Published online Sep 16, 2024. doi: 10.4253/wjge.v16.i9.533
Revised: August 24, 2024
Accepted: August 28, 2024
Published online: September 16, 2024
Processing time: 36 Days and 11 Hours
Esophagopericardial fistula (EPF) is a rare, life-threatening condition with limited scientific literature and no established management guidelines. This case report highlights a successful multidisciplinary approach and the innovative use of endoscopic vacuum assisted closure (endoVAC) therapy in treating this complex condition.
A 16-year-old male with a history of esophageal atresia and colon interposition presented with progressive chest pain, fever, and dyspnea. Imaging revealed an EPF with associated pleural and pericardial effusions. Initial management with an esophageal stent failed, prompting the use of an endoVAC system. The patient underwent multiple endoVAC device changes and received broad-spectrum antibiotics and nutritional support. The fistula successfully closed, and the patient recovered, demonstrating no new symptoms at a 6-month follow-up.
EndoVAC therapy can effectively manage EPF, providing a minimally invasive treatment option.
Core Tip: This case report highlights the successful use of endoscopic vacuum assisted closure (endoVAC) therapy in treating a rare esophagopericardial fistula (EPF) in a 16-year-old male. The innovative approach involved a multidisciplinary strategy, resulting in the resolution of the fistulous tract and significant clinical improvement. Our findings suggest that endoVAC therapy can be an effective minimally invasive alternative for EPF management, offering a promising solution for complex cases where traditional surgical methods may be less feasible. This report emphasizes the need for further research to establish standardized protocols and validate the long-term efficacy of endoVAC therapy.
- Citation: Muñoz-González S, Quejada-Cuesta S, González-Arroyave D, Ardila CM. Endoscopic vacuum assisted closure therapy for esophagopericardial fistula in a 16-year-old male: A case report. World J Gastrointest Endosc 2024; 16(9): 533-539
- URL: https://www.wjgnet.com/1948-5190/full/v16/i9/533.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i9.533
Esophagopericardial fistula (EPF) is an extremely rare and serious condition characterized by an abnormal connection between the esophagus and pericardium. This condition often results in severe complications, including infection and sepsis, due to the passage of esophageal contents into the pericardial cavity[1-3]. The management of EPF poses significant clinical challenges due to its rarity and the lack of well-defined treatment protocols. There are few reported cases of this pathology in the indexed medical literature[2-4], with a variety of clinical presentations but a high burden of morbidity and mortality[5,6]. The anatomical, hemodynamic, and microbiological alterations present in this case make it a notable and complex pathology to treat. In past centuries, successful treatment would have been impossible without the technological advancements and knowledge we have today. It is possible that new endoscopic and minimally invasive therapies may represent an alternative that could replace certain older procedures in the treatment of some pathologies. These new therapies are not without risks or limitations, but they offer the significant advantage of avoiding the trauma and extensive dissections characteristic of traditional surgery.
This case report aims to present a novel and successful approach to treating an EPF in a 16-year-old male using endoscopic vacuum assisted closure (endoVAC) therapy. A non-systematic review of the current literature was also conducted to highlight the potential benefits of this therapy, as well as possible risks and limitations. We hypothesize that minimally invasive therapies, such as the one presented in this case, represent a promising strategy in the management of complex digestive tract pathologies, such as EPF.
A comprehensive literature review was conducted using databases such as PubMed, MEDLINE, Scopus, and Google Scholar. Search terms included "esophagopericardial fistula", "esophageal atresia", "endoVAC therapy", "esophageal stents", and "multidisciplinary approach". The search yielded limited case reports and small case series, underscoring the rarity of the condition and the absence of standardized treatment guidelines.
In this report, we detail the case of a young male patient with a history of esophageal atresia repair who developed a late-onset EPF. This case highlights the utility of a multidisciplinary approach and the innovative application of endoVAC therapy, contributing valuable insights to the scant body of literature on EPF management.
