Published online Nov 16, 2025. doi: 10.4253/wjge.v17.i11.108874
Revised: June 12, 2025
Accepted: October 13, 2025
Published online: November 16, 2025
Processing time: 203 Days and 19.7 Hours
Colonoscopy is a cornerstone in the detection and diagnosis of colorectal tumors, playing a critical role in both screening and clinical evaluation. More recently, its utility has expanded to therapeutic guidance, particularly with the advent of minimally invasive surgical techniques. Preoperative tattoo marking is commonly used for tumor localization; however, it poses challenges such as intraperitoneal ink scattering and difficulty in defining dissection planes in the lower rectum. To address these limitations, a new technology utilizing a near-infrared fluorescence clip placed preoperatively enables accurate intraoperative tumor localization. Intraoperative colonoscopy offers additional advantages, including real-time tumor localization, colonic irrigation, visualization of the proximal colon in obstructive cases, and assessment of anastomosis following colorectal resection. Notably, intraoperative colonoscopy allows for the immediate detection and management of complications, such as anastomotic bleeding and leakage, poten
Core Tip: Colonoscopy has evolved beyond diagnostics to serve as a valuable tool for therapeutic guidance in minimally invasive colorectal surgeries. Traditional preoperative tattoo marking for tumor localization carries risks, including ink scattering and challenges in dissecting the lower rectum. Near-infrared fluorescence clips, placed preoperatively, offer improved intraoperative tumor localization. Intraoperative colonoscopy enables real-time tumor identification, colonic irrigation, and assessment of anastomotic integrity, potentially reducing the risk of leakage. Advanced endoscopic techniques, including endoscopic mucosal resection, endoscopic submucosal dissection, hybrid endoscopic submucosal dissection, and combined endoscopic laparoscopic surgery, have broadened the options for resecting colorectal tumors and appendiceal neoplasms, supporting endoscopy-guided full-thickness resection.
- Citation: Hatsuzawa Y, Tsujinaka S, Miura T, Kitamura Y, Mitamura A, Sawada K, Hikage M, Nakano T, Shibata C. Current roles of colonoscopy in minimally invasive colorectal surgery: Preoperative guidance, intraoperative colonoscopy, and combined endoscopic-laparoscopic surgery. World J Gastrointest Endosc 2025; 17(11): 108874
- URL: https://www.wjgnet.com/1948-5190/full/v17/i11/108874.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i11.108874
The role of colonoscopy has significantly expanded in recent years. Traditionally, colonoscopy has been the primary modality for the detection and diagnosis of colorectal tumors. More recently, its use in therapeutic guidance has gained prominence, driven by advances in minimally invasive surgical techniques. Preoperative tumor assessment and localization are critical for determining the optimal surgical approach[1]. In addition, intraoperative colonoscopy (IOC) allows for precise tumor localization and real-time evaluation of the anastomosis, contributing to the prevention of surgical complications[2]. For endoscopic resection, established techniques such as endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and hybrid ESD remain essential[3]. More recently, combined endoscopic laparoscopic surgery (CELS) and robotic-assisted procedures have broadened the indications for endoscopic intervention and enabled less invasive treatment strategies[4,5]. This article highlights the diverse roles that colonoscopy plays throughout the perioperative period.
Colonoscopy remains the standard modality for colorectal cancer (CRC) screening and for detailed examination in symptomatic patients to detect and diagnose lesions. When early-stage cancer is identified, endoscopic resection using techniques such as EMR or ESD may be appropriate. In cases where endoscopic treatment is not indicated, colonoscopy enables detailed assessment of lesion extent and invasion depth. Typically, invasion depth is estimated based on endoscopic findings; however, achieving an accurate diagnosis remains clinically challenging. Previous studies report the accuracy of endoscopic depth assessment to range between 59% and 84%[6-8]. Endoscopic ultrasonography (EUS) serves as a valuable adjunct, offering enhanced precision in evaluating tumor invasion depth and detecting pericolic or rectal lymph node involvement. EUS facilitates real-time luminal staging of CRC and has demonstrated improved diagnostic accuracy for T staging, ranging from 90% to 96%[9,10]. Additionally, Marone et al[11] reported that EUS could detect lymph node metastasis in rectal cancer with an accuracy of 63% to 85%.
Preoperative evaluation of tumor location is essential for determining the appropriate extent of surgical resection. Tattoo marking is commonly used to mark the location of colorectal tumors prior to surgery, aiding intraoperative identification[12]. Under endoscopic guidance, sterile carbon-based ink is injected into the mucosa or submucosa at designated surgical landmarks. These tattoos act as visual cues that are visible from the serosal surface during laparoscopic procedures, allowing surgeons to accurately identify the tumor site. Tattoo marking is especially useful for tumors located far from anatomical landmarks (e.g., the ileocecal valve) or for lesions without serosal invasion, which cannot be directly visualized during surgery[1].
