Published online Dec 16, 2025. doi: 10.4253/wjge.v17.i12.110136
Revised: June 28, 2025
Accepted: November 4, 2025
Published online: December 16, 2025
Processing time: 200 Days and 2.8 Hours
Endoscopic ultrasound (EUS)-guided biliary drainage (BD) is becoming more common as a secondary drainage method in cases of difficult endoscopic retro
To clarify whether a metallic stent (MS) or a plastic stent (PS) is suitable when per
This was a multicenter retrospective study of patients who underwent EUS-BD between March 2005 and February 2025. The data of patients aged 70 years or older who underwent successful EUS-BD were analyzed, and the long-term outcomes of patients treated with an MS (MS group) and those treated with a PS (PS group) were compared.
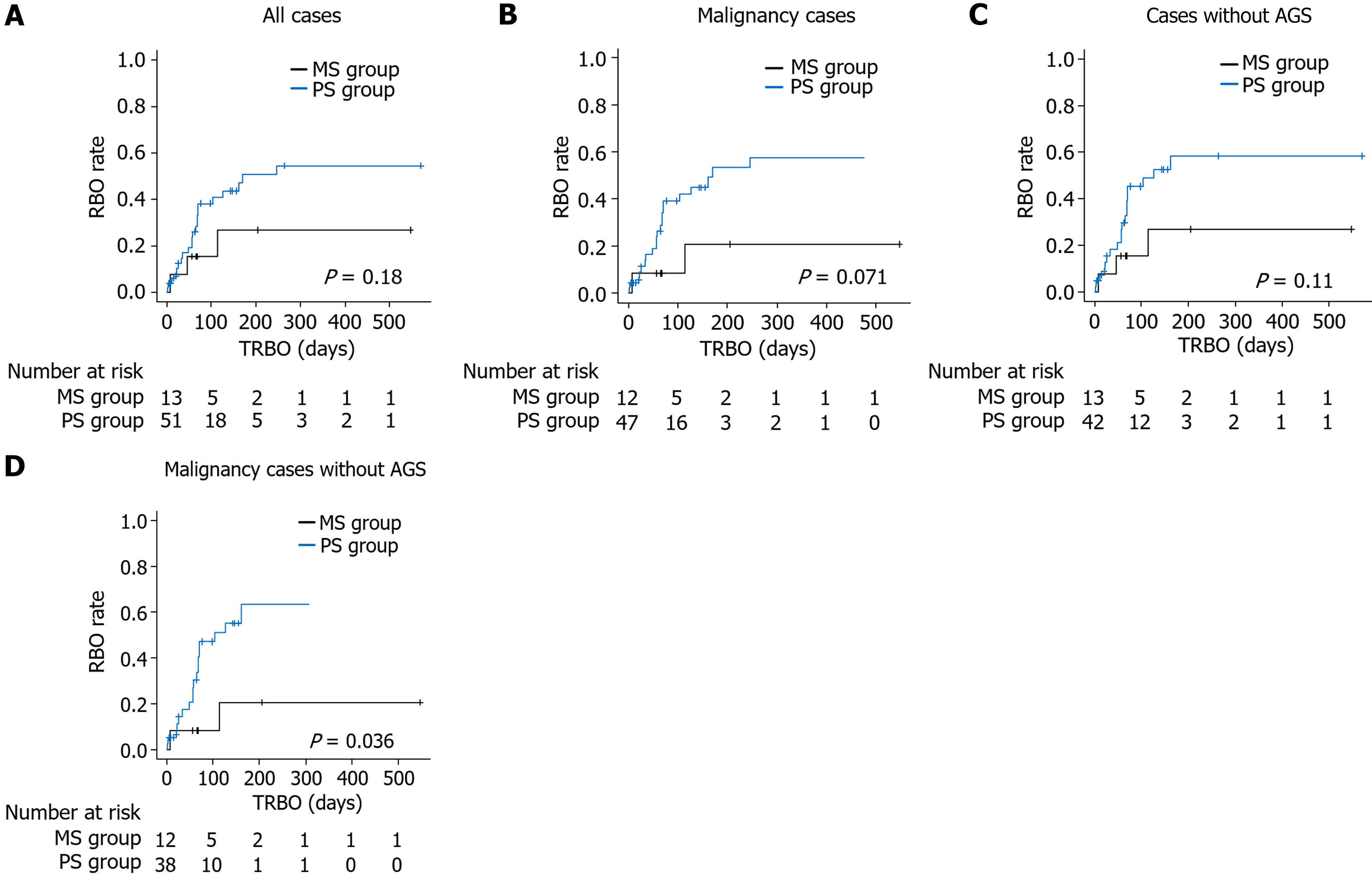
Ninety-four patients underwent successful EUS-BD, of whom 64 were aged 70 years or older. The PS group included 51 patients, and the MS group included 13 patients. The time to recurrent biliary obstruction (TRBO) was not significantly different between the PS group and the MS group (6-month recurrent biliary obstruction rate 50.8% vs 26.8%, P = 0.18). When patients were limited to those with malignancies without antegrade stenting, the TRBO was significantly longer in the MS group than in the PS group (6-month recurrent biliary obstruction rate 63.3% vs 20.7%, P = 0.036).