A 16-year-old patient presented to the emergency department with the onset of severe retrosternal chest pain, accompanied by epigastric pain, subjective fever, and dyspnea.
During the last week, the pain became severe and was accompanied by dyspnea and subjective fever. The patient sought care at a primary-level hospital emergency department, where initial studies were conducted.
A three-month history of progressive epigastric pain, heartburn, and retrosternal chest pain, initially of mild intensity.
The patient has a history of congenital esophageal atresia, which was corrected during the neonatal period, followed by reoperation at the age of 3 with colonic interposition between the proximal and distal esophagus (neoesophagus). During childhood, the patient experienced occasional dysphagia as the only symptom and was on chronic treatment with domperidone and esomeprazole.
Upon hospital admission, the patient was in fair general condition. Vital signs revealed a body temperature of 38.2 °C, blood pressure of 98/52 mmHg, heart rate of 134 beats per minute, and respiratory rate of 28 breaths per minute. On chest auscultation, the patient had rhythmic but distant heart sounds, decreased vesicular breath sounds in both lung fields, and absent breath sounds at the left lung base. The abdomen was soft and non-tender.
Upon admission, severe anemia was documented with an Hb level of 4.9 mg/dL. The electrocardiogram showed peaked T waves, concave ST segment elevation, and sinus tachycardia.
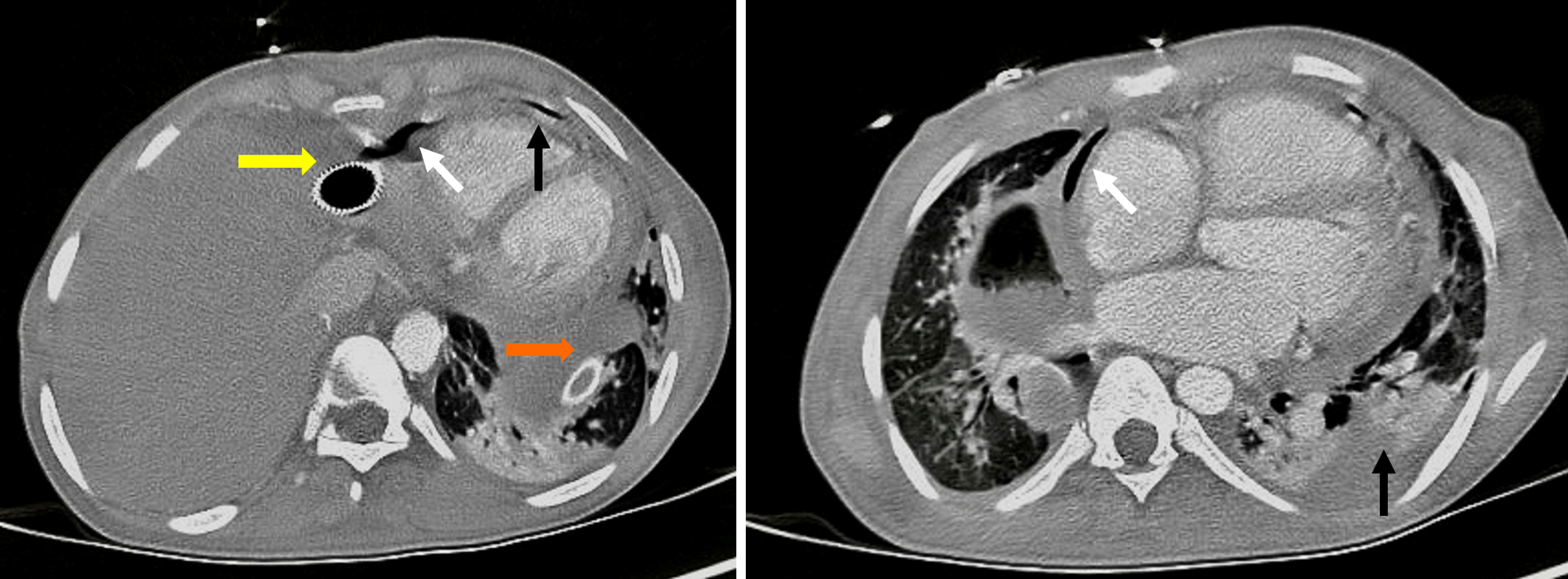
A computed tomography (CT) scan performed at the initial healthcare facility documented a left pleural effusion and pericardial effusion. A diagnosis of sepsis with a probable infectious focus in the pleura and pericardium was made, leading to the early initiation of broad-spectrum antibiotics and an urgent subxiphoid pericardial window and left closed thoracostomy by a general surgeon. Purulent fluid was obtained from both spaces, but microbiological studies did not demonstrate bacterial growth. Initially, his clinical condition deteriorated, requiring invasive mechanical ventilation, vasopressor support, and admission to a high-dependency unit, prompting transfer to a higher-complexity hospital.
