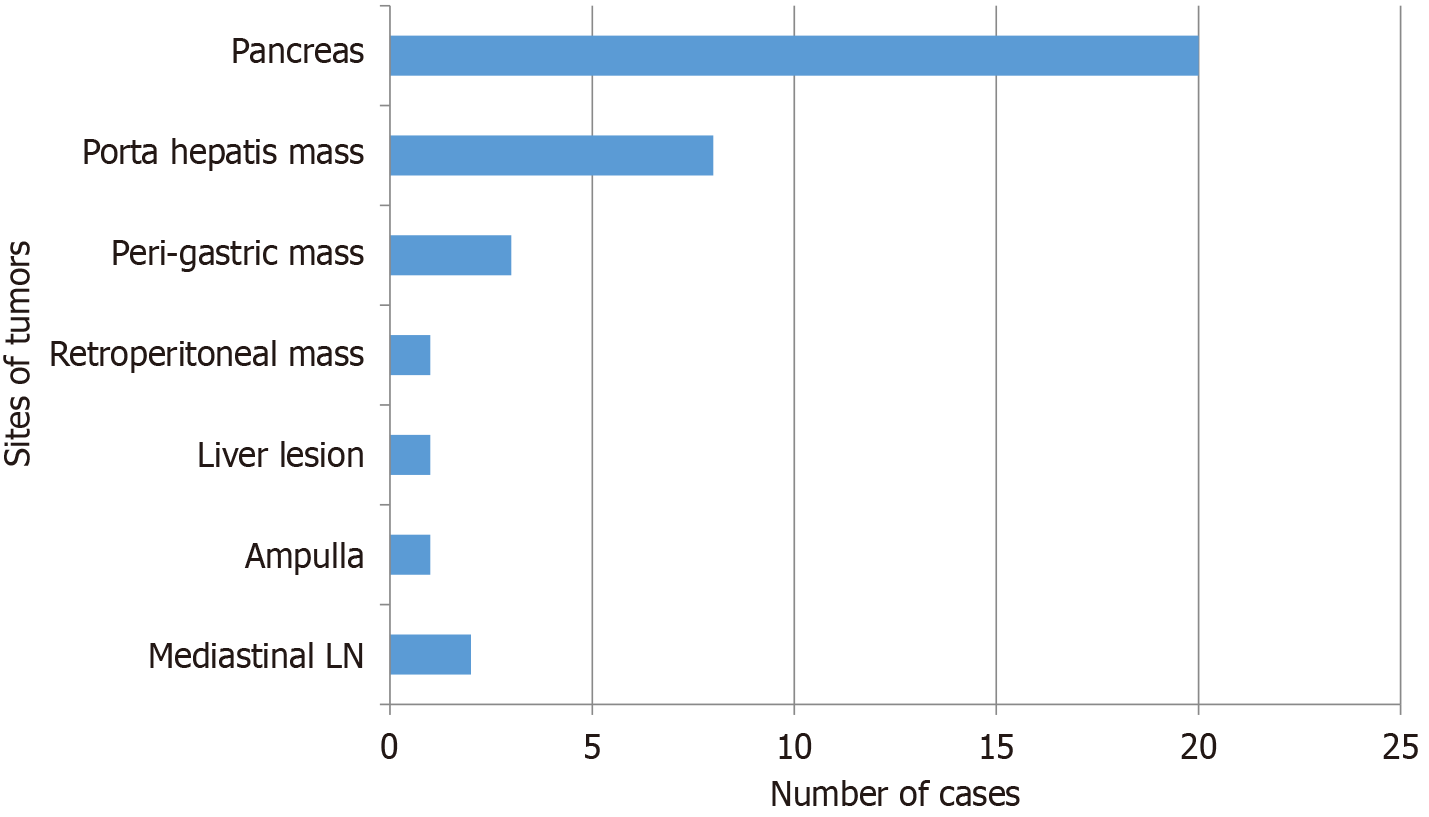
The PHB regions affected by NETs are shown in Figure 1. The most common organ with PHB-NETs was the pancreas accounting for 20 (55.6%) patients. The other tissues whose biopsy showed this diagnosis were porta hepatis mass lesions 8 (22.2%), perigastric mass lesions 3 (8.3%), mediastinal (subcarinal, right and left hilar) lymph nodes 2 (5.4%), left lobe liver lesion 1 (2.7%), ampullary mass 1 (2.7%), and retroperitoneal mass lesion 1 (2.7%). Among the 20 patients with pancreatic NETs, the most commonly affected area was the body of the pancreas accounting for 9 cases followed by tail 5, head 5, and neck of pancreas 1. Among the 8 patients with porta hepatis mass lesions, 5 were adjacent to the portal vein, while 3 were in the subhepatic region, distant from the portal vein (as visualized during EUS). The frequency of different PHB regions affected by benign and malignant NETs is demonstrated in Table 4.