Preoperative tattoo marking has also been associated with improved lymph node retrieval in rectal cancer surgery. Imaoka et al[13] reported that carbon-based tattoo ink, when injected near the tumor, can migrate through lymphatic channels, resulting in visible pigmentation of regional lymph nodes. This provides a clear visual indicator of lymphatic drainage pathways, aiding both surgeons and pathologists in assessing lymph node involvement. In their study, the rate of adequate lymph node retrieval was significantly higher in the tattooed group compared to the non-tattooed group (75.5% vs 55.8%)[13].
Despite its utility, intraoperative tattooing has several limitations and complications that must be considered: (1) Inaccurate tumor localization - misplacement of the tattoo can lead to incorrect intraoperative guidance and may alter the surgical plan; (2) Excessive tattooing - overuse of ink can obscure tumor margins, complicating precise identification; (3) Reduced visibility in obese patients - ink deposits may be obscured by adipose tissue, limiting their effectiveness during surgery; (4) Intraperitoneal ink scattering - ink diffusion into the peritoneal cavity can obscure dissection planes, particularly in the lower rectum[14]; (5) Transmural injection - the most common complication, occurring in approximately 2.4%-13% of cases, can result in ink leakage beyond the intestinal wall[15]; and (6) Focal peritonitis and hematoma - these inflammatory and hemorrhagic reactions represent potential adverse effects of the procedure.
The diagnostic accuracy of tumor localization based on endoscopic findings has been reported to range from approximately 81% to 83%[14,16,17]. However, inaccurate localization has led to intraoperative changes in the surgical plan in 6.3%-16.7% of cases[14,16,17]. Even when preoperative endoscopic tattoo marking is performed, the markings may not be identifiable during surgery in 17%-21.5% of patients[18,19]. Lee et al[20] suggested that performing tattoo marking within two days prior to surgery may enhance tattoo visibility and improve localization accuracy.
Computed tomography (CT) colonography or endoscopic radiographic studies can also aid in accurate tumor loca
As a newer technique, magnetic endoscopic imaging (MEI) has demonstrated improved accuracy in lesion localization. In one study, correct localization was achieved in 95% of cases using MEI, compared to 83% in the control group without MEI[23]. MEI is an advanced colonoscopic visualization technology that enhances localization by providing continuous, real-time three-dimensional tracking of the endoscope’s configuration and position within the abdominal cavity. It operates by integrating low-intensity magnetic fields with sensor-based detection, enabling non-radiographic mapping of the endoscope’s trajectory. This allows for precise navigation during colonoscopy without the need for fluoroscopy[24].
A new technology employing near-infrared fluorescence clips (NIRFCs), placed the day before surgery, enables accurate intraoperative tumor localization. This technique is particularly valuable for determining the appropriate extent of bowel resection and selecting the optimal operative approach during laparoscopic gastrointestinal surgery[25-28]. In rectal cancer, NIRFCs have been detected intraoperatively in 85% of cases, facilitating precise distal resection margins and adequate lymph node dissection[26]. However, a major limitation of fluorescent clips is their relatively high cost, approximately 100 dollars per clip. To optimize clinical utility, further studies are needed to establish the minimum effective number of clips required for reliable tumor localization while minimizing expenses. Additionally, clip dislodgement remains a concern, as unintended displacement may compromise surgical guidance and therapeutic outcomes[28]. Overcoming these challenges will require cost-reduction strategies and technological advancements to support broader clinical adoption.
As a relatively new technique, NIRFCs have not yet been extensively studied with respect to the learning curve asso
A major advantage of IOC is its ability to provide real-time evaluation of tumor status and bowel condition. In cases of early CRC or adenoma, tumor localization can be challenging due to the inability to visualize lesions laparoscopically. IOC serves as an effective alternative to preoperative tattoo marking in such cases. By integrating laparoscopic and colonoscopic findings, surgeons can more accurately determine the appropriate extent of colorectal resection. One report indicated that IOC resulted in changes to the surgical approach in 5 cases out of 98 cases[2,29]. This technique is especially valuable in rectal cancer surgery, where achieving an adequate distal resection margin is critical. Direct intraluminal visualization of the tumor during IOC helps ensure precise determination of the rectal transection line[29]. Gorgun et al[30] reported that IOC does not increase procedural complexity nor adversely affect surgical outcomes. There were no significant differences between patients with and without IOC in operative time, blood loss, conversion to open surgery, nor length of hospital stay, and no colonoscopy-related complications were reported. Thus, IOC is a safe and valuable intraoperative tool.
In cases of left-sided obstructive CRCs, preoperative colonoscopy often cannot visualize the proximal colon, making IOC a valuable tool for comprehensive colonic evaluation. This is particularly important given the reported incidence of synchronous primary CRCs, which occur in approximately 5%-10% of cases[31]. Sasaki et al[32] demonstrated that IOC in patients with obstructive CRC identified synchronous adenomatous polyps in 26.8% of cases, carcinoma in 4%, and obstructive colitis in 2%, without increasing patient morbidity. Real-time intraoperative diagnosis enables more informed surgical decision-making and treatment planning.