A PS might be sufficient for performing EUS-BD in elderly patients aged 70 years or older with benign biliary disease because it is easily replaced. However, an MS might be more effective for elderly individuals with malig
Core Tip: In a recent study, endoscopic ultrasound-guided biliary drainage reportedly demonstrated similar safety between elderly patients and all other patients. However, the appropriate stent for placement in elderly patients is unknown. This work reports that plastic stents are sufficient for patients with benign biliary diseases because they result in good outcomes and allow easier reintervention, whereas metallic stents are appropriate for older patients with malignancies because they offer better outcomes and a reduced physical burden of endoscopic reintervention.
- Citation: Sugimoto M, Nakajima Y, Takeda Y, Sato Y, Takagi T, Suzuki R, Asama H, Shimizu H, Sato K, Ohira R, Nakamura J, Kato T, Yanagita T, Otsuka M, Hikichi T, Ohira H. Efficacy and safety of plastic and metal stents for endoscopic ultrasound guided-biliary drainage in elderly patients. World J Gastrointest Endosc 2025; 17(12): 110136
- URL: https://www.wjgnet.com/1948-5190/full/v17/i12/110136.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i12.110136
For patients with biliary obstruction or unremovable biliary stones, endoscopic transpapillary drainage, such as endo
Covered metallic stents (MSs) are typically used in EUS-BD, but stent dislocation sometimes occurs[4]. One alternative to an MS in EUS-BD is a plastic stent (PS), which has two flanges at the biliary end and a pig-tail form with the other two flanges at the gastrointestinal end[5]. When a PS is used for EUS-BD, the BD procedure becomes easier because there is no need for shortening, and dislocation is rare. However, according to multiple reports, the time to recurrent biliary obstruction (TRBO) is longer in patients treated with EUS-BD with an MS than in patients treated with EUS-BD with a PS[6-9]. In a study by Yamashige et al[9] that reported the efficacy of PS use in a study involving the largest number of patients to date (MS group, n = 151; PS group, n = 72), the TRBO of patients managed with PSs was equivalent to that of patients managed with MSs for up to 100 days, but PSs resulted in fewer adverse events and superior outcomes in endoscopic reintervention. However, it remains unclear whether a PS, which is a safe and convenient option, or an MS, which has longer patency, should be used for EUS-BD.
As described above, EUS-BD is becoming a more common method for achieving BD. To this end, Ogura et al[10] reported that EUS-BD can be safely performed in elderly patients aged 75 years or older. However, whether a PS or an MS should be used in elderly patients is unclear. In general, elderly patients tend to have more comorbidities and poorer outcomes than younger patients do. Therefore, it is important to assess the factors that affect the safety and convenience of EUS-BD for elderly patients. In this study, we aimed to clarify whether the PS or the MS is the most appropriate stent for performing EUS-BD in elderly patients.
This was a retrospective study of patients with biliary diseases who underwent initial EUS-BD between March 2005 and February 2025 at Fukushima Medical University, Aizu Medical Center, Ohtanishinouchi General Hospital, and Ohara General Hospital. EUS-BD was performed in these patients due to difficult biliary cannulation/drainage, difficulty arriving at the Vater papilla or biliary-digestive anastomosis. Patients younger than 70 years were excluded from the study in accordance with past reports describing the management of older patients with pancreaticobiliary cancer[11,12]. Patients who underwent EUS-BD with a PS were included in the PS group, and those who underwent EUS-BD with an MS were included in the MS group. Informed consent was not necessary because the clinical data used in this study were anonymized. All patients provided written consent to undergo medical treatment. This study was approved by the Institutional Review Board of Fukushima Medical University (Approval No. 2399).
The target biliary tract was visualized with echoendoscope and punctured via an EUS-guided fine needle aspiration needle. Contrast medium was injected into the biliary tract, and a guidewire was inserted. A dilator was inserted via the guidewire, and a biliary stent was placed. If it was necessary to place the guidewire deeply in the duodenum, EUS-guided antegrade stenting (AGS) was performed concurrently with EUS-guided hepaticogastrostomy. For stomach punctures with large puncture angles, 22-gauge (G) needles were used; otherwise, 19 G needles were used.
The choice of a PS or an MS was made as follows: When ascites was observed or the target bile duct was distant from the upper gastrointestinal wall, a covered MS was used; when the gastrointestinal lumen or diameter of the target bile duct was narrow or the end of the stent reached the hilar bile duct, a PS was used.
