Published online Oct 16, 2025. doi: 10.4253/wjge.v17.i10.110920
Revised: July 19, 2025
Accepted: September 16, 2025
Published online: October 16, 2025
Processing time: 120 Days and 0 Hours
Gastrointestinal stromal tumors are the most common mesenchymal tumors of the gastrointestinal tract, and gastric gastrointestinal stromal tumors (gGISTs) account for the majority of these tumors. Currently, endoscopic removal (ER) is increasingly adopted as a minimally invasive treatment. However, postoperative perforation remains a critical complication, necessitating robust prediction tools.
To identify the risk factors and develop a validated nomogram for predicting perforation after ER of gGISTs.
This retrospective study analyzed the patients undergoing ER at Fuyang People’s Hospital from 2019 to 2024. Clinical data, including tumor size, location, and procedural details, were collected and analyzed. The risk factors were identified via univariate and multivariate logistic regression, and a nomogram was de
Among 301 patients, the perforation rate was 6.3%. Multivariate analysis identified tumor size (odds ratio = 4.699, 95% confidence interval: 2.382-9.267, P = 0.001) and cardia/fundus location (odds ratio = 3.492, 95% confidence interval: 1.121-10.875, P = 0.031) as independent predictors. A nomogram was constructed and achieved good predictive performance in both the training (area under the curve = 0.881) and validation sets (area under the curve = 0.878).
This study identified that tumor size and location were independent risk factors, and provides a clinically actionable nomogram for evaluating and predicting postoperative perforation risk in gGISTs treated with ER, facilitating preprocedural planning and risk monitoring.
Core Tip: Gastric gastrointestinal stromal tumors are the most common mesenchymal tumors of the gastrointestinal tract, with endoscopic resection being a preferred minimally invasive treatment. Postoperative perforation is a severe compli
- Citation: Zhang DM, Li PP, Zhang MH, Zhao ZX, Zhou YB. Postoperative perforation risk after endoscopic removal of gastric gastrointestinal stromal tumors: Development and validation of a predictive nomogram. World J Gastrointest Endosc 2025; 17(10): 110920
- URL: https://www.wjgnet.com/1948-5190/full/v17/i10/110920.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i10.110920
Gastrointestinal stromal tumors (GISTs) are a group of tumors originating from the mesenchymal tissue of the gast
However, endoscopic treatment of gGISTs is not without risks, including bleeding, infection, and perforation[5,11,12]. Postoperative perforation - defined as unplanned, accidental perforation that occurs after ER, excluding iatrogenic perforations intentionally induced during procedures such as EFTR for therapeutic purposes - is one of the most severe complications of this procedure. This complication is distinct from intraoperative iatrogenic perforation, as it arises unexpectedly during the postoperative period rather than being a controlled part of the surgical technique. Although its incidence varies across studies and treatment centers, when it occurs, it can lead to serious consequences such as peritonitis, intra-abdominal infection, and hemorrhage, significantly prolonging hospital stays, increasing patient suffering and medical costs, and even threatening patients’ lives[11,13]. Statistics show that approximately 2%-8% of patients develop postoperative perforation after endoscopic treatment of gGISTs, which has become a key issue affecting the clinical efficacy and safety of endoscopic therapy[11,14,15]. Therefore, identifying risk factors for postoperative perforation in gGISTs and constructing effective predictive models are of profound significance for clinical decision-making.
A nomogram, a visual predictive tool, is typically constructed based on logistic regression or other statistical models[16-18]. Characterized by high readability and cost-effectiveness, it can be directly printed or included in clinical work manuals, enabling clinicians to use it readily for real-time clinical decision-making without relying on complex statistical software. This accessibility has made nomograms highly popular among clinicians. This study retrospectively collected clinical and pathological data on patients with gastric stromal tumors treated endoscopically. We aimed to identify risk factors for postoperative perforation, develop a nomogram predictive model, and provide clinicians with a precise risk-assessment tool to enhance treatment safety, efficacy, and prognosis.