Importantly, IOC performed after anastomosis has also been shown to be safe. A comparative study found no significant differences in postoperative outcomes between patients who underwent IOC and those who did not. The overall morbidity was similar between groups (31% vs 39%, P = 0.45), with anastomotic leakage occurring in 1.3% of IOC patients vs 0% in the non-IOC group (P = 0.46). Wound infection rates were also not significantly different (5.1% vs 1.1%, P = 0.18), and the average length of hospital stay was comparable (6.4 days vs 7.3 days)[33]. These findings reinforce the safety profile of IOC in the intraoperative setting. However, to date, no studies have systematically examined the learning curve associated with IOC, particularly in relation to its effectiveness in tumor localization, lesion identification, and synchronous lesion detection. Further research is warranted to assess how procedural experience influences detection rates and overall clinical outcomes.
IOC is also an effective tool for evaluating colorectal anastomose, particularly for identifying complications such as bleeding and leakage, which are among the most serious postoperative risks. Several studies have suggested that IOC may help reduce the incidence of anastomotic complications[34,35]. Postoperative hemorrhage following colorectal anastomosis occurs in approximately 1.8% to 2.3% of cases[36,37]. These events are often missed intraoperatively, as the intraluminal aspect of the anastomosis is not visible through standard laparoscopic approaches[38]. In a study by Li et al[35], intraoperative anastomotic bleeding was identified in 6 patients of 107 patients who underwent IOC. All were managed with direct interrupted suturing, and only 1 patient (0.9%) experienced postoperative bleeding from the staple line. This rate was lower than in patients who did not undergo IOC, where postoperative bleeding occurred in 3.6% of cases (5 patients of 137 patients). Additionally, IOC facilitates immediate hemostasis using endoscopic clipping. Shamiyeh et al[38] reported that IOC detected anastomotic bleeding in 5 patients of 85 patients (5.9%), all of whom were successfully treated intraoperatively with endoscopic clipping.
Anastomotic leakage impacts not only short-term postoperative outcomes but also long-term survival and disease recurrence, through several interrelated pathological mechanisms[39,40]. First, severe abdominal infection and the associated inflammatory response lead to the overexpression of proinflammatory and proangiogenic factors, which can promote tumor progression and angiogenesis. Second, persistent celiac inflammation following leakage may induce systemic immunosuppression, potentially accelerating postoperative metastatic spread. Third, tumor cells shed from the compromised intestine may enter the pelvic cavity via the fistula, facilitating implantation and tumor regrowth. Finally, management of anastomotic leakage often requires prolonged fasting, parenteral nutrition, and broad-spectrum antibiotic therapy, all of which can significantly disrupt the intestinal microbiota, with potential downstream effects on immune function and tumor behavior.
A recent systematic review and meta-analysis demonstrated a significant association between colorectal anastomotic leakage and adverse oncologic outcomes, including increased rates of local recurrence [odds ratio (OR) = 1.93; 95% confidence interval (CI): 1.57-2.38; P < 0.00001], reduced overall survival (OR = 1.64; 95%CI: 1.37-1.95; P < 0.00001), decreased disease-free survival (OR = 2.07; 95%CI: 1.50-2.87; P < 0.00001) and decreased cancer-specific survival
Several reports suggest that combining the intraoperative air leakage test with IOC facilitates timely identification of anastomotic defects and allows for additional suturing during surgery, potentially reducing the incidence of postoperative anastomotic leakage[42,43]. In a study by Kishiki et al[43], 3 patients of 94 patients who underwent the combined air leak test and IOC were found to have positive leak results and received additional intraoperative suturing. Notably, the incidence of postoperative anastomotic leakage was significantly lower in the IOC group compared to the non-IOC group (4% vs 11%). The air leakage test with IOC is a relatively simple technique that can be readily performed by surgeons. Although concerns have been raised regarding potential stress on the anastomosis from endoscopic manipulation, prior studies have reported no adverse effects on short-term postoperative outcomes[33]. However, the re
EMR and ESD are widely utilized techniques for the endoscopic resection of gastrointestinal lesions. Hybrid ESD, a modified approach that incorporates snaring after limited submucosal dissection, has been recently developed to simplify the procedure and reduce associated risks[44,45]. It has demonstrated shorter procedure times compared to conventional ESD, with one study reporting a mean duration of 108 minutes vs 122 minutes, respectively[44]. ESD offers advantages over EMR, including a lower recurrence rate and the ability to achieve en bloc resection, particularly for larger or fibrotic lesions. Its minimally invasive nature also contributes to improved postoperative quality of life compared to surgical resection[3,46,47]. Training availability varies across institutions: Structured programs for EMR are present in approximately 59% of institutions, whereas ESD training programs are available in about 82% of institutions. These programs typically employ a stepwise curriculum, progressing based on lesion size and location to support the development of technical proficiency among endoscopists[48].
