Published online Jan 16, 2025. doi: 10.4253/wjge.v17.i1.103404
Revised: December 15, 2024
Accepted: January 7, 2025
Published online: January 16, 2025
Processing time: 59 Days and 5.5 Hours
Patients diagnosed with esophageal mucosal bridges often experience symptoms such as chest pain and dysphagia, which pose considerable challenges for endo
We present a case involving early-stage esophageal cancer discovered in a resting room, notable for the rare manifestation of esophageal mucosal bridging. Following a comprehensive multidisciplinary discussion and the development of a treatment strategy, we proceeded with endoscopic submucosal dissection for the patient. During the procedure, we encountered operational challenges due to the presence of a diverticulum and a partial absence of the muscularis propria. To facilitate the retraction of a portion of the resected specimen, we utilized dental floss. Ultimately, we successfully excised the entire lesion. After a three-day period of fasting with a water-only diet, subsequent iodine water cholangiography did not indicate any perforations, and the patient was advised to transition to a liquid diet. The patient was discharged five days post-operation. A follow-up endoscopy conducted three months later revealed scar-like changes in the mid-esophagus, with the patient reporting no significant discomfort.
In summary, although esophageal mucosal bridges are rarely documented, they should be considered in the differential diagnosis of mechanical dysphagia. Furthermore, endoscopic therapy represents a feasible approach for their mana
Core Tip: Currently, there exists a paucity of documented cases of submucosal bridges in the esophagus, and no instances have been reported linking submucosal bridges to carcinogenesis within diverticula. In the case under discussion, the tubular structure of the submucosal bridge, along with the detailed pathological features indicative of carcinogenesis, was clarified through the application of thin sectioning of the surgical specimen obtained after endoscopic submucosal dissection.
- Citation: Liu YL, Liu J, Wang YT. Early esophageal cancer with mucosal bridging in the resting room: A case report. World J Gastrointest Endosc 2025; 17(1): 103404
- URL: https://www.wjgnet.com/1948-5190/full/v17/i1/103404.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i1.103404
Esophageal mucosal bridges (EMBs) are anatomical structures that traverse the lumen of the esophagus. EMBs are considered a rare phenomenon, typically arising as a consequence of various factors including trauma from nasogastric tubes, inflammatory conditions such as Crohn’s disease and lupus, infections like human immunodeficiency virus, herpes simplex virus, esophageal candidiasis, and tuberculosis, as well as complications from radiation therapy and sclerotherapy for esophageal varices[1]. Patients with EMBs may exhibit no symptoms or may present with clinical manifestations such as chest pain and dysphagia. In a recent case report, the authors highlighted endoscopic management of EMB and demonstrated that EMB management using a scissor-type knife was safe and provided durable clinical improvement[2]. In this report, we describe a case involving a 57-year-old male patient diagnosed with early esophageal cancer during a routine examination, which was notably associated with the uncommon presentation of esophageal mucosal bridging. Endoscopic submucosal dissection (ESD) was performed as part of the treatment approach.
The patient is a 57-year-old male who presented with a history of dysphagia and was referred to our hospital for further management after an upper endoscopy at an outside hospital revealed a high-grade intramucosal neoplasm of the esophagus.
The patient presented to an outside hospital with upper abdominal discomfort after an upper endoscopy revealed a patchy erythema in the mid-esophagus. Pathology suggested a high-grade intramucosal neoplasm.
Upon questioning the patient’s history, he reported no difficulty swallowing or chest discomfort, and denied a history of trauma, foreign body impaction, or radiation therapy. He also denied a history of smoking or drinking.
The patient denied any family history of malignant tumors.
No significant findings were noted on physical examination.