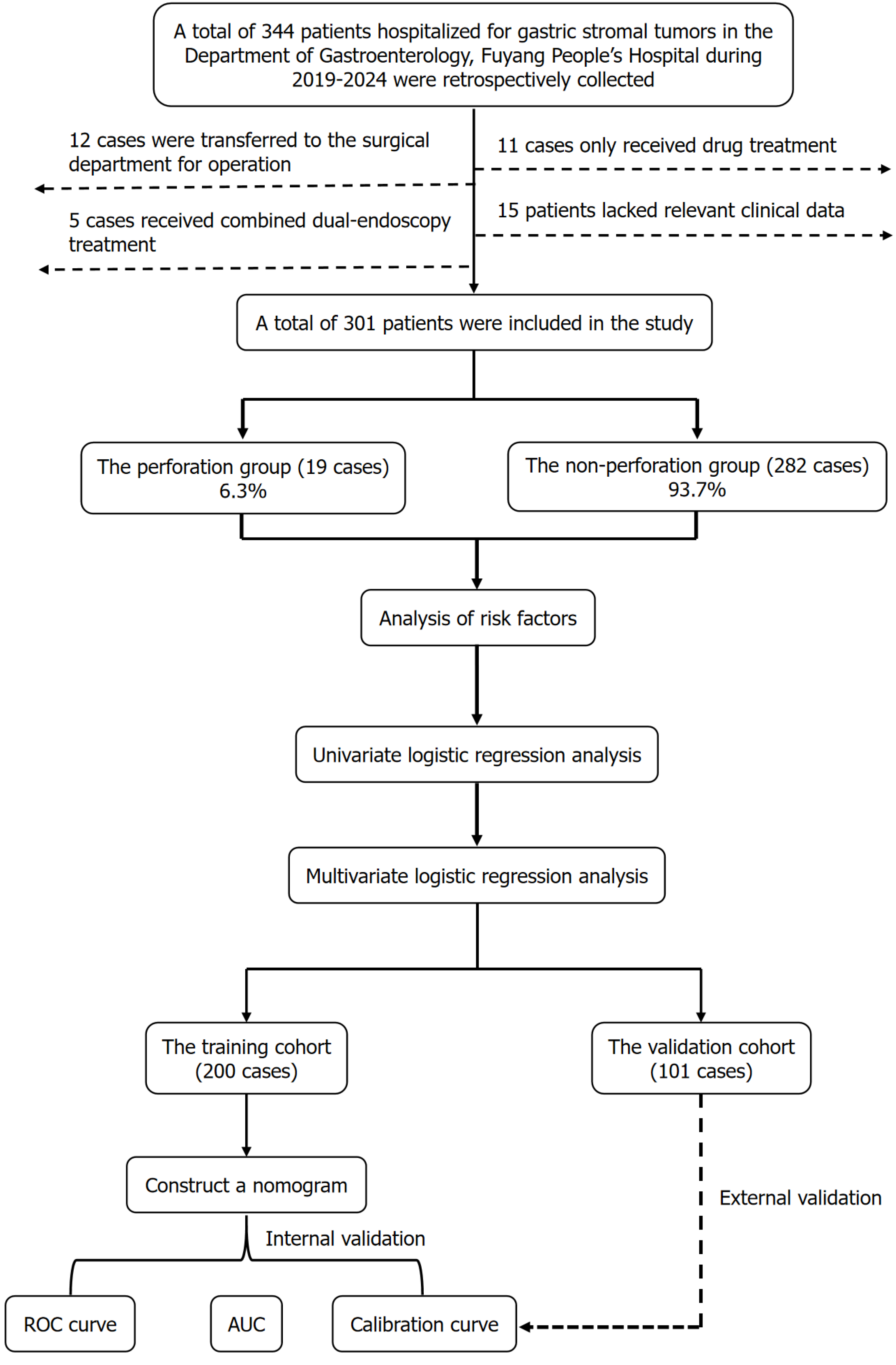
This retrospective study enrolled gGISTs patients admitted to the Department of Gastroenterology, Fuyang People’s Hospital Affiliated to Anhui Medical University from January 2019 to December 2024. The research design and flowchart are shown in Figure 1. Patients were included in the study if they met the following criteria: (1) Diagnosed with gGISTs and treated at the Department of Gastroenterology, Fuyang People’s Hospital; (2) Aged between 18 and 80 years old; (3) Had relatively complete clinical data, including basic demographics, laboratory test results, enhanced computed tomography (CT) imaging characteristics, gastroscopy images, operation time, surgical approach; (4) Underwent ER of gGISTs (e.g., ESD, endoscopic submucosal excavation, EFTR); (5) Provided written informed consent; and (6) Were pathologically confirmed to have stromal tumors postoperatively. Patients were excluded if any of the following criteria was met: (1) Lack of pathological confirmation of a gastric stromal tumor; (2) Combined with other tumors; (3) In
Clinical parameters, including sex, age, height, weight, body mass index, laboratory biochemical indices, CT imaging characteristics, gastroscopy images and information, operation time, surgical approach, and occurrence of postoperative perforation, were collected. Active intraoperative perforation (such as in EFTR) was not defined as accidental perforation and was excluded from this study.
All clinical parameters [such as tumor size, hemoglobin (HGB) level, and tumor location] were subjected to statistical analysis, and potential risk factors were preliminarily identified. Univariate logistic regression analysis was then performed on the potential risk factors to identify those factors associated with postoperative perforation. Variables with a P-value < 0.05 were retained for subsequent multivariate analysis to identify independent predictors of perforation.
The patients were randomly divided into a training cohort and a validation cohort at a ratio of 2:1 (200:101). Based on the results of multivariate logistic regression analysis, a nomogram for predicting the probability of postoperative perforation was constructed using the training cohort. The nomogram was internally validated using 1000 resampling bootstrapping to assess its discriminative ability and calibration. Discriminative ability was evaluated by the receiver operating characteristic (ROC) curve and the area under the curve (AUC). Calibration was assessed by comparing the predicted probabilities with the observed outcomes through calibration curves. Subsequently, external validation of the nomogram was performed using the validation cohort, where ROC, AUC, and calibration curves were similarly calculated to confirm the generalizability of the model.
All statistical analyses were conducted using GraphPad Prism 8 and R.4.2.1 software. The measurement data were analyzed using the Student’s t-test or Mann-Whitney U test, while enumeration data were analyzed using the χ2 or Fisher’s exact tests. The logistic regression model was constructed using the “glm” function, and the nomogram was plotted using the “rms” package in R. All statistical tests were two-sided, and a P-value < 0.05 was considered statistically significant.