A GF-UCT260, GF-UCT240T-AL5, GF-UC260J (Olympus, Tokyo, Japan), or EG-740UT endoscope (Fujifilm Medical, Tokyo, Japan) was used for the operation. The needles used included a 19 G SonoTip (Medi-Grobe, Rosenheim, Germany), 19 G Echotip Ultra (Cook Medical, Tokyo, Japan), 19 G NA-11J-KB (Olympus Medical Systems), 19 G or 22 G Expect, 22 G Acquire (Boston Scientific Japan, Tokyo, Japan), 19 G EUS Sonopsy CY (R) or 19 or 22 G Ez shot3 plus (Olympus Medical Systems, Tokyo, Japan). The guidewires used were a 0.018 Fielder 18, 0.025 Fielder 25, 0.025 VisiGlide, VisiGlide 2 (Olympus Medical Systems, Tokyo, Japan), 0.025 Jagwire, or 0.025 EndoSelector (Boston Scientific Japan, Tokyo, Japan). The dilator used was a 6-Fr Cysto-Gastro-Set (Endo-Flex GmbH, Voerde, Germany), an ES dilator (Zeon Medical Co., Tokyo, Japan), an MTW ERCP tapered catheter (MTW endoskopie, Wesel, Germany), a Tornus ES (Olympus Medical Systems, Tokyo, Japan), a 4 mm REN (KANEKA, Osaka, Japan) or a 4 mm Hurricane RX Biliary Balloon Dilatation Catheter (Boston Scientific Japan, Tokyo, Japan). The biliary PSs used were a 7 Fr Flexima Plus (Boston Scientific, MA, United States), 7 Fr CX-T (SILUX, Saitama, Japan), 7 Fr IT (Gadelius Medical Co., Ltd., Tokyo, Japan), or 7 Fr Zimmon double pig-tail stent (Cook Medical). The biliary self-expandable MS used was an 8 mm Niti-S covered Spring Stopper (Taewoong Medical, Gyoenggi-do, Korea), 10 mm fully covered HANARO, 6-8 mm fully covered HANARO Benefit or a 10 mm partially covered WallFlex Biliary RX stent (Boston Scientific, MA, United States). The biliary self-expandable MS used for EUS-AGS was a 10 mm Zilver 635 (COOK Medical), an 8-10 mm BileRush (PIOLAX, Kanagawa, Japan), or a 10 mm Niti-S Large Cell slim delivery (Taewoong Medical, Gyeonggi-do, S Korea).
The primary outcome of this study was the comparison of the TRBO between patients treated with a PS and those treated with an MS. In previous reports that focused on all adults, the combination of EUS-AGS and hepaticogastrostomy contributed to the extension of TRBO[8,13]. Therefore, the TRBO was compared across four cohorts (all patients, patients with malignancies, patients without AGS, and patients with malignancies without AGS). In accordance with the TOKYO criteria of 2014 reported by Isayama et al[14], the TRBO was defined as the time between EUS-BD stent placement and recurrent biliary obstruction (RBO), which was defined as hepatic dysfunction, recurrent jaundice, biliary tract dilation on percutaneous ultrasonography, or the need for additional BD according to Computed tomography.
The secondary outcomes were EUS-BD procedure details and the duration of follow-up. Patient characteristics were also compared between patients treated with a PS and those treated with an MS. These outcomes were selected on the basis of past reports on the use of EUS-BD for elderly patients[10]. Patient characteristics included age, sex, Eastern Cooperative Oncology Group performance status, disease state (malignant or benign), target biliary portion (distal or hilar), anamnesis (cardiovascular diseases, diabetes mellitus, pulmonary diseases, and renal diseases), past history of upper gastrointestinal surgery, serum white blood cell count, C-reactive protein level, and bilirubin level. The details of the EUS-BD procedures included the needles used (22 G or 19 G), puncture location (stomach or duodenum), type of dilator used (mechanical or cautery), use of AGS, procedural time, adverse events, chemotherapy before RBO, and duodenal stent placement before RBO. Adverse events were defined according to the American Society for Gastrointestinal Endoscopy lexicon[15]. The duration of follow-up was defined as the time between EUS-BD stent placement and the last consultation date or death.
Continuous variables and ordinal variables are presented as medians (interquartile ranges). Group comparisons were analyzed with the Mann-Whitney U test. Nominal variables are presented as n or n (%) and were compared with Fisher’s exact test. The duration of follow-up was evaluated via Kaplan-Meier curves, which were compared with the log-rank test. The TRBO values were compared with Gray’s test. A P value < 0.05 indicated statistical significance. All statistical analyses were performed with EZR version 1.68.