These advanced endoscopic techniques are generally recommended for intramucosal lesions or carcinomas with minimal submucosal invasion. However, certain clinical scenarios carry an elevated risk of complications and warrant careful patient selection[49-51]. High-risk conditions include: (1) Diverticulum-associated lesions: ESD is contraindicated due to the increased risk of perforation; (2) Severe fibrosis: Submucosal scarring complicates the dissection plane and significantly raises the risk of perforation; and (3) Suspected deep submucosal invasion: These lesions often require oncologically appropriate surgical resection with regional lymph node dissection. In such high-risk cases, the establishment of a system for immediate intervention is essential to manage unexpected complications, such as uncontrolled hemorrhage or perforation. The presence of a well-coordinated multidisciplinary team, including ex
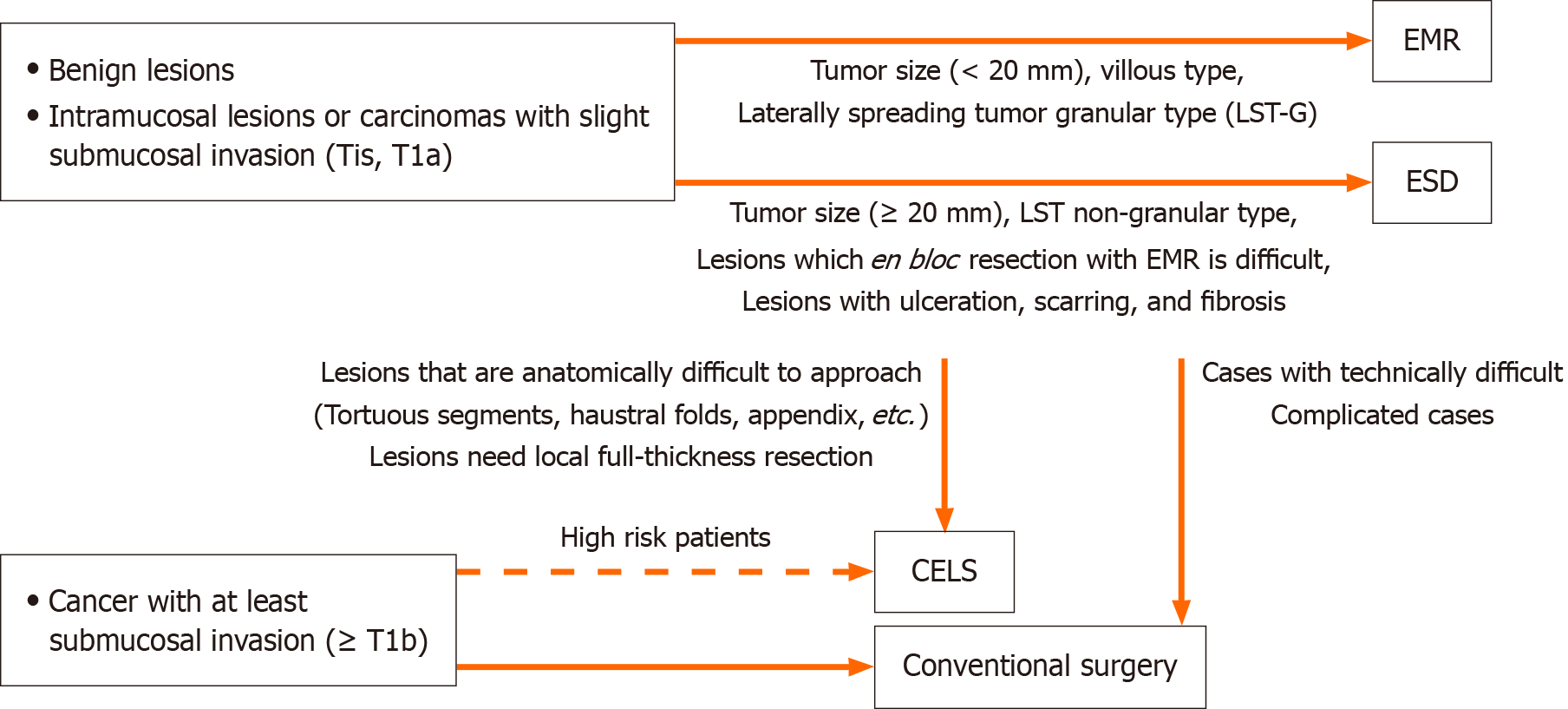
CELS is a safe, effective, and minimally invasive approach for managing colorectal lesions that are unsuitable for endoscopic resection alone[4]. Figure 1 presents a treatment selection algorithm for colorectal lesions, and Table 1 summarizes the characteristics of the previously reported endoscopic procedures. While CELS typically involves a longer procedure time compared to standard endoscopic resection, it achieves a higher en bloc resection rate and facilitates more definitive lesion removal. This technique is particularly advantageous for anatomically challenging lesions, such as broad-based, sessile polyps, and those located in tortuous colonic segments or within haustral folds. The laparoscopic component enables straightening of colonic flexures and improved access to difficult sites. Moreover, laparoscopic bowel graspers can be used to apply external pressure, effectively invaginating flat sessile polyps and enhancing exposure for resection[52]. In a prior study, CELS was deemed feasible in 74% of cases where endoscopic treatment was limited by lesion size (> 2 cm) or challenging anatomical location[53]. Additionally, laparoscopic visualization allows for real-time assessment of colonic perforations or injuries, enabling surgical repair when necessary[52].