As shown in Figure 1, this study retrospectively included 344 patients with gGISTs who visited the Department of Gastroenterology, Fuyang People’s Hospital from January 2019 to December 2024. Among them, 12 cases were transferred to the Department of Surgical for surgical procedures, 11 cases only received drug treatment, 5 cases received combined dual-endoscopy treatment, and 15 cases lacked relevant clinical data; these patients were excluded. Finally, 301 patients with gGISTs who received ER were included. Among the included patients with gGISTs, 19 cases (6.3%) had postoperative perforation, and 282 cases (93.7%) did not. Of the patients with postoperative perforation, 14 cases improved after conservative treatment, and 5 cases, for whom conservative treatment was ineffective, received laparoscopic perforation repair. Baseline data of the relevant parameters are shown in Table 1.
| Characteristics | Perforation group (n = 19) | Non-perforation group (n = 282) | t/χ2 | P value |
| Age (years) | 59.89 ± 9.50 | 59.96 ± 11.07 | 0.024 | 0.981 |
| Gender | 0.806 | 0.369 | ||
| Male | 5 | 103 | ||
| Female | 14 | 179 | ||
| History of hypertension | 0.228 | 0.633 | ||
| Yes | 5 | 89 | ||
| No | 14 | 193 | ||
| History of diabetes | / | 0.141 | ||
| Yes | 4 | 29 | ||
| No | 15 | 253 | ||
| History of stroke | / | 0.169 | ||
| Yes | 3 | 20 | ||
| No | 16 | 262 | ||
| Height (m) | 1.62 ± 0.04 | 1.63 ± 0.08 | 0.017 | 0.987 |
| Weight (kg) | 62.61 ± 7.27 | 61.49 ± 10.50 | 0.453 | 0.651 |
| BMI (kg/m2) | 23.57 ± 2.12 | 23.126 ± 3.21 | 0.584 | 0.553 |
| HGB (g/L) | 117.47 ± 15.17 | 125.27 ± 16.40 | 2.008 | 0.046 |
| WBC (× 109) | 5.43 ± 1.34 | 5.04 ± 1.56 | 1.066 | 0.287 |
| ALB (g/L) | 39.07 ± 4.69 | 39.80 ± 3.26 | 0.915 | 0.361 |
| Glu (mmol/L) | 5.16 ± 0.51 | 5.30 ± 1.20 | 0.503 | 0.616 |
| Tumor location | 4.830 | 0.028 | ||
| Cardia/fundus | 13 | 120 | ||
| Antrum/body | 6 | 162 | ||
| Tumor size (cm) | 3.53 ± 1.15 | 1.56 ± 0.85 | 10.73 | 0.001 |
| Growth pattern | 5.879 | 0.015 | ||
| Exophytic type | 13 | 113 | ||
| Non-exophytic type | 6 | 169 | ||
| Operative approach | 1.458 | 0.483 | ||
| ESD | 5 | 47 | ||
| ESE | 4 | 85 | ||
| EFTR | 10 | 150 | ||
| Operation duration (minutes) | 135.12 ± 79.80 | 73.55 ± 51.19 | 4.752 | 0.001 |
| Risk assessment | 80.35 | 0.001 | ||
| Very low risk | 1 | 210 | ||