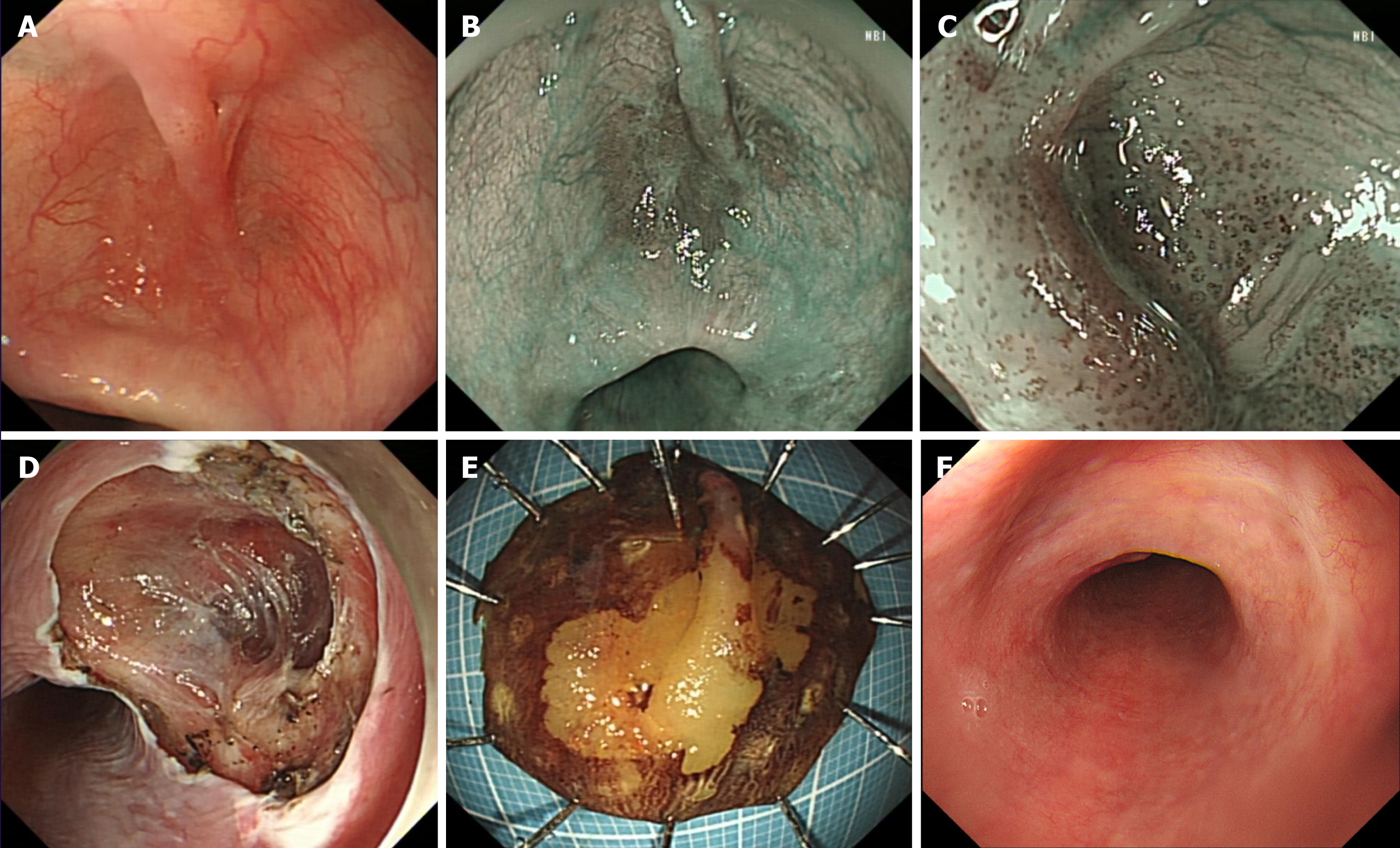
An upper endoscopy revealed a 2.5 cm submucosal cystic lesion at the distal esophagus near the posterior wall (Figure 1A), with a 2.0 cm × 2.0 cm patchy erythema in the central area and a 1.0 cm × 0.2 cm bridging mucosal change (Figure 1B).
Narrow band imaging showed a tea-colored lesion (Figure 1B). After admission, we conducted preoperative examinations. Under magnifying endoscopy with narrow band imaging, we observed intrapapillary capillary loops in the epithelial tufts of the esophagus, which were classified as type B1 (Figure 1C). Ultrasonographic endoscopy suggested an intact mucosal layer, but a thinner muscularis propria. Chest computed tomography did not suggest any lesions or enlarged lymph nodes.
After a multidisciplinary discussion and formulation of a treatment plan, we performed ESD for the patient.
In conjunction with the patient’s medical history, a pre-operative diagnosis of early esophageal cancer characterized by mucosal bridging was established in the recovery room.