| Ref. | Procedure type | Number of lesions | Location (right/Left/rectum) | Mean tumor size (mm) | En bloc resection (%) | Mean procedure time (minute) | Complications (%) | Local recurrence (%) |
| Saito et al[46], 2014 | EMR | 228 | 89/52/110 | 28 | 74 (33) | 29 | 10 (4) | 33 (14) |
| ESD | 145 | 44/28/73 | 37 | 122 (84) | 108 | 11 (8) | 3 (2) | |
| Fuccio et al[45], 2017 | ESD | 97 | N/A | 33 | 88 (91) | 88 | 8 (8) | 2 (2) |
| Wagner et al[47], 2018 | ESD | 80 | 41/22/17 | 33 | 68 (85) | 131 | 7 (9) | 2 (3) |
| Suzuki et al[49], 2021 | CELS | 22 | 20/1/1 | 20 | 22 (100) | 174 | 1 (4.5) | NA |
| Tamegai et al[50], 2018 | CELS | 17 | 15/1/1 | 22 | 17 (100) | 183 | 0 (0) | 0 |
| Lee et al[53], 2013 | CELS | 48 | 41/7/0 | 30 | 48 (100) | 145 | 2 (4.2) | 5 (10) |
| Hartwig et al[56], 2023 | CELS | 20 | 19/1/0 | 25 | 20 (100) | 70 | 1 (5) | 0 |
| Fukunaga | CELS | 3 | 3/0/0 | 27 | 3 (100) | 205 | 0 (0) | NA |
CELS facilitates local full-thickness resection of benign colonic lesions, which is often difficult to achieve with endoscopic resection alone. This approach is particularly beneficial in cases with severe fibrosis or for older patients and those with poor general health who require a less invasive treatment strategy. Van Marle et al[54] demonstrated the safety and efficacy of colonoscopy-assisted laparoscopic wedge resection for colonic neoplasms deemed unresectable via endoscopy. Their study reported technical success rates of 93%-100% and R0 resection rates of 91%-100%, with morbidity rates ranging from 6% to 20%. CELS has also been applied to appendiceal neoplasms, achieving a 100% R0 resection rate and a low complication rate of 2.4%[55].
In high-risk patients with early-stage CRC, local resection using CELS has proven to be a safe effective alternative, potentially reducing hospital stays. Hartwig et al[56] reported that among 25 high-risk patients (defined by an American Society of Anesthesiologists score ≥ 3 and/or performance score ≥ 1) with clinical stage 1 disease, CELS without lymph node dissection was feasible in 22 cases (88%). Only 1 patient experienced postoperative complications, and no local recurrences were observed during the follow-up period.
Laparoscopy and endoscopy cooperative surgery for colorectal tumors represents an effective combined approach for the resection of endoscopically unresectable colorectal tumors. This technique involves an endoscopic mucosal incision and submucosal dissection with adequate safety margins, followed by complete full-thickness dissection and excision using a coordinated endoscopic-laparoscopic strategy[57]. Several studies have reported excellent outcomes with laparoscopy and endoscopy cooperative surgery for colorectal tumors, including 100% en bloc and R0 resection rates and low perioperative complication rates ranging from 0% to 4.5%, underscoring its safety and efficacy[49,50].
Okamoto et al[58] demonstrated that robotic and endoscopic cooperative surgery (RECS) may reduce intraoperative intestinal content leakage and postoperative infections. In an experimental study comparing RECS and LECS performed in pigs, the level of Escherichia coli contamination in the abdominal cavity was significantly lower in the RECS group. The mean number of Escherichia coli colonies was 36.7 ± 30.2 in the RECS group compared to 142.2 ± 78.4 in the LECS group
Despite its versatility, CELS has several limitations. Unlike standard surgical resections, CELS does not permit regional lymph node dissection, limiting its use primarily to benign lesions or select early-stage malignancies. Intraoperative identification of features suggestive of invasive cancer necessitates immediate conversion to conventional oncological surgery to ensure appropriate oncological management. Moreover, CELS typically requires a longer operative time than standard endoscopic procedures such as EMR or ESD. As its use expands, there is a growing need to standardize advanced endoscopic techniques to ensure consistent clinical outcomes across institutions. Establishing structured training programs, akin to those available for ESD, will be essential in maintaining procedural efficiency and safety. Effective implementation of CELS also requires close collaboration between gastroenterologists and surgeons, as well as sufficient endoscopic proficiency among surgeons.
Although the integration of robotic platforms into CELS (e.g., RECS) may enhance procedural capabilities, high equipment costs and increased technical complexity remain substantial barriers to widespread adoption. A stepwise introduction of such technologies, supported by accumulating clinical experience and outcome data, may offer a more feasible path toward broader implementation. It would be more practical to implement such an approach gradually, following further clinical experience and supporting data.