| PHB region affected | n (%) | Benign NET (n) | NEC (n) | Benign NET (%) (no/total) | NEC (%) (no/total) | |
| Pancreas (n = 20) | Body | 9 (45) | 7 | 2 | 85%; (17/20) | 15%; (3/20) |
| Tail | 5 (25) | 5 | 0 | |||
| Head | 5 (25) | 4 | 1 | |||
| Neck | 1 (5) | 1 | 0 | |||
| Extra-pancreatic PHB-NETs (n = 16) | Porta hepatis mass | 8 (50) | 2 | 6 | 43.7%; (7/16) | 56.3%; (9/16) |
| Peri-gastric mass | 3 (18.7) | 3 | 0 | |||
| Liver | 1 (6.2) | 0 | 1 | |||
| Ampulla | 1 (6.2) | 1 | 0 | |||
| Retroperitoneal mass | 1 (6.2) | 1 | 0 | |||
| Mediastinal LN1 | 2 (12.5) | 0 | 2 | |||
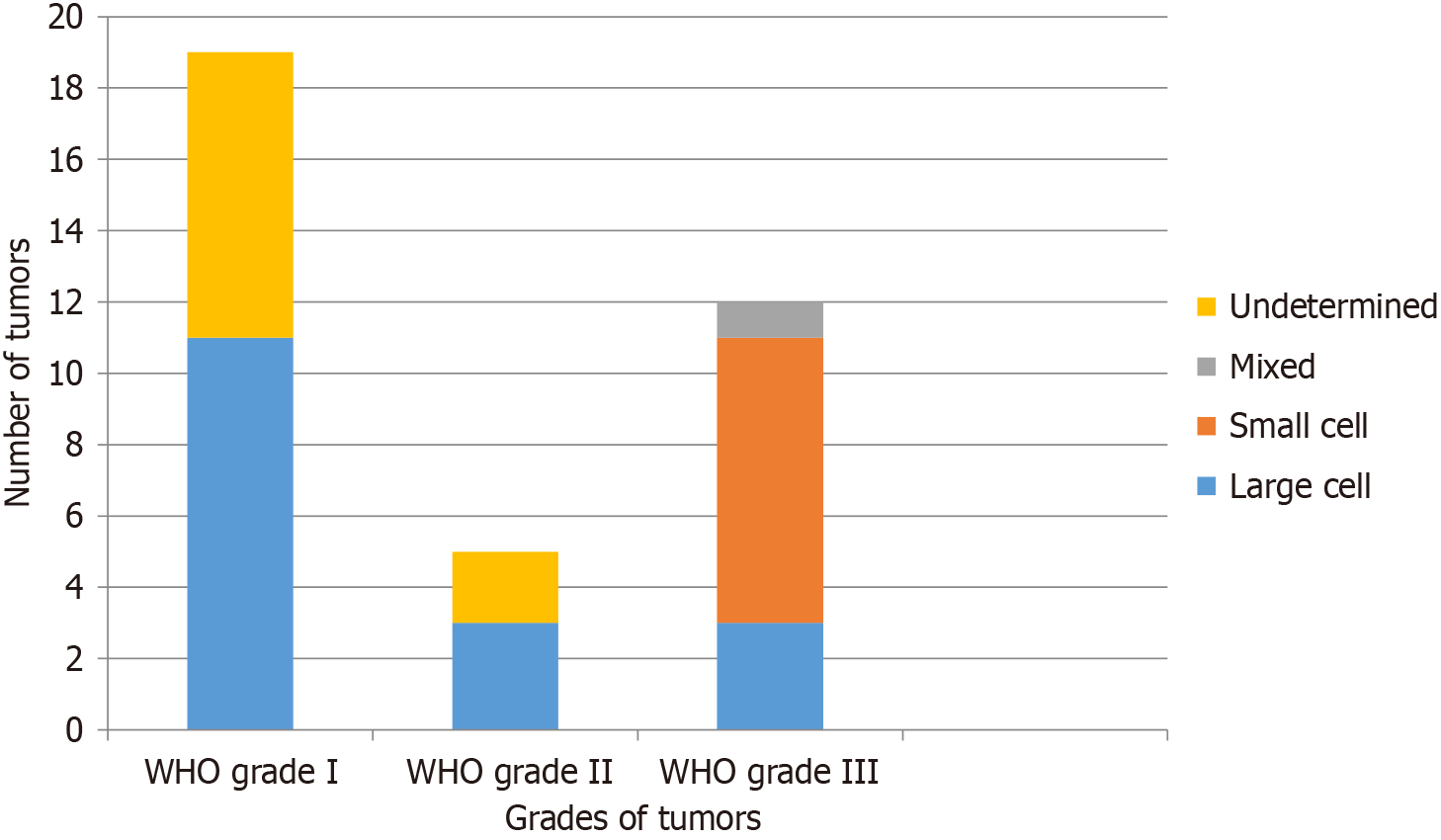
Two-thirds (24/36; 66.7%) of the patients with PHB-NETs had benign tumors (Ki-67 ≤ 20%). WHO grade I (Ki-67 of < 2%) NETs were found in 19 (52.8%) while WHO grade II (Ki-67 of 2%-20%) in 5 (13.9%) patients. NECs, having WHO grade III (Ki-67 of > 20%), were found in 12 (33.3%) patients. The proportion of the patients with different WHO grades of NETs having various histologies is shown in Figure 2. As shown, all small cell cancers were found among patients with WHO grade III NETs. Functioning NETs were found to be present in only 4 (11.1%) patients, while the remaining were non-functioning tumors. All functioning NETs were insulinomas that involved the body and tail of the pancreas. All patients with insulinomas were symptomatic, having recurrent hypoglycemic episodes, and requiring frequent hospitalization. All patients with insulinoma and one patient who had NET WHO grade I underwent EUS-guided ablation with injection of ethanol 99%.
Univariate analysis was performed to identify the factors associated with NECs i.e., NETs with WHO grade III (Ki-67 > 20%). Clinical parameters found to be associated with NECs were age > 40 years (P = 0.016), extrapancreatic origin of NETs (P = 0.014), and small cell histologic type (P < 0.0001). No statistically significant association of NECs was found with gender, size, or functionality of NETs (Table 5).