At our higher-complexity center, an upper gastrointestinal endoscopy was performed after observing the discharge of alimentary material through the previously placed pericardial drain.
This study revealed a perforation of the neoesophagus, 40 cm from the dental arch, with confirmation of a fistulous tract to the pericardium and left pleural cavity via a new contrast-enhanced chest CT (Figure 1).
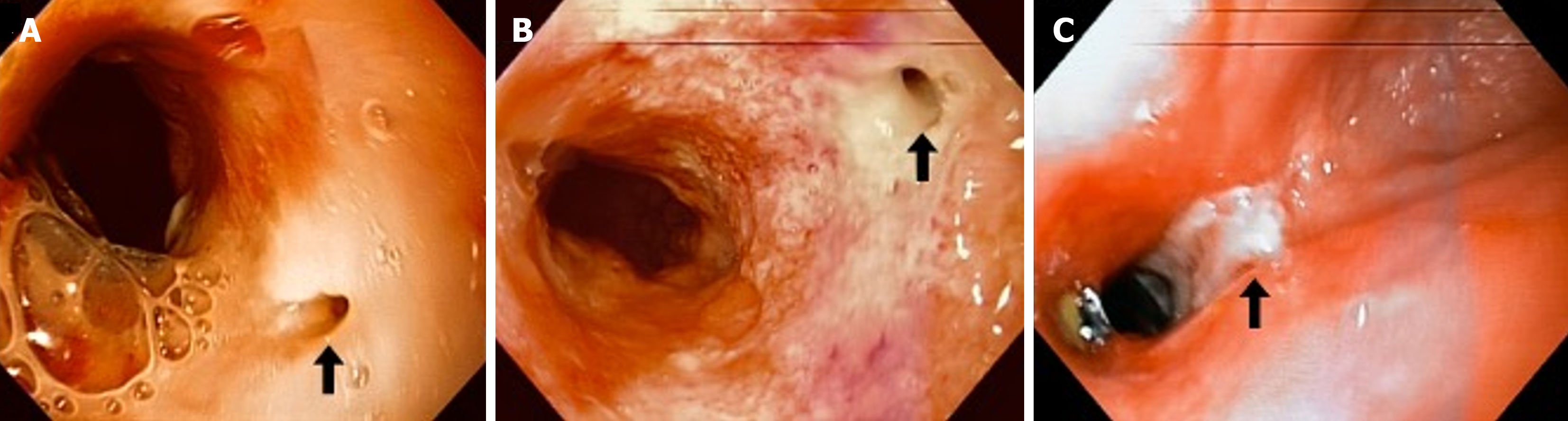
Initially, a self-expanding metallic esophageal stent and total parenteral nutrition were used to control the fistula, but the stent migrated without achieving the intended result. Due to persistent fistulous tract, an endoluminal esophageal device with continuous subatmospheric pressure was inserted to control effluent. This device is handcrafted using a 14 Fr nasogastric tube, to which five additional fenestrations are made, and the distal third (the last 10 cm) is covered with black polyurethane foam. The foam is secured with a 3-0 silk transfixion suture that allows for precise positioning and prevents displacement (Figure 2). After construction, the device is inserted under endoscopic visualization using a foreign body forceps into the esophagus at the desired segment, connecting the tube to a hospital suction system at continuous negative pressure (-100 mmHg). Periodic changes of the device were performed at the bedside every 4 to 5 days via endoscopy. Additional treatment included complete suspension of oral intake and the use of total parenteral nutrition. Empirical antimicrobial therapy with broad-spectrum antibiotics was also administered.