The primary aim of this study was to clarify whether the TRBO was longer following EUS-BD with a PS or an MS in elderly patients. In a previous study, the six-month RBO rate was approximately 80% in patients who underwent EUS-BD with an MS and approximately 30% in those who underwent EUS-BD with a PS. On the basis of an α error of 5% and a β error of 20%, at least 16 patients were required for this study. The sample size was calculated using web application software released by Kengo Nagashima (Biostatistics Unit, Clinical and Translational Research Center, Keio University Hospital, Tokyo, Japan).
Ninety-four patients underwent successful EUS-BD. Among them, 64 years were 70 years of age or older, 51 patients underwent EUS-BD with a PS (PS group), and 13 patients underwent EUS-BD with an MS (MS group). The patient characteristics are presented in Table 1, which shows that no characteristics were significantly different between the PS group and the MS group. The factors related to EUS-BD procedures are presented in Table 2. A 22 G needle was used more often in the PS group than in the MS group [30 (58.8%) vs 1 (7.7%), P value < 0.01]. More gastric punctures were performed in the PS group than in the MS group [45 (88.2%) vs 4 (30.8%), P value < 0.01]. A mechanical dilator was used more often in the PS group than in the MS group [50 (98.0%) vs 7 (53.8%), P value < 0.01]. Adverse events were not significantly different between the PS group and the MS group [1 (2.0%) (bleeding) vs 0 (0%), P value = 1.0]. One patient in the PS group was treated with transfusion.
| PS group (n = 51) | MS group (n = 13) | P value | |
| Age, years | 78 (73-83) | 79 (71-84) | 0.79 |
| Sex (male/female) | 30/21 | 8/5 | 1.0 |
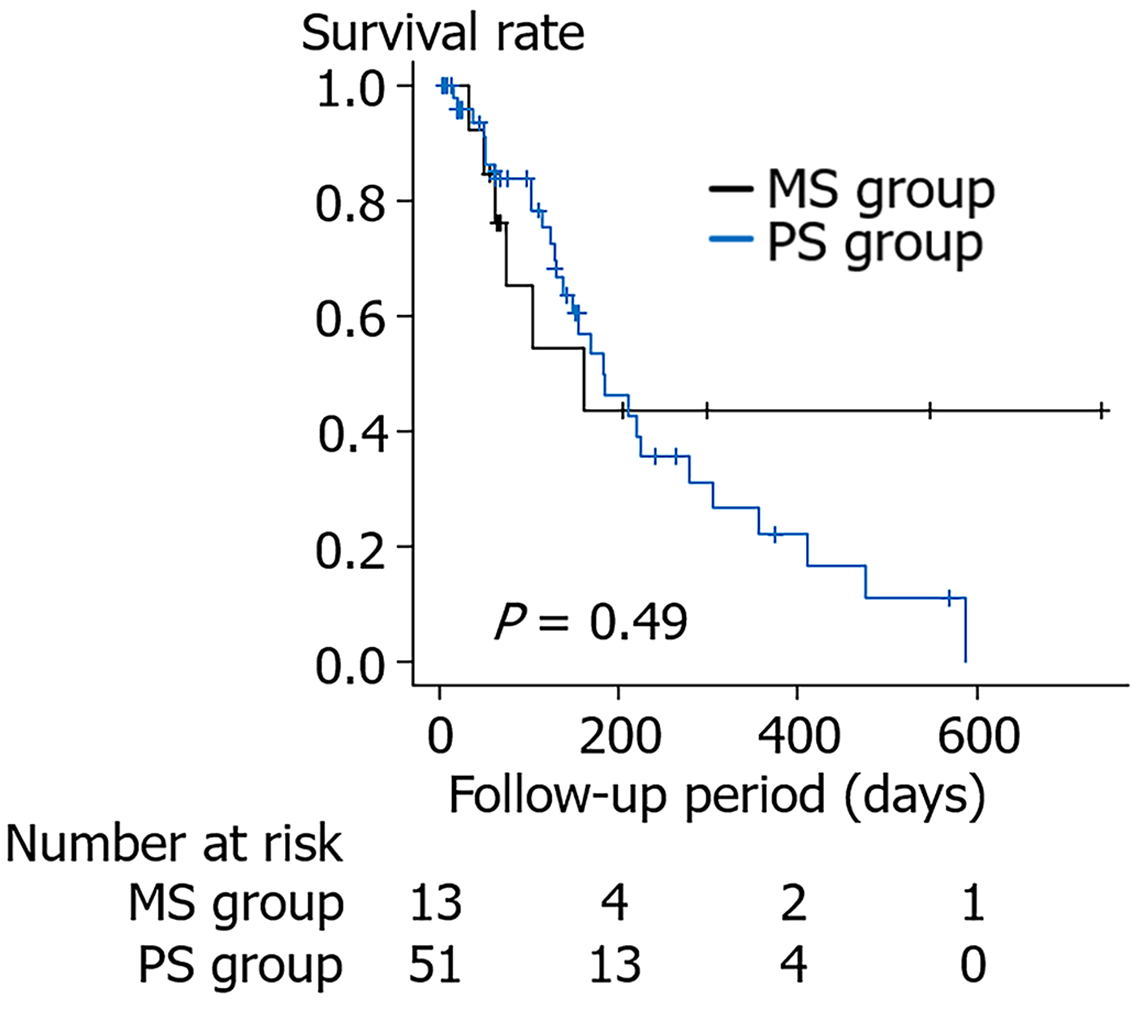
| Performance status | 0 (0-1.0) | 0 (0-0.5) | 0.49 |
| Diseases (malignant/benign) | 47/4 | 12/1 | 1.0 |
| Pancreatic cancer | 22 | 10 | |
| Biliary tract cancer | 16 | 2 | |
| Duodenal cancer | 2 | ||
| Gastric cancer | 2 | ||
| Colon cancer | 2 | ||
| Urothelial cancer | 1 | ||
| Liposarcoma | 1 | ||
| Biliary tract stone | 4 | ||
| Cholecystitis | 1 | ||