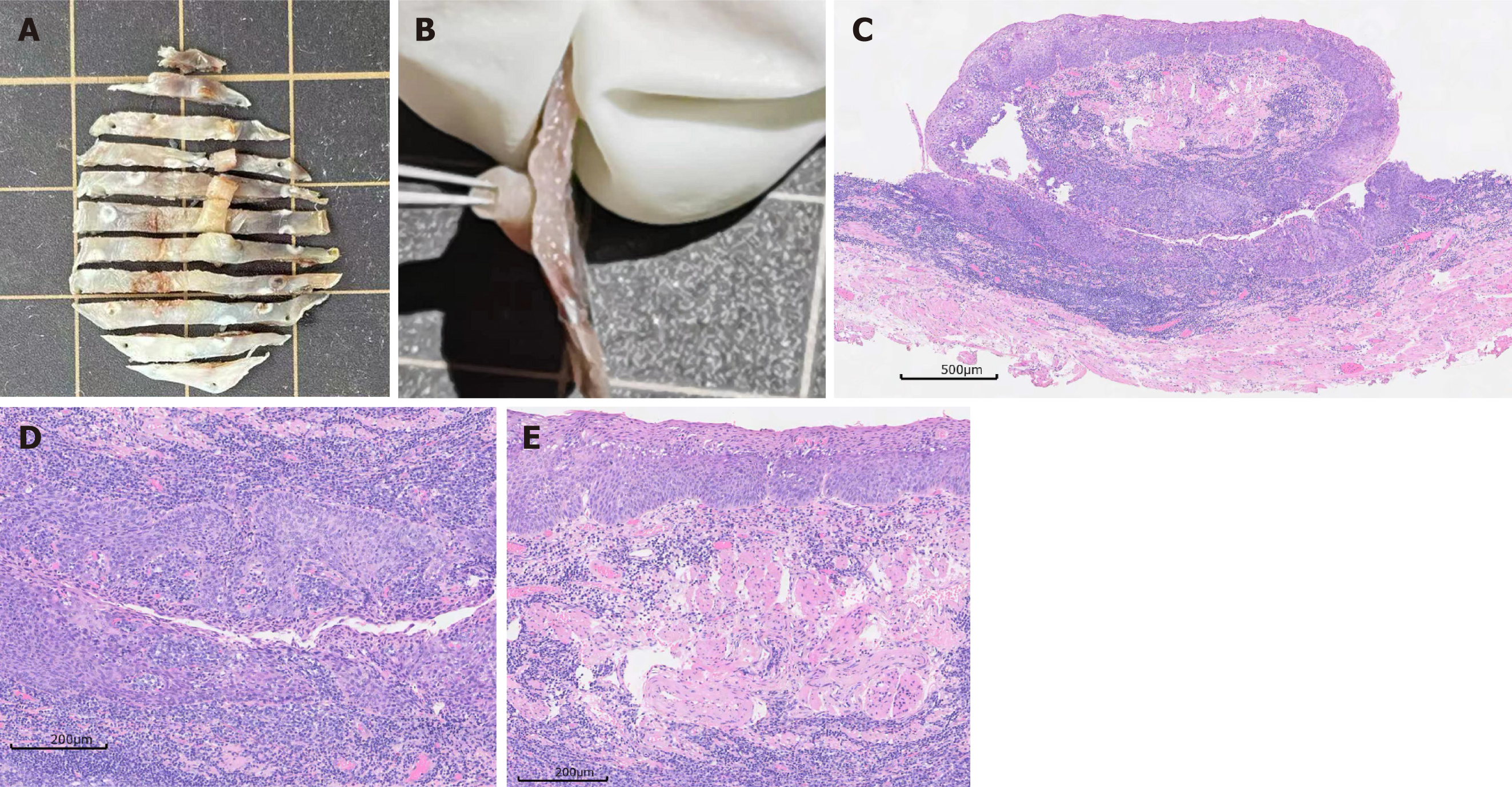
After a multidisciplinary discussion and formulation of a treatment plan, we performed ESD for the patient. During the procedure, due to operational difficulties within the diverticulum and partial absence of the muscularis propria, we assisted with dental floss to pull back part of the resected specimen (Figure 1D and E). We then successfully removed the entire lesion (Figure 1D and E). After a 3-day fasting and water-only diet, follow-up iodine water cholangiography did not reveal any perforations, and we instructed the patient to consume a liquid diet. The patient was discharged 5 days after the operation. While processing the specimen, we noticed that the submucosal bridge was a solid tubular structure spanning the esophageal mucosa (Figure 2A and B). Histological sections showed that the tubular structure had a circumferential epithelial lining consisting of stratified squamous epithelium, which was found both in the submucosal bridge and in the underlying esophageal mucosa. Both regions showed high-grade intramucosal neoplasia, with some areas showing in situ carcinoma changes. The tubular structure was composed of a central vascular axis surrounded by a continuous and contiguous epithelial layer, resembling a fibroepithelial polyp (Figure 2C, D and E).
Three months later, follow-up endoscopy showed scar-like changes in the mid-esophagus (Figure 1F), with no specific discomfort (Figure 1F).