| Low risk | 10 | 63 | ||
| Intermediate risk | 1 | 3 | ||
| High risk | 7 | 6 |
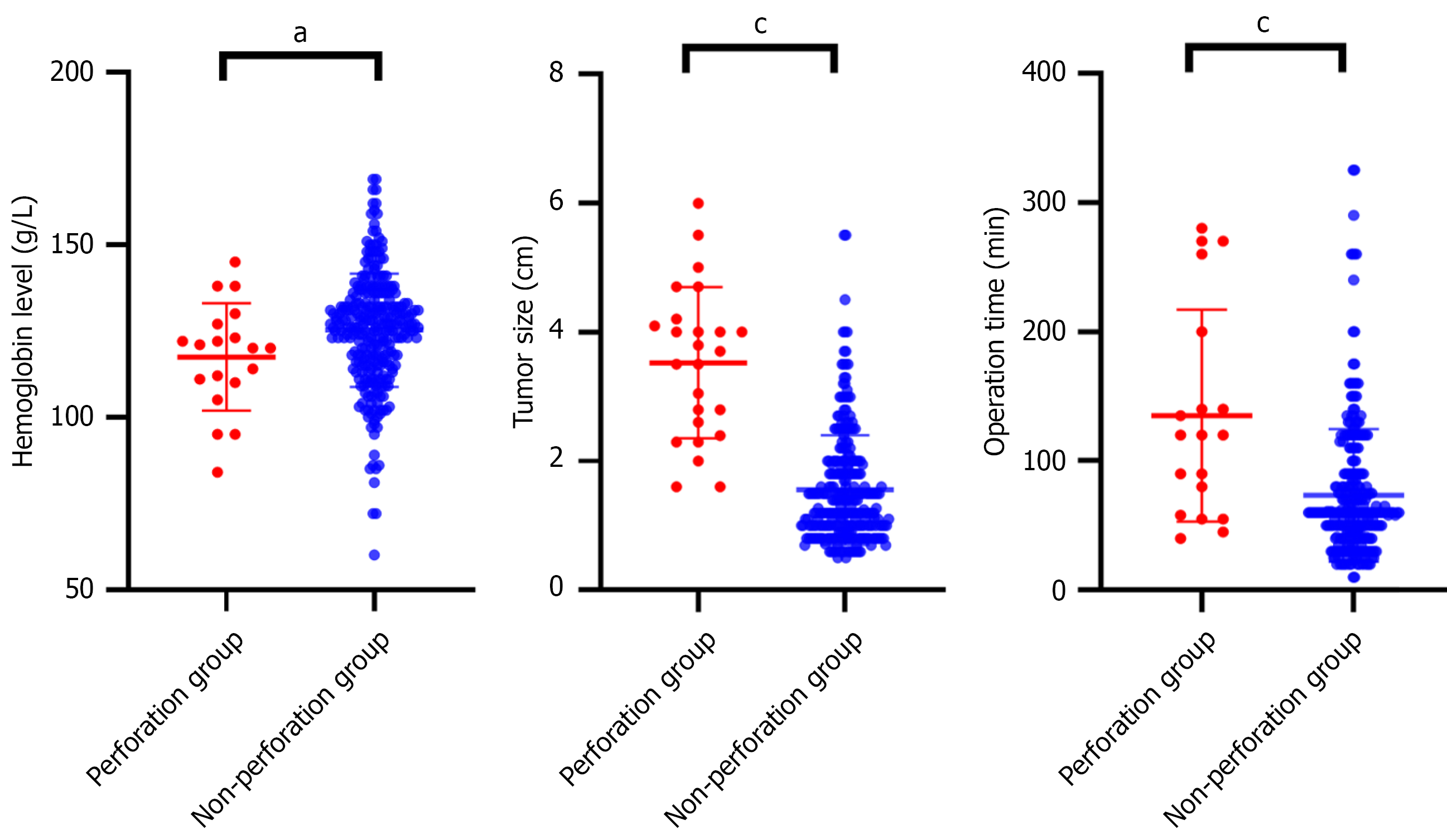
As shown in Table 1 and Figure 2, higher preoperative HGB levels were associated with reduced postoperative perforation risk (P = 0.046), and tumors located in the gastric fundus/body significantly reduced perforation risk compared to the cardia/antrum (P = 0.028). In addition, larger tumor size (P = 0.001), exophytic growth pattern (P = 0.015), and longer operation duration (P = 0.001) were associated with higher postoperative perforation risk.
As shown in Table 2, univariate analysis demonstrated that lower HGB level [odds ratio (OR) = 0.974, 95% confidence interval (CI): 0.948-0.999, P = 0.046], cardia/fundus location (OR = 2.925, 95%CI: 1.081-7.917, P = 0.035), larger tumor size (OR = 3.366, 95%CI: 2.173-5.211, P = 0.001), and longer operation duration (OR = 1.012, 95%CI: 1.006-1.018, P = 0.001) were associated with higher postoperative perforation risk. However, tumor growth pattern (exophytic type vs non-exophytic type) was not associated with postoperative perforation risk in this study. Multivariate analysis showed that cardia/fundus location (OR = 3.492, 95%CI: 1.121-10.875, P = 0.031) and larger tumor size (OR = 4.699, 95%CI: 2.382-9.267, P = 0.001) were independent risk factors.
| Characteristics | Univariate logistic regression analysis | Multivariate logistic regression analysis | ||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| HGB (g/L) | 0.974 | 0.948-0.999 | 0.046 | 1.025 | 0.993-1.058 | 0.129 |
| Tumor location | ||||||
| Antrum/body | 1 | |||||
| Cardia/fundus | 2.925 | 1.081-7.917 | 0.035 | 3.492 | 1.121-10.875 | 0.031 |
| Tumor size (cm) | 3.366 | 2.173-5.211 | 0.001 | 4.699 | 2.382-9.267 | 0.001 |
| Growth pattern | / | |||||
| Exophytic type | 1 | |||||
| Non-exophytic type | 0.390 | 0.149-1.021 | 0.055 | |||
| Operation duration (minutes) | 1.012 | 1.006-1.018 | 0.001 | 0.999 | 0.989-1.008 | 0.804 |
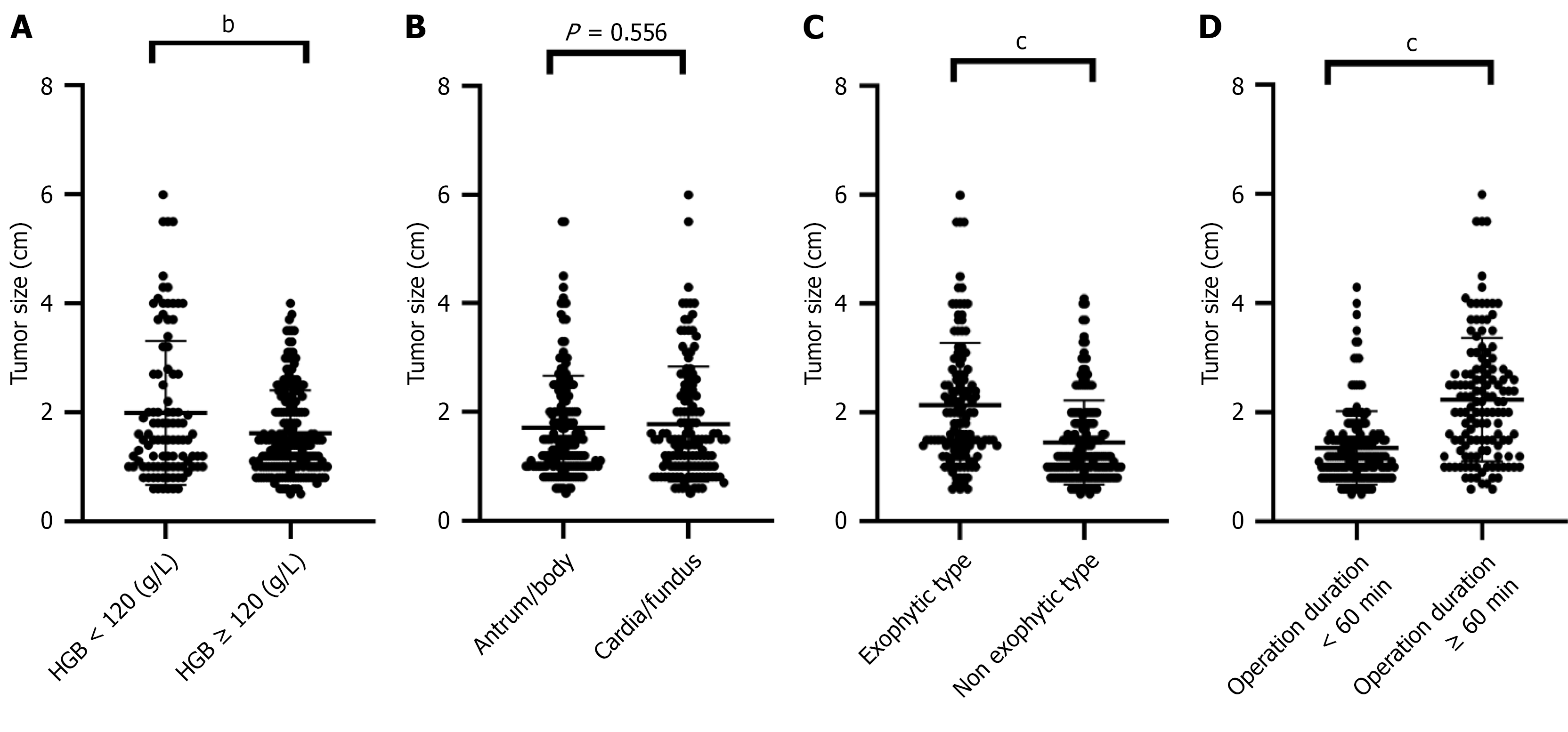
To further analyze the results of the logistic regression, we completed a corresponding subgroup analysis (Figure 3). The analysis showed that patients with anemia (HGB < 120 g/L) (P = 0.003), exophytic growth type (P < 0.001), and operation time ≥ 60 minutes (P < 0.001) had significantly larger gastric stromal tumors, with a demonstrated correlation between these factors and tumor size. In contrast, tumor location (cardia/fundus vs antrum/body) was not associated with tumor size (P = 0.556).