After 3 device changes the fistulous tract closed (Figure 3). Clinical improvement was noted, with recovery of spontaneous ventilation and oral intake. The patient was discharged 50 days after admission to our institution. At 6 months, a clinical evaluation and echocardiography were performed, showing no pericardial effusion or other abnormalities in cardiac anatomy or function. At the 36-month follow-up, the patient reported only occasional dysphagia with the ingestion of meat, without chest pain or dyspnea. Up to this last follow-up, no additional hospital admissions have been required.
The spectrum of esophageal surgical pathologies is broad, including congenital abnormalities such as esophageal atresia (with or without tracheoesophageal fistula) and acquired conditions such as perforation, rupture, cancer, and some motor disorders and strictures.
An EPF is an acquired abnormal communication between the esophageal lumen and the pericardium. It is a rare condition with few reports and case series in the scientific literature[1-3], which hinders the establishment of statistical and epidemiological measures such as prevalence and incidence[3-6]. Mortality has been estimated to be between 50%-80%[2]. Recent reports on EPFs have shown lower morbidity and mortality rates, with patient survival in 85% of cases[5].
Its etiology is diverse, but benign esophageal disorders are the most common cause of this entity, accounting for approximately 77% of all cases[7]. Of these, 35% are due to chronic esophagitis from gastroesophageal reflux disease and 16% to foreign body ingestion. Malignant causes such as esophageal carcinoma appear to be the second most common, and thoracic trauma and iatrogenic causes such as radiotherapy, digestive endoscopy, and surgical interventions for congenital pathologies like atresia with bowel interposition represent only 2%-6% of the total[8]. Recently, there has been an increase in case reports associated with endovascular procedures, such as radiofrequency ablations for the management of cardiac arrhythmias[5].
The clinical presentation of an EPF is variable, ranging from asymptomatic in early stages to noisy semiology due to cardiovascular or systemic compromise[7]. The most common symptoms are related to pneumopericardium extension, associated infection, and the underlying etiology[9]. A clinical diagnosis is rarely made before confirmation by imaging or direct visualization of the fistula, likely due to the low incidence of this entity[8]. The final diagnostic method varies among case reports, but contrast-enhanced chest CT is the most informative study. Echocardiography, digestive endo
The rarity of this pathology means that there is no standard treatment and, therefore, no baseline for comparing new therapies. In reported cases[2,3,6,7,10-12], the initial management of similar entities typically focuses on addressing infectious complications secondary to the communication between the digestive tract and sterile structures such as the pericardium and pleura, in addition to draining pericardial fluid to prevent subsequent hemodynamic compromise. Microbiological isolation is usually polymicrobial, and treatment includes broad-spectrum drugs targeting normal upper digestive tract microbiota, including gram-positive, gram-negative, anaerobic bacteria, and fungi. Crucially, infection control involves surgical pericardial drainage and insertion of drainage tubes[6].