| Benign biliary stricture | 1 | ||
| Target biliary part (distal/hilar) | 41/10 | 12/1 | 0.44 |
| Anamnesis | 21 (41.2) | 7 (53.8) | 0.53 |
| Cardiovascular diseases | 13 (25.5) | 3 (23.1) | 1.0 |
| Diabetes mellitus | 5 (9.8) | 4 (30.8) | 0.07 |
| Pulmonary diseases | 3 (5.9) | 0 (0) | 1.0 |
| Renal diseases | 0 (0) | 1 (7.7) | 0.2 |
| Past history of upper gastrointestinal surgery | 9 (17.6) | 1 (7.7) | 0.67 |
| Serum WBC count, (× 103/μL) | 6.8 (5.0-8.7) | 6.8 (4.9-9.3) | 0.92 |
| Serum CRP level, mg/L | 3.2 (1.1-6.7) | 5.1 (2.4-8.1) | 0.33 |
| Serum bilirubin level, mg/dL | 4.9 (1.6-11.5) | 5.9 (0.3-24.8) | 0.97 |
| PS group (n = 51) | MS group (n = 13) | P value | |
| 22 G needle use | 30 (58.8) | 1 (7.7) | < 0.01 |
| Punctured organ (stomach/duodenum) | 45/6 | 4/9 | < 0.01 |
| Dilator (mechanical/cautery/unknown) | 50/0/1 | 7/6/0 | < 0.01 |
| Use of antegrade stenting | 10 (19.6) | 0 (0) | 0.11 |
| Procedural time, minutes | 38.5 (25.8-60.0) | 50.0 (41.8-59.8) | 0.31 |
| Adverse events | 1 (2.0) | 0 (0) | 1.0 |
| Bleeding | 1 | ||
| RBO | 22 (43.1) | 3 (23.1) | 0.22 |
| Reason for RBO | |||
| Debris | 16 | 1 | |
| Dislocation | 4 | 2 | |
| Blood clot | 1 | ||
| Overgrowth | 1 | ||
| Chemotherapy before RBO | 21 (41.2) | 4 (30.8) | 0.54 |
| Duodenal stent placement before RBO | 11 (21.6) | 3 (23.1) | 1.0 |
Comparisons with respect to the follow-up period are presented in Figure 1. The cumulative survival rate was not significantly different between the PS group and the MS group (one-year survival rate 22.2% vs 43.5%, P value = 0.49). Additionally, the cumulative RBO rate was not significantly different between the PS group and the MS group (6-month RBO rate 50.8% vs 26.8%, P value = 0.18) (Figure 2A). The results were similar when the patient cohort was limited to those with a malignancy or patients for whom EUS-guided AGS was not performed (Figure 2B and C). When the patient cohort was further limited to patients with malignancies without AGS, the cumulative RBO rate was significantly higher in the PS group than in the MS group (6-month RBO rate 63.3% vs 20.7%, P value = 0.036; Figure 2D).