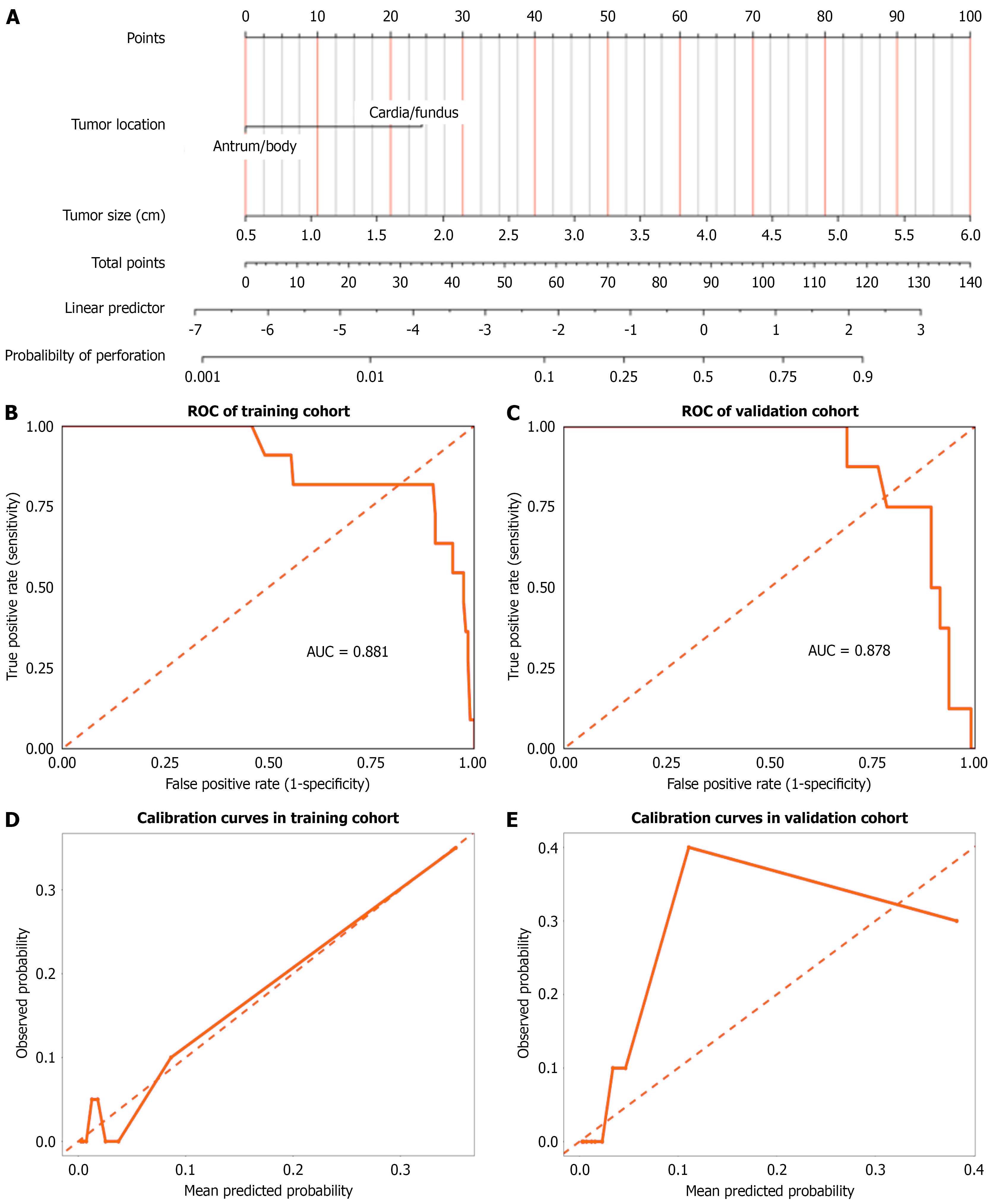
Guided by the multivariate logistic regression analysis results, tumor size and tumor location were selected as key predictors to develop the nomogram model in the training cohort (Figure 4A). The model exhibited robust discriminative capability, as evidenced by the training set ROC curve (Figure 4B), which yielded an AUC of 0.881, indicating a strong ability to distinguish perforation probability. The validation cohort ROC curve showed an AUC of 0.878, closely comparable to the training results, further validating the model’s generalizability (Figure 4C). Calibration curves in both the training (Figure 4D) and validation (Figure 4E) cohorts demonstrated good agreement between predicted and ob
According to the GISTs-related clinical guidelines, ER is the preferred minimally invasive approach for gGISTs[19,20]. However, postoperative perforation, as a severe complication, directly affects patient prognosis, increases medical burden, and may even lead to serious adverse events such as peritonitis and intra-abdominal infection. Through this single-center retrospective analysis, the study enrolled 301 patients with gGISTs who underwent endoscopic resection in our hospital, systematically explored the risk factors for postoperative perforation, and constructed a clinical prediction model to assess perforation risk. The results confirmed that tumor size and anatomical location (cardia/fundus) were independent high-risk factors for postoperative perforation. The constructed nomogram, validated by external ve
In this study, we confirmed that tumor size was significantly positively correlated with the incidence of postoperative perforation, a finding that is highly consistent with previous studies. A multicenter study analyzing ESD for early gastric cancer found that a tumor diameter > 20 mm was an independent risk factor for postoperative perforation, which is highly consistent with the results of this study[21]. In addition, a large-sample retrospective study has shown that a tumor diameter > 2 cm is an independent risk factor for perforation after ER of gGISTs[22]. When gastric stromal tumors are too large, the area of muscularis defect formed after resection increases correspondingly, making it difficult to achieve precise three-layer (mucosa-muscularis-serosa) apposition with endoscopic clips or purse-string sutures. Intraluminal pressure generated by gastric peristalsis can gradually expand the incompletely closed wound, eventually leading to perforation. Additionally, larger tumors are often associated with more complex blood supply systems, and incomplete electrocoagulation during surgery can easily induce secondary hemorrhage. Repeated hemostatic procedures further damage the muscularis tissue, weakening its healing capacity. Also, larger-diameter tumors typically require longer operative times, not only increasing operator fatigue-related errors but also elevating gastric wall tension due to continuous intra-luminal insufflation, exacerbating muscularis injury.
In this study, the antrum and body were grouped together, as were the cardia and fundus. This is because the antrum and body are similar in muscular layer thickness, blood supply, and difficulty of endoscopic procedures; while the cardia and fundus share common features such as a thinner muscularis propria and the need for retroflexion during endoscopic observation, all of which affect surgical risk. This study found that gGISTs located in the gastric fundus and cardia are more prone to postoperative perforation, a conclusion that is highly consistent with previous studies[22]. The muscularis layer of the gastric fundus is significantly thinner than that of other gastric regions, with a sparse submucosal vascular plexus and relatively poor blood supply. Even if perforation is sutured during the operation, the healing capacity of the muscularis defect remains poor and prone to delayed perforation. The cardia is located at the esophagogastric junction, requiring retroflexion for endoscopic surgery, which reduces the precision of clip deployment and increases suture difficulty. Additionally, the cardia’s rich submucosal nerve plexus is susceptible to vagal reflexes during electrocoagulation, causing abrupt heart rate drops or gastrointestinal motility disorders, with frequent intraoperative peristaltic interference further exacerbating precise suture challenges.
Subgroup analysis further revealed significant correlations between tumor size and factors including HGB level, tumor growth pattern, and operation duration[23]. Studies have shown that larger tumors are often associated with intratumoral vascular malformations or hemorrhage, leading to lower HGB levels[24]. Exophytic growth patterns, unrestricted by gastric lumen space, tend to develop into larger volumes; conversely, excessively large tumors often penetrate the serosa to grow extraluminally, forming an exophytic phenotype, indicating a bidirectional correlation[25]. Additionally, larger tumors require more complex resection techniques (such as EFTR) and extensive, difficult suturing, significantly prolonging operation time.
The final model in this study retained only two variables: Tumor size and location. While this enhances the simplicity of the model, potentially predictive variables such as operator experience, tumor growth pattern, and HGB level were not included. Regarding operator experience, the exclusion was due to the standardized training system implemented in our center for the ER of gGISTs. All doctors have over 5 years of endoscopic operation experience and hold the title of associate chief physician, with complex cases (e.g., EFTR) performed by a fixed team. This setup minimizes the impact of differences in experience and technical proficiency among operators on outcomes, thus weakening their independent predictive value. As for tumor growth pattern and HGB level, they were not included in the final model because subgroup analysis revealed their significant correlation with tumor size: Larger tumors are more likely to present with exophytic growth and be associated with clinical manifestations such as anemia. To avoid multicollinearity and prevent the inclusion of excessively weakly correlated variables from reducing the model’s accuracy, these variables were excluded. However, from a clinical perspective, these factors may be related to perforation. Future multicenter studies with large sample sizes, incorporating standardized recording of variables like operator experience, are needed to further validate their predictive value in larger datasets.
The nomogram model constructed in this study included tumor size and location as independent predictors, with an AUC of 0.881 in the training set and 0.878 in the validation set, indicating good discriminative ability. The nomogram model established in this study uses tumor size as the core predictor, with a stepwise risk assignment based on tumor size. The anatomic tumor location is also incorporated as a key auxiliary predictor, enabling precise quantification of risk assessment. Calibration curves showed high consistency between predicted probabilities and actual perforation rates. However, single-center data may have bias and are more susceptible to confounding factors, and molecular markers were not included. Multicenter studies and integration of gene testing are needed in the future to optimize the model.
This study has certain limitations. Firstly, as a single-center retrospective study, the data were derived from cases at Fuyang People’s Hospital between 2019 and 2024, with obvious regional characteristics and an insufficient proportion of perforation cases, which may affect the generalizability of the model. Secondly, some clinical data were missing, variables such as surgeons’ experience were not recorded in a standardized manner, and the development of endoscopic techniques during the study period may affect the stability of the results. In addition, genetic risk factors were not explored, the measurement of tumor volume based on preoperative enhanced CT had certain errors, the classification of tumor growth patterns was rough, and more detailed radiomics indicators were not included, affecting the accuracy of risk assessment. Future research can be optimized in multiple aspects. Multicenter studies should be conducted to include more cases for model validation. Genetic testing data and radiomics data should be integrated to construct a more accurate model. Prospective cohort studies should be performed to develop practical clinical tools. Mechanistic studies on high-risk factors should be carried out, and randomized controlled trials should be designed to explore intervention strategies to reduce the perforation rate.
This retrospective study identified tumor size and location as independent risk factors for postoperative perforation after ER of gGISTs. In addition, a nomogram was developed for predicting postoperative perforation risk after ER of gGISTs. The nomogram model showed good predictive performance, facilitating clinical risk assessment.