The role of colonoscopy during the perioperative period is expanding, particularly in the context of minimally invasive colorectal surgeries. Endoscopic techniques have become essential not only for diagnosis and disease assessment, but also as integral components of therapeutic interventions. When combined with laparoscopic or robotic approaches, colo
| 1. | Yeung JM, Maxwell-Armstrong C, Acheson AG. Colonic tattooing in laparoscopic surgery - making the mark? Colorectal Dis. 2009;11:527-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 2. | Liu ZH, Liu JW, Chan FS, Li MK, Fan JK. Intraoperative colonoscopy in laparoscopic colorectal surgery: A review of recent publications. Asian J Endosc Surg. 2020;13:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Saito Y, Fukuzawa M, Matsuda T, Fukunaga S, Sakamoto T, Uraoka T, Nakajima T, Ikehara H, Fu KI, Itoi T, Fujii T. Clinical outcome of endoscopic submucosal dissection versus endoscopic mucosal resection of large colorectal tumors as determined by curative resection. Surg Endosc. 2010;24:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 438] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 4. | Placek SB, Nelson J. Combined Endoscopic Laparoscopic Surgery Procedures for Colorectal Surgery. Clin Colon Rectal Surg. 2017;30:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Broome JM, Coonan EE, Jones AT, Zelhart MD. Combined Endoscopic Robotic Surgery for Complex Colon Polyps. Dis Colon Rectum. 2023;66:1132-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Minami S, Saso K, Miyoshi N, Fujino S, Kato S, Sekido Y, Hata T, Ogino T, Takahashi H, Uemura M, Yamamoto H, Doki Y, Eguchi H. Diagnosis of Depth of Submucosal Invasion in Colorectal Cancer with AI Using Deep Learning. Cancers (Basel). 2022;14:5361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Yanai S, Nakamura S, Matsumoto T. Role of magnifying colonoscopy for diagnosis of colorectal neoplasms: From the perspective of Japanese colonoscopists. Dig Endosc. 2016;28:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kawaguti FS, Franco MC, Martins BC, Segateli V, Marques CFS, Nahas CSR, Pinto RA, Safatle-Ribeiro AV, Ribeiro-Junior U, Nahas SC, Maluf-Filho F. Role of Magnification Chromoendoscopy in the Management of Colorectal Neoplastic Lesions Suspicious for Submucosal Invasion. Dis Colon Rectum. 2019;62:422-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Hurlstone DP, Brown S, Cross SS, Shorthouse AJ, Sanders DS. Endoscopic ultrasound miniprobe staging of colorectal cancer: can management be modified? Endoscopy. 2005;37:710-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Hünerbein M, Totkas S, Ghadimi BM, Schlag PM. Preoperative evaluation of colorectal neoplasms by colonoscopic miniprobe ultrasonography. Ann Surg. 2000;232:46-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Marone P, de Bellis M, D'Angelo V, Delrio P, Passananti V, Di Girolamo E, Rossi GB, Rega D, Tracey MC, Tempesta AM. Role of endoscopic ultrasonography in the loco-regional staging of patients with rectal cancer. World J Gastrointest Endosc. 2015;7:688-701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 12. | Acuna SA, Elmi M, Shah PS, Coburn NG, Quereshy FA. Preoperative localization of colorectal cancer: a systematic review and meta-analysis. Surg Endosc. 2017;31:2366-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Imaoka K, Yano T, Yoshimitsu M, Fukuhara S, Oshita K, Nakano K, Kunihiro M, Idani H, Okajima M. Preoperative endoscopic tattoo marking improves lymph node retrieval in laparoscopic rectal resection: a retrospective cohort study. Ann Coloproctol. 2023;39:115-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 14. | Fernandez LM, Ibrahim RNM, Mizrahi I, DaSilva G, Wexner SD. How accurate is preoperative colonoscopic localization of colonic neoplasia? Surg Endosc. 2019;33:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Trakarnsanga A, Akaraviputh T. Endoscopic tattooing of colorectal lesions: Is it a risk-free procedure? World J Gastrointest Endosc. 2011;3:256-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Johnstone MS, Moug SJ; West of Scotland Surgical Research Network. The accuracy of colonoscopic localisation of colorectal tumours: a prospective, multi-centred observational study. Scott Med J. 2014;59:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Yap R, Ianno D, Burgess A. Colonoscopic localization accuracy for colorectal resections in the laparoscopic era. Am J Surg. 2016;212:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Letarte F, Webb M, Raval M, Karimuddin A, Brown CJ, Phang PT. Tattooing or not? A review of current practice and outcomes for laparoscopic colonic resection following endoscopy at a tertiary care centre. Can J Surg. 2017;60:394-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Saleh A, Ihedioha U, Babu B, Evans J, Kang P. Audit of preoperative localisation of tumor with tattoo for patients undergoing laparoscopic colorectal surgery. Scott Med J. 2016;61:160-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Lee SJ, Sohn DK, Han KS, Kim BC, Hong CW, Park SC, Kim MJ, Park BK, Oh JH. Preoperative Tattooing Using Indocyanine Green in Laparoscopic Colorectal Surgery. Ann Coloproctol. 