Some authors have proposed additional interventions to those mentioned above to resolve the fistulous tract[13,14]. Conservative options have been proposed for those who are not good surgical candidates, such as patients with disseminated neoplastic disease. In recent years, the management of fistulas has significantly improved with the advent of new technologies that allow early detection and timely intervention[15,16]. This is evident in replacing traditional surgical approaches with a conservative approach, including endoscopic therapy with clips, covered stents, fibrin glue, endo
EndoVAC is a useful therapy in managing luminal perforations and upper gastrointestinal leaks. It involves placing a polyurethane sponge with continuous suction at a negative pressure of 75 to 125 mmHg. This therapy promotes healing and scarring through improved granulation tissue formation, increased vascularization, and decreased venous stasis, associated with infectious effluent control and cavity collapse[20]. The sponge needs to be replaced every 72 hours until granulation leads to cavity obliteration, typically within 2 to 3 weeks on average[15]. This therapy reports variable success rates of up to 80%-90% in some studies[15,18]. EndoVAC is considered a useful complement to conventional treatments for upper gastrointestinal leaks and perforations when there is a contained cavity smaller than 8 cm (4 cm for some authors)[18]. In our case, endoVAC therapy was successful in closing the EPF, as have some cases managed with reconstructive surgery. However, unlike those cases, our patient did not undergo the risks associated with major conventional surgeries, which for this condition could involve simultaneous cervical, thoracic, and abdominal approaches.
Despite its advantages and relatively easy implementation, there are factors that could limit the use of endoVAC therapy, such as the need for oral intake restriction. In some scenarios, this involves the risks and potential complications of total parenteral nutrition and enteral nutrition via gastrostomies or jejunostomies[14]. Additionally, trained nursing staff is required for monitoring and managing complications associated with the device. At times, maintaining a stable suction level can be challenging.
The use of these devices is not without risk. Real risks have been described in case reports and other studies. The primary risk associated with repeated endoscopic procedures is esophageal perforation and oral cavity lacerations. Aspiration events are also documented, which can lead to ventilatory failure, pneumonia, or even death. The use of esophageal suction devices has been associated with excessive granulation and stenosis in up to 9.1% of cases[17], necessitating additional procedures for esophageal dilation. There is also the risk of iatrogenic fistula formation, with catastrophic consequences such as massive hemorrhage from aortoesophageal fistulas[19].
Despite the above, endoluminal subatmospheric pressure devices, such as EndoVAC therapy, could be a promising minimally invasive option for managing complex esophageal fistulas (EPF). This case demonstrates its successful application in a young patient, highlighting its potential as a therapeutic alternative. The use of EndoVAC therapy, combined with targeted antibiotic use, nutritional support, and management of underlying comorbidities, may be considered within the therapeutic arsenal for this rare pathology. However, there is a need to acknowledge the lack of comprehensive information regarding its use, safety, and current therapeutic effect. Further research and clinical trials with greater epidemiological weight are necessary to validate the effectiveness of EndoVAC therapy and establish precise indications and comprehensive management guidelines.
EPF is a rare and potentially fatal complication associated with upper digestive tract pathologies. This case underscores the effectiveness of endoVAC therapy as a minimally invasive treatment option. Based on our findings, we recommend considering endoVAC therapy for EPF management due to its promising outcomes in fistula resolution. Further studies are warranted to establish standardized treatment protocols and validate the long-term efficacy and safety of endoVAC therapy in diverse patient populations.