| 1. | Mantese G. Gastrointestinal stromal tumor: epidemiology, diagnosis, and treatment. Curr Opin Gastroenterol. 2019;35:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (1)] |
| 2. | Stamatakos M, Douzinas E, Stefanaki C, Safioleas P, Polyzou E, Levidou G, Safioleas M. Gastrointestinal stromal tumor. World J Surg Oncol. 2009;7:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 3. | Schaefer IM, Mariño-Enríquez A, Fletcher JA. What is New in Gastrointestinal Stromal Tumor? Adv Anat Pathol. 2017;24:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Blay JY, Kang YK, Nishida T, von Mehren M. Gastrointestinal stromal tumours. Nat Rev Dis Primers. 2021;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 296] [Article Influence: 59.2] [Reference Citation Analysis (1)] |
| 5. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (9)] |
| 6. | Huang LY, Cui J, Liu YX, Wu CR, Yi DL. Endoscopic therapy for gastric stromal tumors originating from the muscularis propria. World J Gastroenterol. 2012;18:3465-3471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Deprez PH, Moons LMG, OʼToole D, Gincul R, Seicean A, Pimentel-Nunes P, Fernández-Esparrach G, Polkowski M, Vieth M, Borbath I, Moreels TG, Nieveen van Dijkum E, Blay JY, van Hooft JE. Endoscopic management of subepithelial lesions including neuroendocrine neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2022;54:412-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 8. | Jacobson BC, Bhatt A, Greer KB, Lee LS, Park WG, Sauer BG, Shami VM. ACG Clinical Guideline: Diagnosis and Management of Gastrointestinal Subepithelial Lesions. Am J Gastroenterol. 2023;118:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 103] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 9. | Rajan E, Wong Kee Song LM. Endoscopic Full Thickness Resection. Gastroenterology. 2018;154:1925-1937.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Landin MD, Guerrón AD. Endoscopic Mucosal Resection and Endoscopic Submucosal Dissection. Surg Clin North Am. 2020;100:1069-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Oda I, Suzuki H, Nonaka S, Yoshinaga S. Complications of gastric endoscopic submucosal dissection. Dig Endosc. 2013;25 Suppl 1:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Shichijo S, Uedo N, Sawada A, Hirasawa K, Takeuchi H, Abe N, Miyaoka M, Yao K, Dobashi A, Sumiyama K, Ishida T, Morita Y, Ono H. Endoscopic full-thickness resection for gastric submucosal tumors: Japanese multicenter prospective study. Dig Endosc. 2024;36:811-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Liu Z, Li H, Deng J, Wu R. Endoscopic submucosal excavation for gastric muscularis propria tumours less than 10 mm in diameter: What are the risk factors responsible for perforation? PLoS One. 2025;20:e0319245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Kim HH. Endoscopic treatment for gastrointestinal stromal tumor: Advantages and hurdles. World J Gastrointest Endosc. 2015;7:192-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (4)] |
| 15. | Watari J, Tomita T, Toyoshima F, Sakurai J, Kondo T, Asano H, Yamasaki T, Okugawa T, Ikehara H, Oshima T, Fukui H, Miwa H. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: A prospective pilot study. World J Gastrointest Endosc. 2013;5:281-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Li S, Nahar A, Zhang Q, Xing J, Li P, Zhang S, Sun X. Risk factors and a nomogram for predicting local recurrence in adult patients with early gastric cancer after endoscopic submucosal dissection. Dig Liver Dis. 2024;56:1921-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 17. | Li X, You L, Liu Q, He W, Cui X, Gong W. A nomogram for predicting survival in patients with gastrointestinal stromal tumor: a study based on the surveillance, epidemiology, and end results database. Front Med (Lausanne). 2024;11:1403189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Zhou G, Xiao K, Gong G, Wu J, Zhang Y, Liu X, Jiang Z, Ma C. A novel nomogram for predicting liver metastasis in patients with gastrointestinal stromal tumor: a SEER-based study. BMC Surg. 2020;20:298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Serrano C, Martín-Broto J, Asencio-Pascual JM, López-Guerrero JA, Rubió-Casadevall J, Bagué S, García-Del-Muro X, Fernández-Hernández JÁ, Herrero L, López-Pousa A, Poveda A, Martínez-Marín V. 2023 GEIS Guidelines for gastrointestinal stromal tumors. Ther Adv Med Oncol. 2023;15:17588359231192388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 20. | von Mehren M, Kane JM, Riedel RF, Sicklick JK, Pollack SM, Agulnik M, Bui MM, Carr-Ascher J, Choy E, Connelly M, Dry S, Ganjoo KN, Gonzalez RJ, Holder A, Homsi J, Keedy V, Kelly CM, Kim E, Liebner D, McCarter M, McGarry SV, Mesko NW, Meyer C, Pappo AS, Parkes AM, Petersen IA, Poppe M, Schuetze S, Shabason J, Spraker MB, Zimel M, Bergman MA, Sundar H, Hang LE. NCCN Guidelines® Insights: Gastrointestinal Stromal Tumors, Version 2.2022. J Natl Compr Canc Netw. 2022;20:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 21. | Mimura T, Yamamoto Y, Suzuki H, Takizawa K, Hirasawa T, Takeuchi Y, Ishido K, Hoteya S, Yano T, Tanaka S, Kudara N, Nakagawa M, Mashimo Y, Ishigooka M, Fukase K, Shimazu T, Ono H, Tanabe S, Kondo H, Iishi H, Ninomiya M, Oda I; J‐WEB/EGC group. Risk factors for intraoperative and delayed perforation related with gastric endoscopic submucosal dissection. J Gastroenterol Hepatol. 2024;39:1358-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Ni M, Tang D, Ren W, Meng R, Yang J, Yan P, Ding X, Xu G, Lv Y, Chen M, Yang H, Wang L. Risk factors of perforation in gastric stromal tumors during endoscopic resection: a retrospective case-control study. Gastric Cancer. 2023;26:590-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Bai S, Sun Y, Xu H. Impact of Gastrointestinal Bleeding on Prognosis and Associated Risk Factors in Gastrointestinal Stromal Tumors: A Systematic Review and Meta-Analysis. Am Surg. 2025;91:434-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 24. | Liu Q, Kong F, Zhou J, Dong M, Dong Q. Management of hemorrhage in gastrointestinal stromal tumors: a review. Cancer Manag Res. 2018;10:735-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Lin C, Sui C, Tao T, Guan W, Zhang H, Tao L, Wang M, Wang F. Prognostic analysis of 2-5 cm diameter gastric stromal tumors with exogenous or endogenous growth. World J Surg Oncol. 2023;21:139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/