2018;34:206-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Cho YB, Lee WY, Yun HR, Lee WS, Yun SH, Chun HK. Tumor localization for laparoscopic colorectal surgery. World J Surg. 2007;31:1491-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Offermans T, Vogelaar FJ, Aquarius M, Janssen-Heijnen MLG, Simons PCG. Preoperative segmental localization of colorectal carcinoma: CT colonography vs. optical colonoscopy. Eur J Surg Oncol. 2017;43:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Szura M, Pasternak A, Solecki R, Matyja M, Szczepanik A, Matyja A. Accuracy of preoperative tumor localization in large bowel using 3D magnetic endoscopic imaging: randomized clinical trial. Surg Endosc. 2017;31:2089-2095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Wehrmann K, Frühmorgen P. Evaluation of a new three-dimensional magnetic imaging system for use during colonoscopy. Endoscopy. 2002;34:905-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Takahashi J, Yoshida M, Ohdaira H, Nakaseko Y, Nakashima K, Kamada T, Suzuki N, Sato T, Suzuki Y. Efficacy and Safety of Gastrointestinal Tumour Site Marking with da Vinci-Compatible Near-Infrared Fluorescent Clips: A Case Series. World J Surg. 2023;47:2386-2391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 26. | Narihiro S, Nakashima S, Kazi M, Kumamoto T, Kitagawa K, Toya N, Eto K. Fluorescence guidance using near-infrared fluorescent clips in robotic rectal surgery: a case series. Int J Colorectal Dis. 2024;39:41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Kumagai K, Yoshida M, Ishida H, Ishizuka N, Ohashi M, Makuuchi R, Hayami M, Ida S, Yoshimizu S, Horiuchi Y, Ishiyama A, Yoshio T, Hirasawa T, Fujisaki J, Nunobe S. Diagnostic Performance of Near-Infrared Fluorescent Marking Clips in Laparoscopic Gastrectomy. J Surg Res. 2024;300:157-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 28. | Ryu S, Okamoto A, Nakashima K, Hara K, Ishida K, Ito R, Nakabayashi Y, Eto K, Ikegami T. Usefulness of Preoperative Endoscopic Fluorescent Clip Marking in Laparoscopic Gastrointestinal Surgery. Anticancer Res. 2020;40:6517-6523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Cerdán Santacruz C, Esteban López-Jamar JM, Sánchez López E, Cerdán Miguel J. Contribution of intraoperative colonoscopy in a colorectal surgery unit(). Scand J Gastroenterol. 2017;52:1292-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Gorgun IE, Aytac E, Manilich E, Church JM, Remzi FH. Intraoperative colonoscopy does not worsen the outcomes of laparoscopic colorectal surgery: a case-matched study. Surg Endosc. 2013;27:3572-3576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Pajares JA, Perea J. Multiple primary colorectal cancer: Individual or familial predisposition? World J Gastrointest Oncol. 2015;7:434-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 32. | Sasaki K, Kazama S, Sunami E, Tsuno NH, Nozawa H, Nagawa H, Kitayama J. One-stage segmental colectomy and primary anastomosis after intraoperative colonic irrigation and total colonoscopy for patients with obstruction due to left-sided colorectal cancer. Dis Colon Rectum. 2012;55:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Simmerman EL, King RS, Ham PB 3rd, Hooks VH 3rd. Feasibility and Safety of Intraoperative Colonoscopy after Segmental Colectomy and Primary Anastomosis. Am Surg. 2018;84:1175-1179. [PubMed] |
| 34. | Ishihara S, Watanabe T, Nagawa H. Intraoperative colonoscopy for stapled anastomosis in colorectal surgery. Surg Today. 2008;38:1063-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Li VK, Wexner SD, Pulido N, Wang H, Jin HY, Weiss EG, Nogeuras JJ, Sands DR. Use of routine intraoperative endoscopy in elective laparoscopic colorectal surgery: can it further avoid anastomotic failure? Surg Endosc. 2009;23:2459-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Hébert J, Eltonsy S, Gaudet J, Jose C. Incidence and risk factors for anastomotic bleeding in lower gastrointestinal surgery. BMC Res Notes. 2019;12:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Cirocco WC, Golub RW. Endoscopic treatment of postoperative hemorrhage from a stapled colorectal anastomosis. Am Surg. 1995;61:460-463. [PubMed] |