| Clinical characteristics | NEC | NET | Odds ratio | Confidence interval | P value | |
| Age | > 40 | 12 | 15 | 1.80 | 1.285-2.522 | 0.016 |
| < 40 | 0 | 09 | ||||
| Gender | Male | 5 | 13 | 1.655 | 0.408-6.714 | 0.725 |
| Female | 7 | 11 | ||||
| Origin of NET | Extra pancreatic | 9 | 7 | 0.137 | 0.028-0.663 | 0.014 |
| Pancreatic | 3 | 17 | ||||
| Largest lesion diameter | > 20 mm | 11 | 15 | 0.152 | 0.017-1.378 | 0.115 |
| < 20 mm | 1 | 9 | ||||
| Functionality | Functional | 0 | 4 | 0.625 | 0.478-0.817 | 0.278 |
| Non-functional | 12 | 20 | ||||
| Histology | Small cell | 8 | 0 | 7.000 | 2.825-17.343 | < 0.0001 |
| Non-small cell | 4 | 24 | ||||
In our study, the sample size was small, primarily because it focused on a specific variety of the rare NETs, i.e., of the PHB region, and diagnosed only with EUS. Besides, the majority of the patients were of age greater than 40 years with the mean age being 48.4 years. This is because the majority of these patients were those with sporadic NETs while among the young patients, only one had von Hippel Lindau syndrome, a condition that predisposes to pancreatic neoplasms in the earlier part of life. Also, in this study, there was no gender predominance among patients with NETs. A study done in China showed that there was no significant gender predominance among NET patients; however, there were slightly higher numbers of females (52.8% vs 47.2%), with the largest subgroup being 40-60 years of age (54.9%)[7]. Interestingly, a study from England showed that females diagnosed with NETs tend to survive longer than males[8]. This data shows that NETs can affect any gender and age; although, the majority are those above age 40 years.
Generally, pancreatic NETs are responsible for less than 10% of all pancreatic tumors while those of the biliary tract are extremely rare and are found in not more than 2% of all gastrointestinal cancers[9]. Also, pancreatic NETs are much less common than pancreatic exocrine tumors and have a better prognosis[10]. In this research, which studied only the PHB-NETs, more than half of these affected the pancreas; however, quite a significant number was also detected in extra-pancreatic sites like porta hepatis, peri-gastric, liver, ampulla & peri-ampullary duodenum and retroperitoneal region. The medical literature shows varying information with some sources reporting that hepatobiliary NETs are extremely rare with excellent prognosis[11], while others reporting gall bladder NET as another rare cause of NET and having a prognosis even worse than gall bladder adenocarcinoma[12]. It is likely that the porta hepatis and peri-gastric masses seen through EUS in our study have their origins in the liver, gall bladder, and retroperitoneal regions. All such cases were later discussed in the institutional tumor board meeting for further management according to the latest guidelines.
The majority of the NETs in this study were benign tumors. This finding is in contrast to other studies which show that NETs are predominantly malignant[13,14]. A possible reason why most of these patients had early, benign lesions is that these cases were referred to us for an incidental diagnosis of pancreatic lesions on a computed tomography scan that was done for some other complaint. These lesions were small in size and caused no symptoms. At the time of EUS-guided biopsy, these NETs were in the early period of their development. Besides, we found that the large cell type was more common than the small-cell NETs. This is consistent with the worldwide observation whereby the large cell type has been observed rather more commonly than the small cell type[15,16]. Also, in this study, the most commonly affected area of the pancreas was the body followed by the tail and head. Studies done elsewhere in the world also show that pancreatic NETs are more common in the body/tail region as compared to the head. Interestingly, a study from China further demonstrated that the prognosis of pancreatic head NETs sized 21-40 mm was worse than that of pancreatic body/tail NETs[17].
In this study, high-grade NETs (NECs; Ki-67 > 20%) were associated with advanced age, extra-pancreatic site of origin and small cell histology of the tumor. The grade of tumor was not associated with any specific gender, size, or functionality of the tumor. Various studies have shown that small-cell NETs, particularly those arising from the lung, are very aggressive tumors[18]. In contrast, a study by Yachida et al[16] shows that both small and large cell NETs of pancreatic origin may be aggressive tumors. Interestingly, a study from Karachi reveals that high-grade (grade 3) well-differentiated NETs were more frequent in the pancreatobiliary tract than the gastrointestinal tract[19]. Our study showed that while pancreatic NETs were largely benign, the majority of the porta hepatis masses were malignant. A plausible explanation for this finding is that the porta hepatis masses may represent metastatic lymph nodal masses from NECs in the surrounding hepatobiliary regions which may have been missed on imaging. Besides, lymph node metastases have also been shown to be a significant predictor of recurrence and survival in gastroenteropancreatic NETs[20]. Not much data is available in medical literature for comparing pancreatic small and large-cell NETs. Although our study showed that small-cell NETs are associated with high-grade lesions, a study comparing the survival of gastric small-cell and large-cell NEC found no statistical significance[21]. Similarly, a study comparing the survival of gall bladder NECs with that of adenocarcinomas, also found no statistically significant difference[22]. A study comparing the pulmonary small and large cell NETs, demonstrated that expression of VGF (nerve growth factor inducible) may be associated with advanced stage and poor prognosis[23]. Whether or not this is true for PHB neoplasms, will only be evident with further studies in this regard.
Our research demonstrated that although a small number of large-cell NETs were malignant, a majority of these were benign; while all cases with small-cell NETs were malignant, high-grade tumors. Furthermore, this study is unique because it compares the behavior of pancreatic with extra-pancreatic NETs of the PHB region. This important piece of information is a valuable addition to the already existing but scarce literature regarding the characteristics and behavior of PHB-NETs.
There are some limitations in the study as well. These include its retrospective design, small sample size, single-center origin, lack of evaluation for hereditary syndromes, and lack of follow-up data on the outcomes of the tumors. Nevertheless, it is an important piece of evidence regarding the clinicopathological characteristics of a rare type of PHB tumors.
In summary, the most common site of PHB-NET detected through EUS was the pancreas. Although most were benign, about one-third were high-grade cancers. Insulinoma was the most common functioning tumor. NECs were associated with advanced age, extra-pancreatic origin, and small-cell histology.
| 1. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (20)] |
| 2. | Kim JY, Hong SM. Recent Updates on Neuroendocrine Tumors From the Gastrointestinal and Pancreatobiliary Tracts. Arch Pathol Lab Med. 2016;140:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 11.1] [Reference Citation Analysis (2)] |
| 3. | Pellat A, Cottereau AS, Terris B, Coriat R. Neuroendocrine Carcinomas of the Digestive Tract: What Is New? Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Fujiwara W, Yotsukura M, Yoshida Y, Nakagawa K, Kashima J, Yatabe Y, Watanabe SI. Clinical and Pathologic Differences between Small-Cell Carcinoma and Large-Cell Neuroendocrine Carcinoma of the Lung. Ann Surg Oncol. 2024;31:5697-5705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Zhang XB, Fan YB, Jing R, Getu MA, Chen WY, Zhang W, Dong HX, Dakal TC, Hayat A, Cai HJ, Ashrafizadeh M, Abd El-Aty AM, Hacimuftuoglu A, Liu P, Li TF, Sethi G, Ahn KS, Ertas YN, Chen MJ, Ji JS, Ma L, Gong P. Gastroenteropancreatic neuroendocrine neoplasms: current development, challenges, and clinical perspectives. Mil Med Res. 2024;11:35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 6. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 623] [Article Influence: 155.8] [Reference Citation Analysis (2)] |