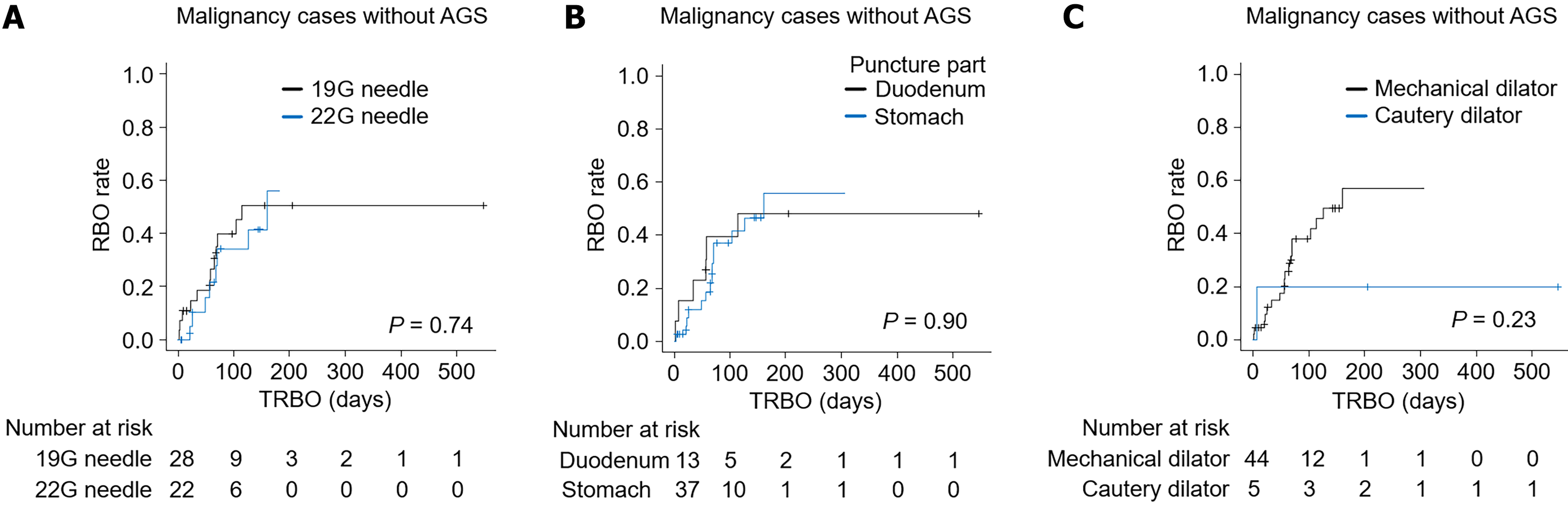
The influence of factors that were significantly different between the PS group and the MS group on the TRBO (Table 2) was investigated. In the cohort of patients with malignancies without AGS, the TRBO was also compared between patients for whom a 19 G needle or a 22 G needle was used to puncture the location (duodenum or stomach) and between patients for whom a mechanical dilator or a cautery dilator was used (Figure 3). For both comparisons, the TRBO was not significantly different between the groups.
EUS-BD is comparable to ERCP-BD. Although ERCP can be safely performed on very old (≥ 90 years) patients[16], the efficacy and safety of EUS-BD for elderly patients have not been evaluated. In a multicenter retrospective study, Ogura et al[10] were the first to report that EUS-BD could be performed safely for patients aged ≥ 75 years. However, hypoxemia and severe hypoxemia are more common in older patients, and capnographic monitoring might be necessary for safety. This study did not compare the safety of EUS-BD between older patients and younger patients, and oxygenation was unknown. EUS-BD was performed with overall safety, but the anesthetic state should receive special attention in older patients. The present study is the first to identify the appropriate stent for elderly patients undergoing EUS-BD. In all cases involving benign biliary strictures, the TRBO was not significantly different between the groups. In patients with malignancies without AGS, the TRBO was significantly longer in the MS group than in the PS group. When patients with AGS were involved, the TRBO of the PS became comparable to that of the MS (AGS was not combined with the MS). However, technical success decreased and the procedure time increased with the addition of AGS[13]. Therefore, an MS might be preferable to a PS in elderly patients with malignancies to decrease the burden.
For patients with benign biliary strictures, a PS may be sufficient. However, for patients with benign biliary strictures, endoscopic reintervention is essential, and this procedure is easier if a PS was used than if an MS was used. Furthermore, although reintervention is not limited to elderly patients, easier reintervention is suitable for older patients with physical limitations and coexisting diseases. The difficulty of reintervention following EUS-BD with an MS has been attributed to various causes. For example, Minaga et al[17] reported 33 cases of endoscopic reintervention for EUS-BD involving a partially covered MS. To prevent dislocation of the abdominal cavity, a long partially covered MS is often used for EUS-BD. In such cases, the standard approach to the bile duct cannot be easily used. Guidewire and catheter insertion to the distal end of the existing MS, which is the first choice of approach, was successful in only 60.6% of patients, whereas an approach through the stent mesh of the existing MS was successful in 30.3% of patients, and an approach via the fistula after MS removal was successful in 9.1% of patients. However, MSs that have been in place for extended periods cannot be easily removed. Tissue hyperplasia is another reason for the difficulty of reintervention in patients managed with an MS. The insertion of an additional stent was prevented by stenosis due to tissue hyperplasia, and partially covered MS removal was not feasible because of hyperplasia in the uncovered region[9,17].
In contrast, an MS may be desirable for treating malignant biliary strictures. Older age is a risk factor for a poor outcome[12,18], and the unnecessary burden of endoscopic stenting can be avoided by using an MS. The main causes of malignant biliary stricture are pancreatic cancer and biliary tract cancer. When patients aged ≥ 70 years with advanced pancreatic cancer underwent gemcitabine monotherapy, the overall survival (OS) was 4.7-10.4 months (4.7 months for metastatic disease)[11], whereas in patients aged ≥ 75 years with advanced biliary tract cancer who received mono
This study has several limitations. First, this was a retrospective cohort study that was performed in a single country. Second, the detailed strategy for performing EUS-BD might differ across hospitals, but the details of the procedure for the other cooperating institutions are unknown because of the retrospective nature of the study. Third, the puncture route was significantly different between the PS group and the MS group. In a recent meta-analysis, the puncture route was not related to the TRBO[20]. In this study, the TRBO did not differ significantly because of differences in the puncture route. Fourth, multiple stents were used. This may have influenced the study results and may make the results nonreproducible. The sample size might also be too small to account for these differences. In the future, prospective studies involving the use of the same stent across institutions in multiple regions should be performed. Fifth, the conditions of patients with a PS differ from those of patients with an MS. As described in the methods section, MSs tend to be placed for patients with advanced cancer with ascites or dilated bile ducts. However, the TRBO of the MS was longer than that of the PS in patients with malignancies without AGS. Therefore, the superiority of MSs is certain.
A PS may be sufficient for elderly patients with benign biliary diseases, whereas an MS may be more effective for patients with malignant biliary strictures, especially those who have previously undergone monotherapy or received BSC.
We thank all the staff at the Department of Gastroenterology of Fukushima Medical University, the Department of Endoscopy of Fukushima Medical University Hospital, the Department of Gastroenterology of Ohara General Hospital, the Department of Gastroenterology of Aizu Medical Center, Fukushima Medical University, and the Department of Gastroenterology of Ohtanishinouchi Hospital. We also thank American Journal Experts for providing English language editing services.
| 1. | Mukai S, Itoi T, Baron TH, Takada T, Strasberg SM, Pitt HA, Ukai T, Shikata S, Teoh AYB, Kim MH, Kiriyama S, Mori Y, Miura F, Chen MF, Lau WY, Wada K, Supe AN, Giménez ME, Yoshida M, Mayumi T, Hirata K, Sumiyama Y, Inui K, Yamamoto M. Indications and techniques of biliary drainage for acute cholangitis in updated Tokyo Guidelines 2018. J Hepatobiliary Pancreat Sci. 2017;24:537-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 135] [Article Influence: 15.0] [Reference Citation Analysis (1)] |
| 2. | Marzioni M, Crinò SF, Lisotti A, Fuccio L, Vanella G, Amato A, Bertani H, Binda C, Coluccio C, Forti E, Fugazza A, Ligresti D, Maida M, Marchegiani G, Mauro A, Mirante VG, Ricci C, Rizzo GEM, Scimeca D, Spadaccini M, Arvanitakis M, Anderloni A, Fabbri C, Tarantino I, Arcidiacono PG; i-EUS Group. Biliary drainage in patients with malignant distal biliary obstruction: results of an Italian consensus conference. Surg Endosc. 2024;38:6207-6226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Gopakumar H, Singh RR, Revanur V, Kandula R, Puli SR. Endoscopic Ultrasound-Guided vs Endoscopic Retrograde Cholangiopancreatography-Guided Biliary Drainage as Primary Approach to Malignant Distal Biliary Obstruction: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Am J Gastroenterol. 2024;119:1607-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 4. | Martins FP, Rossini LG, Ferrari AP. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: fatal complication. Endoscopy. 2010;42 Suppl 2:E126-E127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Umeda J, Itoi T, Tsuchiya T, Sofuni A, Itokawa F, Ishii K, Tsuji S, Ikeuchi N, Kamada K, Tanaka R, Tonozuka R, Honjo M, Mukai S, Fujita M, Moriyasu F. A newly designed plastic stent for EUS-guided hepaticogastrostomy: a prospective preliminary feasibility study (with videos). Gastrointest Endosc. 2015;82:390-396.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 6. | Hashimoto S, Iwashita Y, Taguchi H, Tanoue S, Ohi T, Shibata R, Haraguchi T, Kamikihara Y, Toyodome K, Kojima I, Araki N, Tsuneyoshi K, Nakamura Y, Fujita T, Hinokuchi M, Iwaya H, Arima S, Sasaki F, Kanmura S, Ido A. Comparison of recurrent biliary obstruction with the use of metal and plastic stents in EUS-guided biliary drainage: A propensity score-matched analysis. Endosc Ultrasound. 2023;12:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 7. | Shibuki T, Okumura K, Sekine M, Kobori I, Miyagaki A, Sasaki Y, Takano Y, Hashimoto Y. Covered self-expandable metallic stents versus plastic stents for endoscopic ultrasound-guided hepaticogastrostomy in patients with malignant biliary obstruction. Clin Endosc. 2023;56:802-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Sugimoto M, Takagi T, Suzuki R, Waragai Y, Konno N, Asama H, Sato Y, Irie H, Nakamura J, Takasumi M, Hashimoto M, Kato T, Kobashi R, Yanagita T, Hikichi T, Ohira H. Comparison of time to recurrent biliary obstruction between plastic stents and metallic stents for endoscopic ultrasoundguided biliary drainage. Exp Ther Med. 2023;25:214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 9. | Yamashige D, Hijioka S, Nagashio Y, Maruki Y, Komori Y, Kuwada M, Fukuda S, Yagi S, Okamoto K, Agarie D, Chatto M, Morizane C, Ueno H, Sugawara S, Sone M, Saito Y, Okusaka T. Metal stent versus plastic stent in endoscopic ultrasound-guided hepaticogastrostomy for unresectable malignant biliary obstruction: Large single-center retrospective comparative study. Dig Endosc. 2025;37:117-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 10. | Ogura T, Ishiwatari H, Fujimori N, Iwasaki E, Ishikawa K, Satoh T, Kaneko J, Sato J, Oono T, Matsumoto K, Fukuhara S, Kayashima A, Hakoda A, Higuchi K. Propensity score matching analysis for adverse events of EUS-guided biliary drainage in advanced elderly patients (PEACE study). Therap Adv Gastroenterol. 2022;15:17562848221092612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 11. | Kobayashi S, Ueno M, Ishii H, Furuse J. Management of elderly patients with unresectable pancreatic cancer. Jpn J Clin Oncol. 2022;52:959-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Renteria Ramirez DE, Knøfler LA, Kirkegård J, Fristrup CW, Stender MT, Nielsen SD, Markussen A, Larsen PN, Akdag D, Al-Saffar HA, Pommergaard HC. Prognosis related to treatment plan in patients with biliary tract cancer: A nationwide database study. Cancer Epidemiol. 2024;93:102688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Imai H, Takenaka M, Omoto S, Kamata K, Miyata T, Minaga K, Yamao K, Sakurai T, Nishida N, Watanabe T, Kitano M, Kudo M. Utility of Endoscopic Ultrasound-Guided Hepaticogastrostomy with Antegrade Stenting for Malignant Biliary Obstruction after Failed Endoscopic Retrograde Cholangiopancreatography. Oncology. 2017;93 Suppl 1:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Isayama H, Hamada T, Yasuda I, Itoi T, Ryozawa S, Nakai Y, Kogure H, Koike K. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc. 2015;27:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 15. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 2022] [Article Influence: 126.4] [Reference Citation Analysis (1)] |
| 16. | Manabe D, Arizumi T, Aoyagi H, Abe K, Kodashima S, Asaoka Y, Yamamoto T, Tanaka A. Risk factors for post-endoscopic retrograde cholangiopancreatography complications in very elderly patients aged 90 years or older-No additional risk. Geriatr Gerontol Int. 2025;25:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Minaga K, Kitano M, Uenoyama Y, Hatamaru K, Shiomi H, Ikezawa K, Miyagahara T, Imai H, Fujimori N, Matsumoto H, Shimokawa Y, Masuda A, Takenaka M, Kudo M, Chiba Y. Feasibility and efficacy of endoscopic reintervention after covered metal stent placement for EUS-guided hepaticogastrostomy: A multicenter experience. Endosc Ultrasound. 2022;11:478-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 18. | Huang X, Chen W, Liu J, Liao Y, Cai J, Zhong D. Clinicopathological features, prognostic factors, and prognostic survival prediction in patients with extrahepatic bile duct cancer liver metastasis. Eur J Gastroenterol Hepatol. 2024;36:1029-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Okamoto T, Takeda T, Sasaki T, Hamada T, Mie T, Ishitsuka T, Yamada M, Nakagawa H, Hirai T, Furukawa T, Kasuga A, Ozaka M, Sasahira N. Safety and Effectiveness of Chemotherapy in Elderly Biliary Tract Cancer Patients. Curr Oncol. 2023;30:7229-7240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Yamazaki H, Yamashita Y, Shimokawa T, Minaga K, Ogura T, Kitano M. Endoscopic ultrasound-guided hepaticogastrostomy versus choledochoduodenostomy for malignant biliary obstruction: A meta-analysis. DEN Open. 2024;4:e274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/