| 38. | Shamiyeh A, Szabo K, Ulf Wayand W, Zehetner J. Intraoperative endoscopy for the assessment of circular-stapled anastomosis in laparoscopic colon surgery. Surg Laparosc Endosc Percutan Tech. 2012;22:65-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Yang J, Chen Q, Jindou L, Cheng Y. The influence of anastomotic leakage for rectal cancer oncologic outcome: A systematic review and meta-analysis. J Surg Oncol. 2020;121:1283-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 40. | Salvans S, Mayol X, Alonso S, Messeguer R, Pascual M, Mojal S, Grande L, Pera M. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg. 2014;260:939-43; discussion 943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Vallicelli C, Pirrera B, Alagna V, Fantini E, Palini GM, Zanini N, Garulli G. Intraoperative endoscopy with immediate suture reinforcement of the defect in colorectal anastomosis: a pilot study. Updates Surg. 2020;72:999-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | Yang SY, Han J, Han YD, Cho MS, Hur H, Lee KY, Kim NK, Min BS. Intraoperative colonoscopy for the assessment and prevention of anastomotic leakage in low anterior resection for rectal cancer. Int J Colorectal Dis. 2017;32:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Kishiki T, Kojima K, Aso N, Iioka A, Wakamatsu T, Kataoka I, Kim S, Ishii S, Isobe S, Sakamoto Y, Abe N, Sunami E. Intraoperative Colonoscopy in Laparoscopic Rectal Cancer Surgery Reduces Anastomotic Leakage. J Anus Rectum Colon. 2022;6:159-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 44. | Okamoto K, Muguruma N, Kagemoto K, Mitsui Y, Fujimoto D, Kitamura S, Kimura T, Sogabe M, Miyamoto H, Takayama T. Efficacy of hybrid endoscopic submucosal dissection (ESD) as a rescue treatment in difficult colorectal ESD cases. Dig Endosc. 2017;29 Suppl 2:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Fuccio L, Hassan C, Ponchon T, Mandolesi D, Farioli A, Cucchetti A, Frazzoni L, Bhandari P, Bellisario C, Bazzoli F, Repici A. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc. 2017;86:74-86.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 46. | Saito Y, Yamada M, So E, Abe S, Sakamoto T, Nakajima T, Otake Y, Ono A, Matsuda T. Colorectal endoscopic submucosal dissection: Technical advantages compared to endoscopic mucosal resection and minimally invasive surgery. Dig Endosc. 2014;26 Suppl 1:52-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 47. | Wagner A, Neureiter D, Kiesslich T, Wolkersdörfer GW, Pleininger T, Mayr C, Dienhart C, Yahagi N, Oyama T, Berr F. Single-center implementation of endoscopic submucosal dissection (ESD) in the colorectum: Low recurrence rate after intention-to-treat ESD. Dig Endosc. 2018;30:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Oka S, Uraoka T, Tamai N, Ikematsu H, Chino A, Okamoto K, Takeuchi Y, Imai K, Ohata K, Shiga H, Raftopoulos S, Lee BI, Matsuda T. Standardization of endoscopic resection for colorectal tumors larger than 10 mm in diameter. Dig Endosc. 2017;29 Suppl 2:40-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 49. | Suzuki K, Saito S, Fukunaga Y. Current Status and Prospects of Endoscopic Resection Technique for Colorectal Tumors. J Anus Rectum Colon. 2021;5:121-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Tamegai Y, Fukunaga Y, Suzuki S, Lim DNF, Chino A, Saito S, Konishi T, Akiyoshi T, Ueno M, Hiki N, Muto T. Laparoscopic and endoscopic cooperative surgery (LECS) to overcome the limitations of endoscopic resection for colorectal tumors. Endosc Int Open. 2018;6:E1477-E1485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Ikezawa N, Toyonaga T, Tanaka S, Yoshizaki T, Takao T, Abe H, Sakaguchi H, Tsuda K, Urakami S, Nakai T, Harada T, Miura K, Yamasaki T, Kostalas S, Morita Y, Kodama Y. Feasibility and safety of endoscopic submucosal dissection for lesions in proximity to a colonic diverticulum. Clin Endosc. 2022;55:417-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Wlodarczyk J, Gupta A, Lee SW. Combined Endoscopy-Laparoscopy Surgery: When and How to Utilize This Tool. Clin Colon Rectal Surg. 2024;37:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Lee SW, Garrett KA, Shin JH, Trencheva K, Sonoda T, Milsom JW. Dynamic article: long-term outcomes of patients undergoing combined endolaparoscopic surgery for benign colon polyps. Dis Colon Rectum. 2013;56:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | van Marle L, Hanevelt J, de Vos Tot Nederveen Cappel WH, van Westreenen HL. Colonoscopic-assisted laparoscopic wedge resection for colonic neoplasms: a systematic review. Scand J Gastroenterol. 2024;59:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 55. | Justiniano CF, Ozgur I, Liska D, Valente MA, Steele SR, Gorgun E. The role of advanced endoscopy in appendiceal polyp management and outcomes. Surg Endosc. 2024;38:2267-2272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Hartwig MFS, Bulut M, Ravn-Eriksen J, Hansen LB, Bojesen RD, Klein MF, Jakobsen HL, Rasmussen M, Rud B, Eriksen JO, Eiholm S, Fiehn AK, Quirke P, Gögenur I. Combined endoscopic and laparoscopic surgery (CELS) for early colon cancer in high-risk patients. Surg Endosc. 2023;37:8511-8521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 57. | Fukunaga Y, Tamegai Y, Chino A, Ueno M, Nagayama S, Fujimoto Y, Konishi T, Igarashi M. New technique of en bloc resection of colorectal tumor using laparoscopy and endoscopy cooperatively (laparoscopy and endoscopy cooperative surgery - colorectal). Dis Colon Rectum. 2014;57:267-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Okamoto N, Al-Taher M, Mascagni P, Vazquez AG, Takeuchi M, Marescaux J, Diana M, Dallemagne B. Robotic endoscopic cooperative surgery for colorectal tumors: a feasibility study (with video). Surg Endosc. 2022;36:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/