Currently, there are fewer reported cases of submucosal bridges in the esophagus, and no reports of submucosal bridges with carcinogenesis occurring within diverticula. In this case, the submucosal bridge’s tubular structure and the pathological complete presentation of carcinogenesis were shown using the advantage of thin section cutting of the surgical specimen after ESD. Submucosal bridges are linear extensions of smooth muscle tissue, which are generally seen in the intestines, especially in ulcerative colitis. There are a few reports showing that submucosal bridges can occur in the esophagus, gastric antrum, and small intestine. Studies have shown that submucosal bridges in the esophagus are tubular band-like structures that connect the esophageal wall at both ends and have a gap below, appearing as “double-lumen” or “pseudomass” on endoscopy[3]. There are two theoretical hypotheses. One is that the inflamed esophageal wall comes into contact with each other, and the connective tissue below each wall forms a bridge by adhesion, thus forming a submucosal bridge. The second is that multiple esophageal ulcers heal with submucosal communication or the formation of granuloma at the front or back of the esophagus after healing. The cause of the carcinogenic lesion in this case is unclear, and it may be related to repeated inflammatory stimulation and temporary food retention in the diverticula. Buchman reported the first case of submucosal bridges in the esophagus caused by the placement of a nasoenteric tube in 1992[4]. Salamun et al[5] reported a case of a patient with a long-term history of smoking and chronic obstructive pul
Endoscopic treatment can also be used to resolve obstructive symptoms in some symptomatic mucosal bridges, some of which are of unknown origin[6], some of which are stimulated by placement of prostheses, such as the placement of articulatory prostheses after laryngeal cancer[2], and the stimulation of mucosal bridging after esophageal fistula. In this patient, the cystoid protrusion of the middle esophageal diverticulum is not very severe, there is no obvious choking, retrosternal pain with acid reflux symptoms, and some endoscopically found diverticula is large, and active intervention and treatment are required for symptomatic ones. At present, diverticular per oral endoscopic myotomy has been widely used to perform complete diverticulectomy under direct endoscopic vision[7]. In this case, esophageal carcinoma in situ in diverticulum is an absolute indication for ESD, and endoscopic resection can be curative treatment, and regular follow-up is sufficient. The occurrence of mucosal bridges is considered to be a bridge-like structure across the mucosal surface formed by the axis of residual epithelium and fibrovascular vessels during the repair of inflammatory lesions.
Recent years have witnessed considerable advancements in the treatment modalities for esophageal disorders. Endoscopic intervention has emerged as the predominant therapeutic strategy for this patient demographic. Argon plasma coagulation has been shown to be both safe and effective across various clinical contexts[8]. Furthermore, electrosurgical techniques, including the utilization of hot biopsy forceps and needle knives, have been successfully employed following the placement of hemoclips at both extremities of the esophageal bridge in numerous instances[9,10]. Presently, there is a paucity of data that directly compares the safety and efficacy of these techniques.
In summary, although EMBs are rarely documented, they should be considered in the differential diagnosis of mechanical dysphagia, and endoscopic therapy represents a feasible option for their management.
| 1. | Antoine M, Krishnan U, Manfredi M, Cervinskiene J, Viala J, Brendel J, Tzivinikos C, Vanrenterghem A, Dimitrov G, Hauser B, Laverdure N, Rohmer B, Behal H, Nicolas A, Gottrand F. Endoscopic management of esophageal mucosal bridges in children with esophageal atresia. Surg Endosc. 2023;37:9167-9172. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Madi MY, Peller M, Presti M, Bazarbashi AN. Endoscopic management of tracheoesophageal prosthesis-induced esophageal mucosal bridge. Endoscopy. 2023;55:E625-E626. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Sunkara T, Then EO, Yarlagadda KS, Jhaveri M, Gaduputi V. An Innocent Esophageal Mucosal Bridge: Case Report and Literature Review. J Investig Med High Impact Case Rep. 2018;6:2324709618767204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Buchman AL, Waring JP. Mucosal bridge formation in the esophagus caused by injury from a nasoenteric feeding tube. JPEN J Parenter Enteral Nutr. 1994;18:278-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Salamun E, Gröchenig HP, Langner C. Mucosal bridge formation in a patient with esophageal epidermoid metaplasia. Endoscopy. 2016;48:E325. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Debnath P, Udgirkar S, Rathi P, Jain S, Nair S, Pawar V, Contractor Q. Esophageal Mucosal Bridge of Unknown Etiology Causing Dysphagia in an Elderly Female: Endoscopic Management and Literature Review. GE Port J Gastroenterol. 2020;27:356-360. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Mandavdhare HS, Praveen Kumar M, Jha D, Kumar A, Sharma V, Desai P, Shumkina L, Gupta P, Singh H, Dutta U. Diverticular per oral endoscopic myotomy (DPOEM) for esophageal diverticular disease: a systematic review and meta-analysis. Esophagus. 2021;18:436-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Mukherjee M, Oh J, Khdair A, Grosman I. Esophageal mucosal bridges associated with idiopathic esophageal ulcer treated with argon plasma coagulation. Gastrointest Endosc. 2008;68:387-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ranketi S, Mwachiro M, Topazian M, Burgert S. Endoscopic treatment of cervical esophageal transluminal bridge. Gastrointest Endosc. 2017;86:561-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Perisetti A, Banerjee D, Tharian B. Endoscopic Resection of Esophageal Mucosal Bridge. Gastroenterology. 2018;154:2033-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/