| 7. | Fu M, Yu L, Yang L, Chen Y, Chen X, Hu Q, Sun H. Gender differences in pancreatic neuroendocrine neoplasms: A retrospective study based on the population of Hubei Province, China. Front Endocrinol (Lausanne). 2022;13:885895. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | White BE, Russell B, Remmers S, Rous B, Chandrakumaran K, Wong KF, Van Hemelrijck M, Srirajaskanthan R, Ramage JK. Sex Differences in Survival from Neuroendocrine Neoplasia in England 2012-2018: A Retrospective, Population-Based Study. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 9. | Nwankwo EC, Khneizer G, Sayuk G, Presti M, Elwing JE. S1770 Neuroendocrine Tumor in the Biliary Tract Presenting With Cholangitis. Am J Gastroenterol. 2022;117:e1245-e1245. [DOI] [Full Text] |
| 10. | Pancreatic Neuroendocrine Tumors (Islet Cell Tumors) Treatment (PDQ®): Patient Version. 2022 Oct 7. In: PDQ Cancer Information Summaries [Internet]. Bethesda (MD): National Cancer Institute (US); 2002– . [PubMed] |
| 11. | Altiti M, Al-Sa'afin AJ, Al-Tawarah T, Suleihat A, Abulhaj S, Mahseeri M. Biliary tree neuroendocrine tumor, an incidental finding. Int J Surg Case Rep. 2021;82:105940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Marak JR, Kumar T, Dwivedi S, Khurana R. Neuroendocrine tumor of the gall bladder: A rare case report with review of literature. Radiol Case Rep. 2023;18:3912-3916. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Ro C, Chai W, Yu VE, Yu R. Pancreatic neuroendocrine tumors: biology, diagnosis,and treatment. Chin J Cancer. 2013;32:312-324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | de Wilde RF, Edil BH, Hruban RH, Maitra A. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev Gastroenterol Hepatol. 2012;9:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Luchini C, Pelosi G, Scarpa A, Mattiolo P, Marchiori D, Maragliano R, Sessa F, Uccella S. Neuroendocrine neoplasms of the biliary tree, liver and pancreas: a pathological approach. Pathologica. 2021;113:28-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, de Wilde RF, Maitra A, Hicks J, Demarzo AM, Shi C, Sharma R, Laheru D, Edil BH, Wolfgang CL, Schulick RD, Hruban RH, Tang LH, Klimstra DS, Iacobuzio-Donahue CA. Small cell and large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol. 2012;36:173-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 17. | Mei W, Ding Y, Wang S, Jia Y, Cao F, Li F. Head and body/tail pancreatic neuroendocrine tumors have different biological characteristics and clinical outcomes. J Cancer Res Clin Oncol. 2020;146:3049-3061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Pandjarova I, Mercieca D, Gijtenbeek RGP, Pereira JO, Fantin A, Castaldo N, Keramida E, Pannu K, Konsoulova A, Aujayeb A. Small cell lung cancer and neuroendocrine tumours. Breathe (Sheff). 2024;20:240004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Hashmi AA, Ali J, Yaqeen SR, Ahmed O, Asghar IA, Irfan M, Asif MG, Edhi MM, Hashmi S. Clinicopathological Features of Primary Neuroendocrine Tumors of Gastrointestinal/Pancreatobiliary Tract With Emphasis on High-Grade (Grade 3) Well-Differentiated Neuroendocrine Tumors. Cureus. 2021;13:e12640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Bonds M, Rocha FG. Neuroendocrine Tumors of the Pancreatobiliary and Gastrointestinal Tracts. Surg Clin North Am. 2020;100:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Li Z, Ren H, Zhang X, Sun C, Fei H, Li Z, Guo C, Shi S, Chen Y, Zhao D. Equivalent Survival between Gastric Large-Cell Neuroendocrine Carcinoma and Gastric Small-Cell Neuroendocrine Carcinoma. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Tang H, Jiang X, Zhu L, Xu L, Wang X, Li H, Gao F, Liu X, Ren C, Zhao Y. Clinicopathologic and molecular characteristics of neuroendocrine carcinomas of the gallbladder. Histol Histopathol. 2025;40:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Yang LH, Lee RK, Kuo MH, Miao CC, Wang YX, Chen A, Jhu YW, Cheng HI, Pan ST, Chou YT. Neuronal survival factor VGF promotes chemoresistance and predicts poor prognosis in lung cancers with neuroendocrine feature. Int J Cancer. 2022;151:1611-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/