| 1. | Cyrlak D, Cohen AJ, Dana ER. Esophagopericardial fistula: causes and radiographic features. AJR Am J Roentgenol. 1983;141:177-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Khader Y, Ghazaleh S, Nehme C, Burlen J, Nawras A. Esophagopericardial Fistula After Esophagectomy. Cureus. 2021;13:e13753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Farkas ZC, Pal S, Jolly GP, Lim MMD, Malik A, Malekan R. Esophagopericardial Fistula and Pneumopericardium From Caustic Ingestion and Esophageal Stent. Ann Thorac Surg. 2019;107:e207-e208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Bowman AW, DiSantis DJ, Frey RT. Esophagopericardial Fistula Causing Pyopneumopericardium. Radiol Cardiothorac Imaging. 2020;2:e200417. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Deneke T, Sonne K, Ene E, Berkovitz A, Nentwich K. Esophagopericardial Fistula: The Wolf in Sheep's Clothing. JACC Case Rep. 2021;3:1136-1138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Kheslat HH, Kelly S, Singh H, Crozier I. Esophagopericardial Fistula Following Radiofrequency Ablation for Atrial Fibrillation: Insights Into Its Management. JACC Case Rep. 2021;3:1132-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Miller HJ. Inguinal Hernia: Mastering the Anatomy. Surg Clin North Am. 2018;98:607-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Abdalwahab A, Al Namshan M, Al Rabeeah A, Laberge JM. Delayed fistulization from esophageal replacement surgery. Semin Pediatr Surg. 2009;18:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Brander L, Ramsay D, Dreier D, Peter M, Graeni R. Continuous left hemidiaphragm sign revisited: a case of spontaneous pneumopericardium and literature review. Heart. 2002;88:e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Xu X, Yan Z, He M. Case report of a 72-year-old man with diaphragmatic hernia and thoracic gastropericardial fistula after esophagectomy for 18 years. J Cardiothorac Surg. 2021;16:193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 11. | Simice P, Zwirewich CV. Gastropericardial fistula complicating benign gastric ulcer: case report. Can Assoc Radiol J. 2000;51:244-247. [PubMed] |
| 12. | Baptiste-castillo HF, Parra-zuluaga R, Niño-andrade F, Rodríguez-sánchez S. Manejo quirúrgico de una fístula gastropleural posterior a manga gástrica. Rev Colomb Cir. 2021;36:712-718. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Pinilla-morales RE, Vélez-bernal J, Guerrero-macías S, Restrepo-lópez J, Briceño-morales C, Manrique-acevedo ME, Rendón-hernández J, Facundo-navia H, Benito-flórez E, Oliveros-wilches R. Manejo de perforaciones, fugas y fístulas del tracto gastrointestinal con clip sobre el endoscopio. Experiencia de un centro oncológico Latinoamericano. Rev Colomb Cir. 2023;. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Winder JS, Pauli EM. Novel endoscopic modalities for closure of perforations, leaks, and fistula in the gastrointestinal tract. Tech Gastrointest Endosc. 2019;21:109-114. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Rubicondo C, Lovece A, Pinelli D, Indriolo A, Lucianetti A, Colledan M. Endoluminal vacuum-assisted closure (E-Vac) therapy for postoperative esophageal fistula: successful case series and literature review. World J Surg Oncol. 2020;18:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Goenka MK, Goenka U. Endotherapy of leaks and fistula. World J Gastrointest Endosc. 2015;7:702-713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Berlth F, Bludau M, Plum PS, Herbold T, Christ H, Alakus H, Kleinert R, Bruns CJ, Hölscher AH, Chon SH. Self-Expanding Metal Stents Versus Endoscopic Vacuum Therapy in Anastomotic Leak Treatment After Oncologic Gastroesophageal Surgery. J Gastrointest Surg. 2019;23:67-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Ooi G, Burton P, Packiyanathan A, Loh D, Chen R, Shaw K, Brown W, Nottle P. Indications and efficacy of endoscopic vacuum-assisted closure therapy for upper gastrointestinal perforations. ANZ J Surg. 2018;88:E257-E263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Omran S, Ardalani L, Beyer K, De Bucourt M, Gombert A, Buerger M, Frese JPB, Greiner A. Management of Tumor- and Nontumor-related Aorto-esophageal and Aorto-bronchial Fistulas. Ann Vasc Surg. 2021;72:419-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Steinbichler TB, Wolfram D, Runge A, Hartl R, Dejaco D, Rauchenwald T, Pototschnig C, Riechelmann H, Schartinger VH. Modified vacuum-assisted closure (EndoVAC) therapy for treatment of pharyngocutaneous fistula: Case series and a review of the literature. Head Neck. 2021;43:2377-2384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |