Published online Aug 16, 2024. doi: 10.4253/wjge.v16.i8.472
Revised: June 16, 2024
Accepted: June 26, 2024
Published online: August 16, 2024
Processing time: 149 Days and 3 Hours
Schistosomiasis, officially named as a neglected tropical disease by The World Health Organization, is a serious parasitic disease caused by trematode flukes of the genus Schistosoma. It is a common infectious disease, endemic in more than 78 countries. The disease can involve various organs and poses far-reaching public health challenges.
Here, we present a series of five patients with variable presentations: an asymp
Intestinal schistosomiasis can present with features mimicking other gastrointestinal conditions. This disease should be a diagnostic consideration in patients who live in or have traveled to endemic areas.
Core Tip: Schistosomiasis still poses significant morbidity to individuals, especially those in endemic areas. Its variable clinical presentation, protracted course, and nonspecific endoscopic findings frequently lead to erroneous diagnoses. Having a high index of clinical suspicion, actively inquiring about exposure or travel history, utilizing epidemiologic surveys to understand disease distribution, correlating symptoms with basic laboratory tests (especially eosinophil count), and obtaining histopathologic examination of colonic mucosa are essential in making a conclusive diagnosis. Confirming the diagnosis is important, as colonic schistosomiasis can be effectively treated with anthelminthic therapy (praziquantel) obviating the need for unnecessary medical treatments and invasive surgical procedures.
- Citation: Mulate ST, Nur AM, Tasamma AT, Annose RT, Dawud EM, Ekubazgi KW, Mekonnen HD, Mohammed HY, Hailemeskel MB, Yimer SA. Colonic schistosomiasis mimicking cancer, polyp, and inflammatory bowel disease: Five case reports and review of literature. World J Gastrointest Endosc 2024; 16(8): 472-482
- URL: https://www.wjgnet.com/1948-5190/full/v16/i8/472.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i8.472
Schistosomiasis is the most prevalent chronic neglected tropical disease (NTDs) affecting people residing in areas with limited access to clean water[1]. It is also one of the NTDs with high morbidity and mortality. It is caused by blood flukes of the genus Schistosoma. Most cases are brought on by three different Schistosoma species, namely S. haematobium, which causes urinary schistosomiasis, and S. mansoni and S. japonicum, which cause intestinal schistosomiasis.
Transmission occurs following contact with a freshwater source where the intermediate hosts (snails) reside. The infectious form of the parasite (cercariae) penetrates the human skin (definitive host). The eggs then migrate to the venules of the liver where they mature into adult worms. From there, the adult worms migrate to the mesenteric vessels of the intestines or the vesical venous plexus where the female starts to deposit eggs, which are subsequently excreted via feces or urine.
Human schistosomiasis is second only to malaria in global infectious disease mortality[2]. It affects 240 million people worldwide, with an estimated 700 million individuals at risk in 76 different countries. Africa accounts for over 85% of global infections, and loses an estimated 280000 people to the disease annually[3]. Approximately 37.5 million individuals in Ethiopia are at an increased risk of contracting schistosomiasis, and confirmed cases have been documented in all administrative regions in the country. While 5.01 million people are currently thought to have the disease, this number is expected to grow quickly over the coming few years[4]. The development of water resources and extensive population movements have contributed to the spread of S. mansoni infection[5].
Colonic schistosomiasis can be asymptomatic or present with acute and/or chronic illness. In some patients, cercarial skin penetration causes a maculopapular dermatitis known as “swimmer's itch.” This is a temporary hypersensitivity reaction induced by the Schistosoma larvae’s migration; maturation, ovipositioning, and release of egg antigens[6]. The chronic form can have a wide spectrum of manifestations ranging from asymptomatic to severe and fatal disease, including portal hypertension and gastro-esophageal varices resulting from periportal fibrosis. Colitis, ulceration, polyp formation, bowel strictures, and occult blood loss with subsequent iron deficiency anemia can also occur in the lower gastrointestinal tract[7].
Case 1: A 20-year-old male patient presented with a 1-year history of recurrent periumbilical abdominal pain.
Case 2: A 39-year-old female patient presented with burning and dull aching lower abdominal pain of a 6-mo duration.
Case 3: A 30-year-old male who had been diagnosed with acromegaly and undergone trans-sphenoidal surgery was referred to the gastroenterology clinic for screening colonoscopy.
Case 4: A 35-year-old male patient presented with a complaint of pain during defecation and difficulty of micturition of several months’ duration.
Case 5: A 10-year-old female patient presented with bloody diarrhea of 4-mo duration.
Case 1: The patient had minimal bright red rectal bleeding occurring during defecation. He visited multiple health institutions for his complaints for which he was given several medications that only resulted in minimal improvement.
Case 2: The patient’s pain worsened following meal ingestion and was associated with passages of 2-3 non-bloody loose stools per day. She also had fever and anorexia. She visited several hospitals and was treated with antibiotics and antacids without experiencing significant improvement in her symptoms. Although she had lived in a schistosomiasis-free region for most of her life, she moved to a schistosomiasis endemic area a few years prior to onset of her current complaints, where she had frequent contact with river water.
Case 3: The patient is from northern Ethiopia, an area known to be endemic for schistosomiasis, and had a history of river water contact as a child. He had mild infrequent flatulence and lower abdominal pain that resolved after defecation. Otherwise, he felt well.
Case 4: The patient recalled a history of river water contact growing up, but claimed to have never been treated for schistosomiasis previously.
Case 5: The patient was originally from Eritrea, and had been living in northern Ethiopia for the past 6 years. Her diarrhea occurred 2-3 times per day and was associated with abdominal pain, poor appetite, and intermittent low-grade fever. She had frequent river water contact after relocating to northern Ethiopia.
Case 1: Eight months before presentation to our hospital, the patient was started on empiric therapy for tuberculosis after his abdominal computed tomography scan showed hepatosplenomegaly with enteritis and segmental lung consolidation. The patient took anti-tuberculosis therapy for 6 mo, following which there was slight improvement in abdominal pain, but the rectal bleeding persisted.
Case 2: Has no pertinent history of hospitalization or treatment for chronic illness like diabetes and hypertension.
Case 3: Undergoing treatment and follow up for acromegaly at the endocrine unit of our hospital.
Case 4: No pertinent history of hospitalization or known chronic illness.
Case 5: Had unremarkable perinatal and childhood medical history, and was vaccinated according to the national vaccination schedule.
Case 1: No other pertinent personal or family history of chronic illness.
Case 2: No other pertinent personal or family history of chronic illness or drug abuse.
Case 3: No other known personal or family history of diabetes, hypertension, or heart disease.
Case 4: No other known personal or family history of similar illness, diabetes, hypertension, or heart disease.
Case 5: No similar illness in family members.
Case 1: Physical examination was notable for hepatomegaly and splenomegaly.
Case 2: Physical examination revealed normal vital signs and mild abdominal tenderness. The liver and spleen were not palpable.
Case 3: On physical examination, typical dysmorphic features of acromegaly were noted. Examination was otherwise unremarkable.
Case 4: On physical examination, vital signs were normal, and abdomen was slightly distended, with no signs of ascites or hepatosplenomegaly. Digital rectal examination showed smooth mucosa with no mass or bleeding.
Case 5: Physical examination was notable for slightly pale conjunctivae and mild peri-umbilical tenderness. Liver and spleen were not enlarged and digital rectal examination was unremarkable.
Case 1: On laboratory evaluation, complete blood count, renal function, serum electrolyte, and liver enzyme tests were all within normal limits.
Case 2: Laboratory tests showed a normal white cell count of 6200 cells/mL with high eosinophil count [600/mL, (9.6%)]. Her hemoglobin was 15.2 g/dL and platelet count was 275000/mL. Multiple stool examinations were negative for ova and stool culture was negative for pathogens. Serologic testing for hepatitis B, hepatitis C, syphilis, and human immunodeficiency virus (HIV) were all negative. Renal and liver function tests and serum electrolytes were within normal limits. Erythrocyte sedimentation rate was 10 mm/h and C-reactive protein was normal.
Case 3: On laboratory evaluation, complete blood count, renal function tests, serum electrolyte, and liver enzyme tests were normal.
Case 4: Upon laboratory evaluation, a comprehensive metabolic panel was normal and serologic tests for hepatitis B, hepatitis C virus, and HIV were all negative.
Case 5: With a clinical diagnosis of inflammatory bowel disease, she was investigated with complete blood count, serum liver tests, and renal function tests, which were all normal. Total protein was 7.05 g/dL and serum albumin was 3.95 g/dL. Repeated stool examination showed no ova or parasites, but there were few white blood cells. Hepatitis B, hepatitis C, and HIV serology tests were all negative.
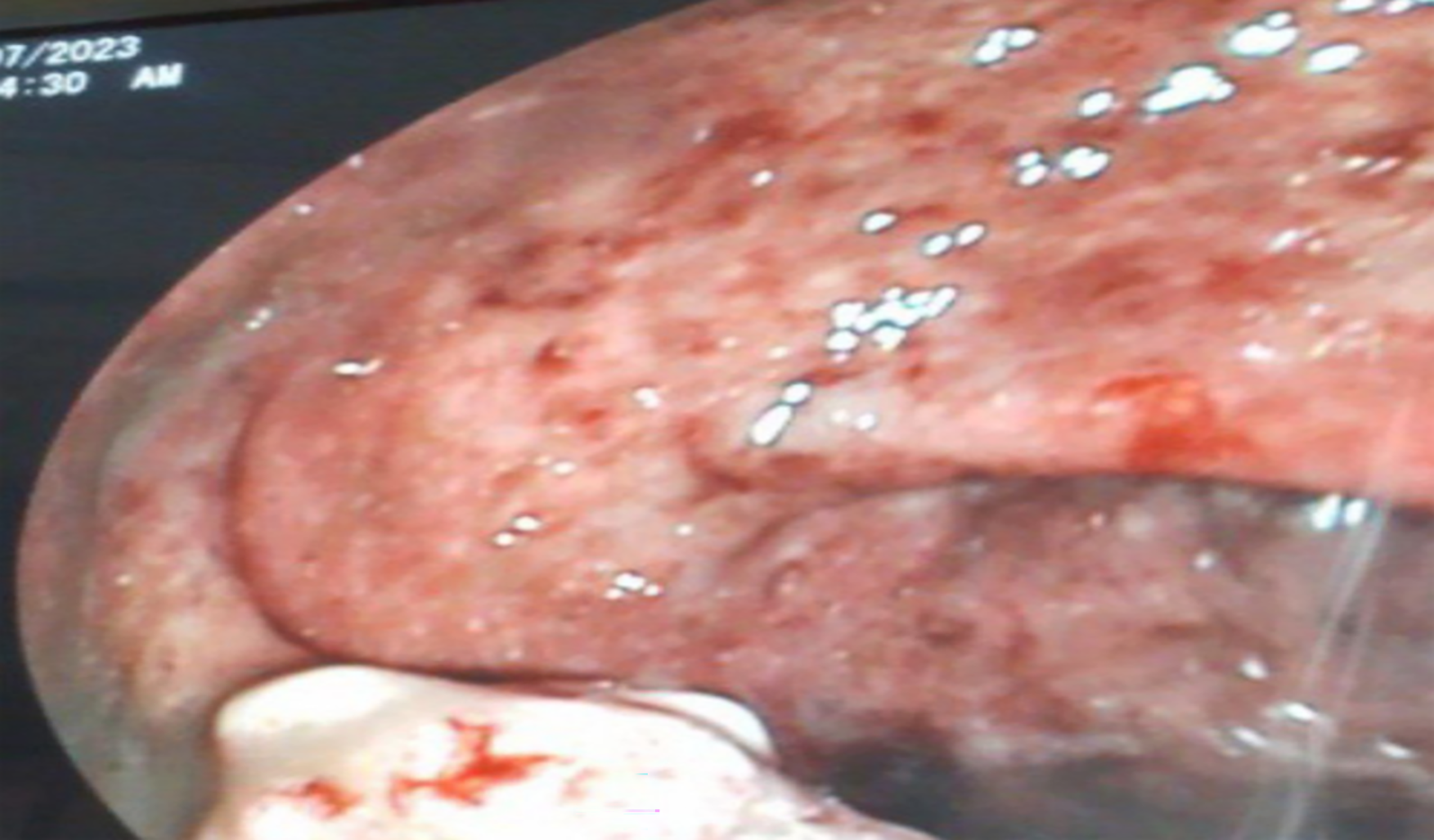
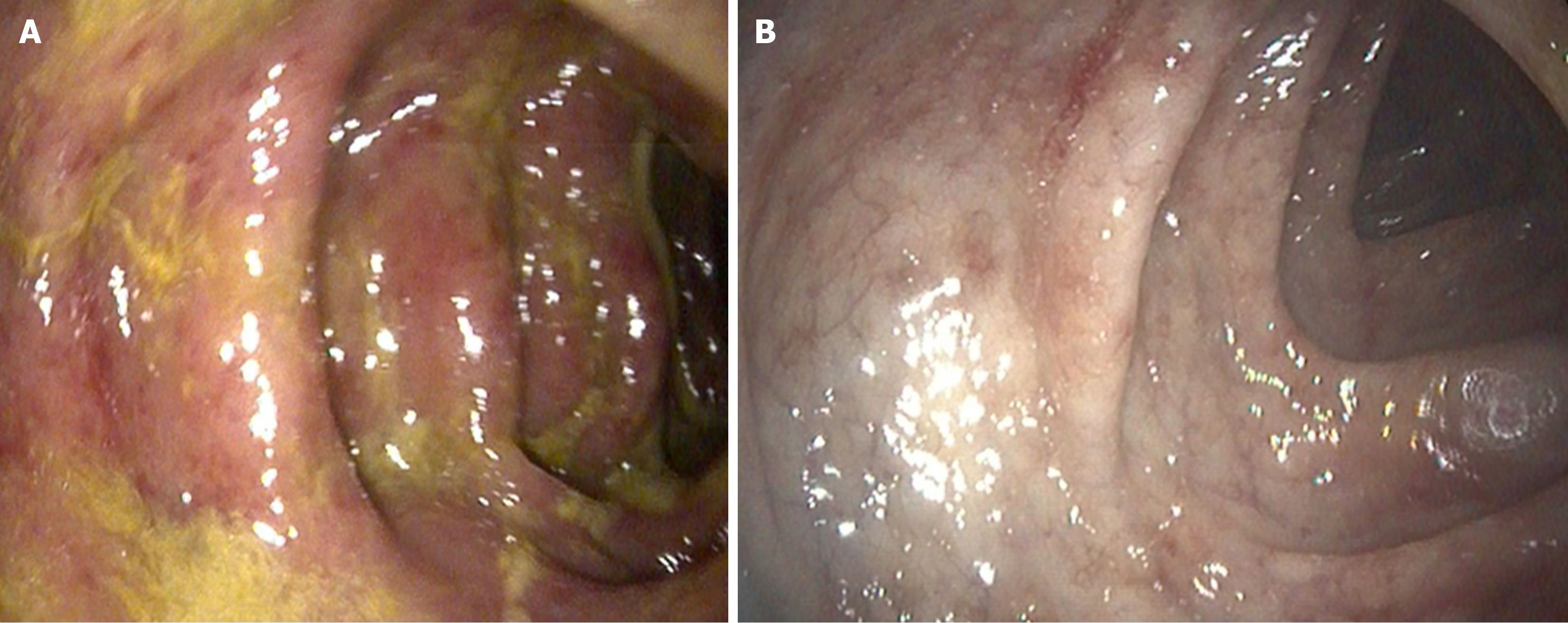
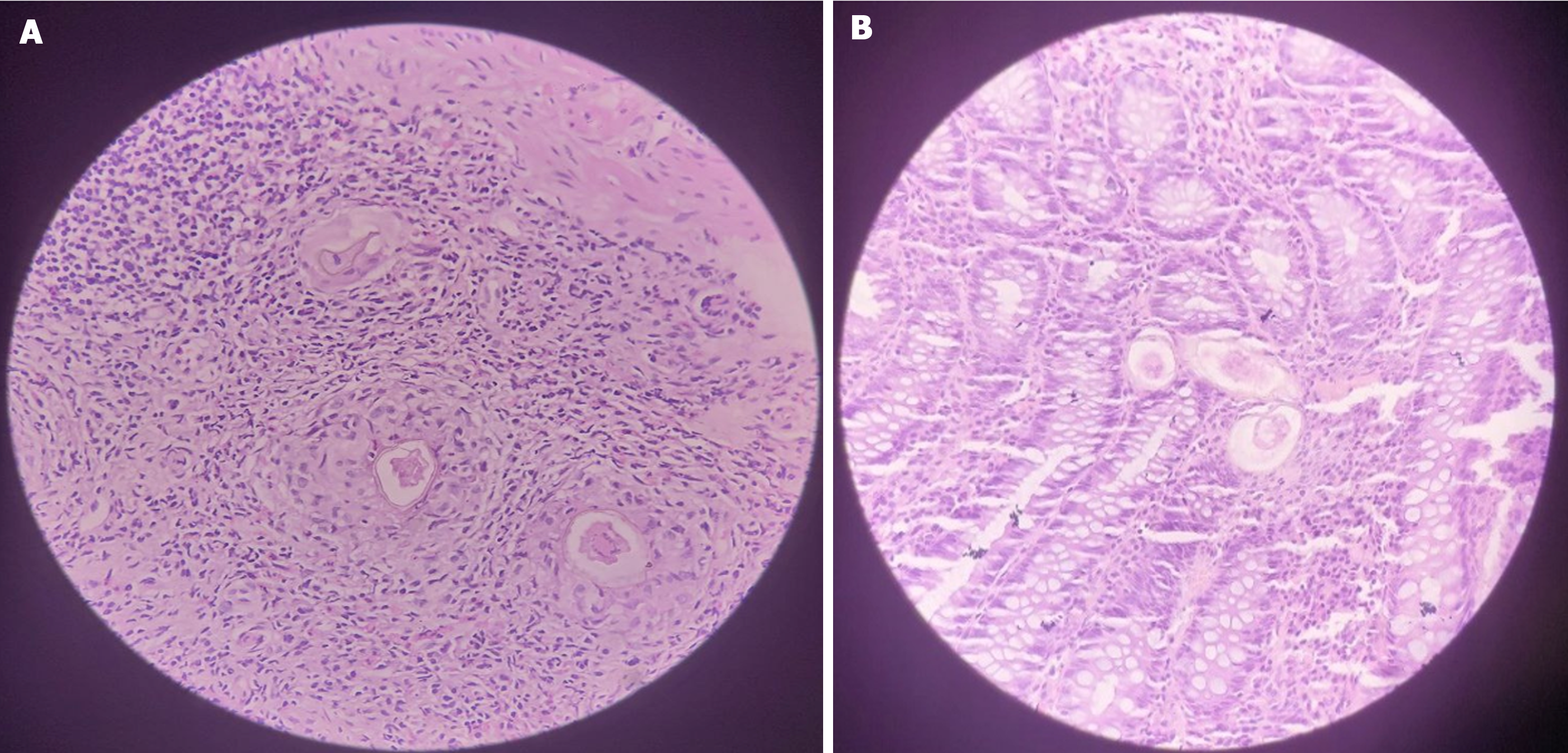
Case 1: Colonoscopy (Figure 1) showed nodular and inflamed colonic mucosa, a mass-like rectal lesion, and multiple ulcers in the terminal ileum. Biopsy from the lesions showed numerous Schistosoma eggs (Figure 2).
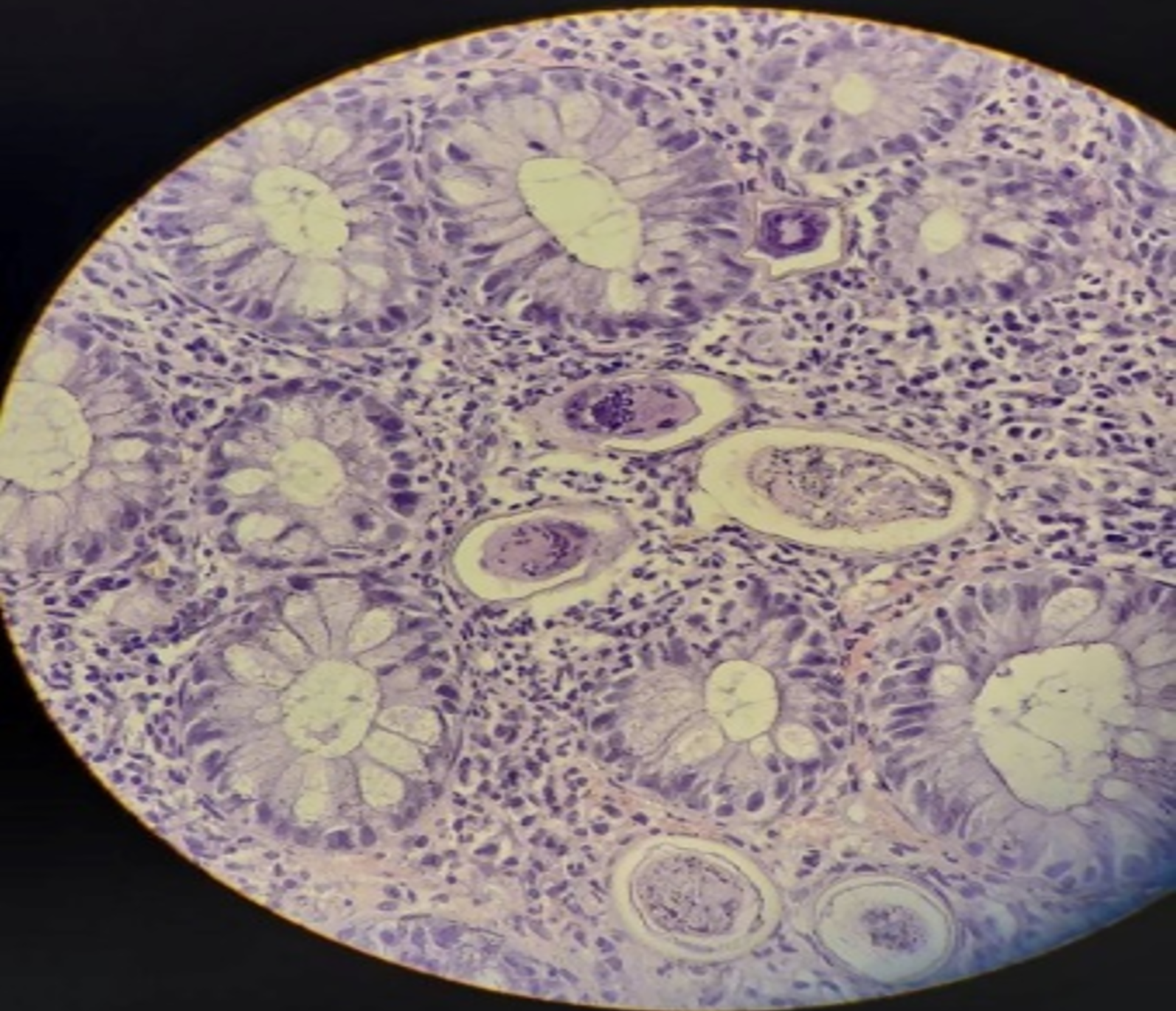
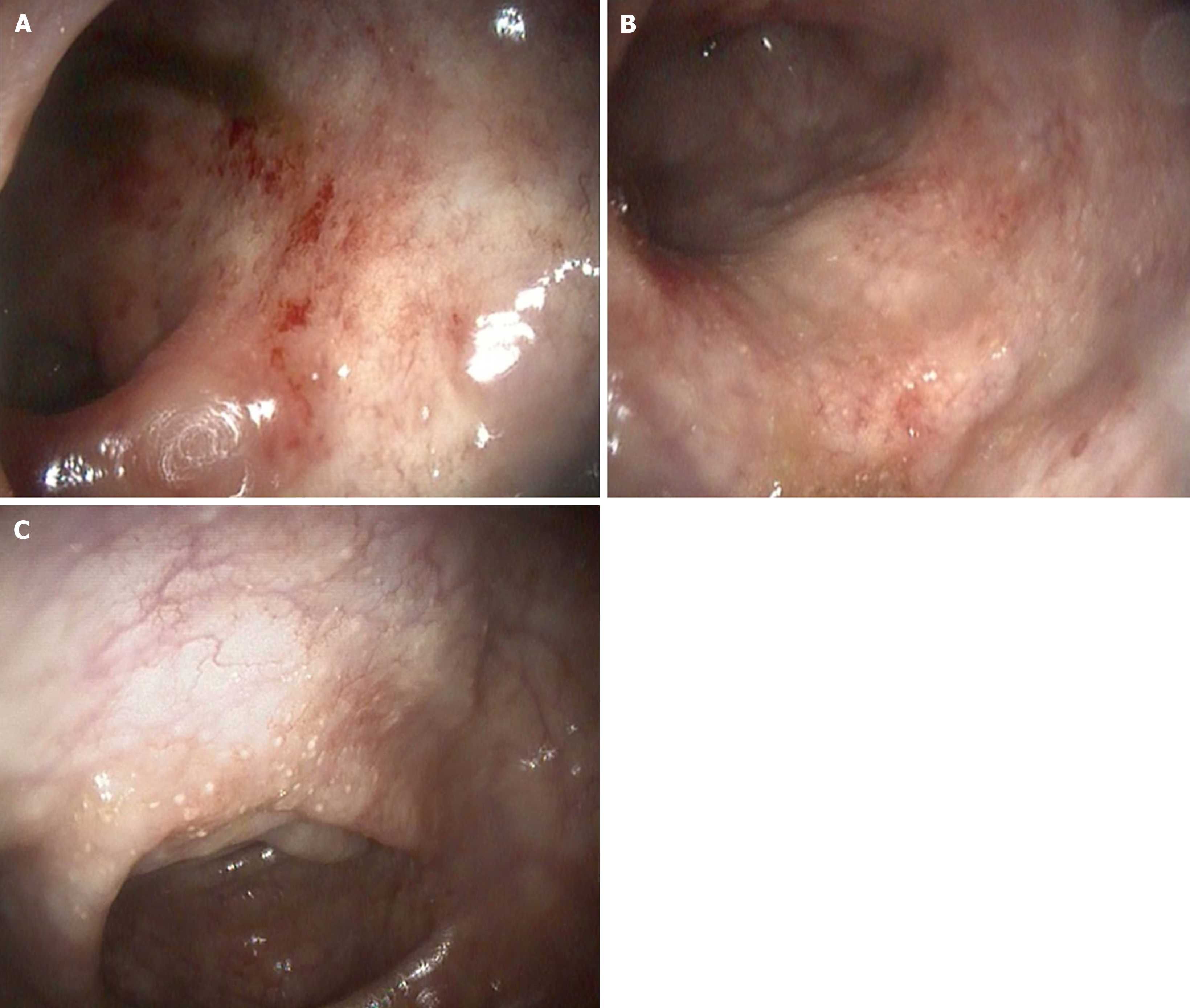
Case 2: Abdominal and pelvic ultrasound were unremarkable. Colonoscopy revealed an edematous and erythematous mucosa with reduced vascular markings and granular areas. Biopsies were taken and showed well-spaced crypts, with dense eosinophil infiltrates and an eosinophilic abscess surrounding a Schistosome egg (Figure 3).
Case 3: The patient underwent screening colonoscopy, which showed recto-sigmoid inflamed mucosa, and biopsy of the lesions revealed colonic mucosal dense eosinophil infiltrates and Schistosoma ova near the lamina propria (Figure 4).
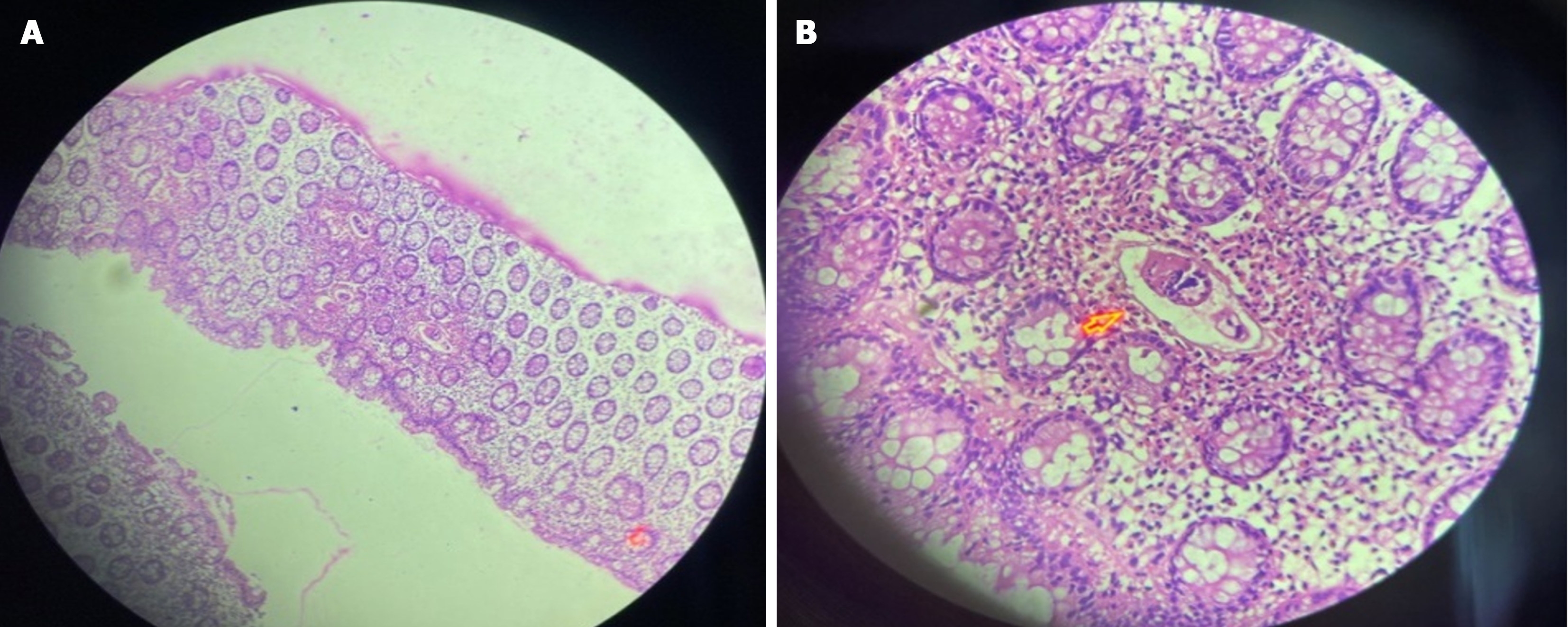
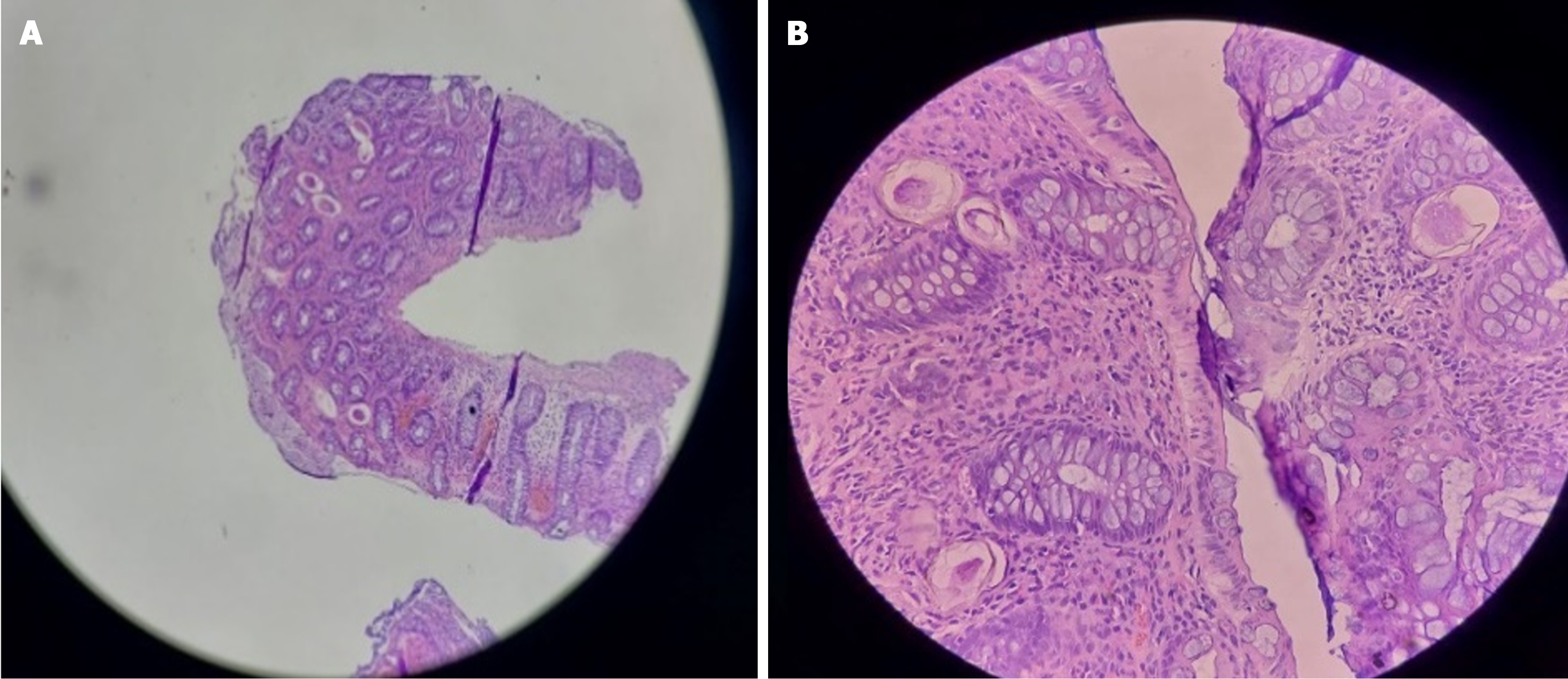
Case 4: Abdominal ultrasound showed, asymmetric distal rectal wall thickening, a distended rectum, and mild right hydronephrosis. With the suspicion of rectal malignancy, a colonoscopy (Figure 5) was performed and revealed patchy mucosal erythema and edema throughout the colon and rectum. Mucosal biopsies showed numerous Schistosoma eggs surrounded by eosinophil-predominant inflammatory cells (Figure 6).
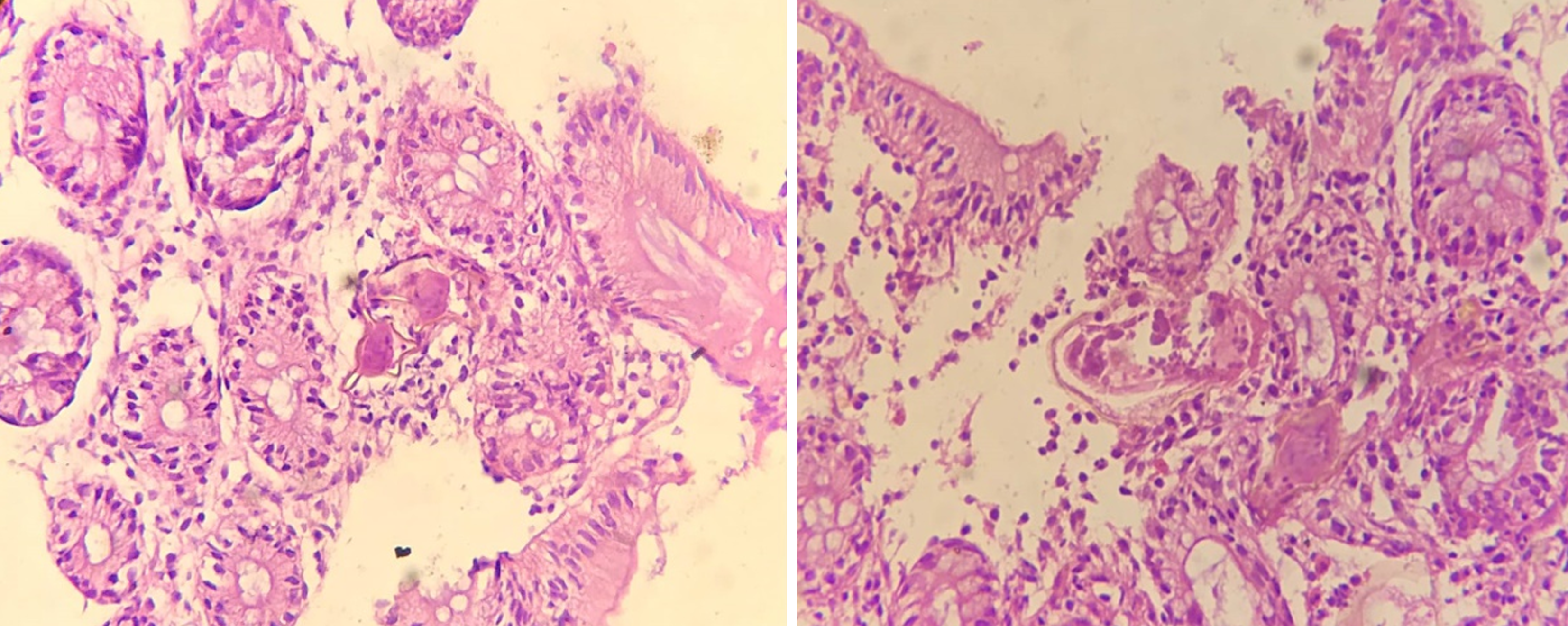
Case 5: Abdominal ultrasound was unremarkable. Her colonoscopy showed patchy mucosal erythema, granularity, and punctuate exudates involving the rectum and colon up to the hepatic flexure (Figure 7). Microscopic examination (Figure 8) of colorectal biopsies showed many Schistosoma ova surrounded by mixed inflammatory cells comprising of eosinophils and mononuclear cells.
A team from general internal medicine, gastroenterology, infectious diseases, and pathology were involved in the management of these patients.
A team of physicians, including general internal medicine, gastroenterology, infectious diseases, and pathology were involved in the management of the patient.
A pathologist and gastroenterologist were involved in the management of the patient.
A team of physicians, including endocrinology, gastroenterology, and infectious diseases were consulted for the evaluation and treatment of the patient.
A team of physicians, including gastroenterology and pathology, were consulted and involved in the evaluation and treatment of the patient.
A team of physicians, including pediatrics, gastroenterology, infectious diseases, and pathology were involved in the management of the patient.
A final diagnosis of colonic schistosomiasis mimicking polyp and colonic malignancy was made.
The final diagnosis was colonic schistosomiasis mimicking inflammatory bowel disease.
A diagnosis of colonic schistosomiasis was made.
The diagnosis of colonic schistosomiasis mimicking colonic malignancy was made.
The diagnosis of colonic schistosomiasis mimicking inflammatory bowel disease was made.
The patient was administered a single dose of praziquantel.
Patient was treated with a single dose of oral praziquantel (40 mg/kg), which led to resolution of her abdominal pain and diarrhea.
A single stat dose of praziquantel was administered.
Patient was administered a single dose of oral praziquantel (40 mg/kg).
Praziquantel (40 mg/kg) was administered.
The outcome of the patient is not known, as he did not return for his follow-up visit.
Her eosinophil count, determined after 6 wk of follow up, was 8% (total count was 600). Another dose of praziquantel (60 mg/kg) was administered, after which the eosinophil count normalized.
During follow up, the patient had no other complaints and investigations were all normal.
After treatment, all symptoms completely resolved.
Four days after treatment, the patient had complete resolution of symptoms. Upon follow up 4 wk later, she had no other complaints.
Schistosomiasis is an infectious disease caused by one of the seven species of the genus Schistosoma. The three major species are S. mansoni, S. japonicum, and S. haematobium. While the former two cause intestinal illness, the latter is often associated with genitourinary disease. Globally, more than 250 million people are infected with schistosomiasis, and 779 million are at risk of infection[4,7]. Over 80% of infected people live in Sub-Saharan Africa, and The World Health Organization considers the disease as an NTD[4-8].
Clinical disease is usually a result of immune reaction to Schistosoma eggs[9], which may cause tissue damage to several organs, including the intestines, liver, portal vein, bladder, brain, and spinal cord[10-13]. Intestinal schistosomiasis typically presents with intermittent abdominal pain, loss of appetite, and diarrhea. With a higher parasite load, the symptoms tend to be more severe, leading to bloody diarrhea and iron deficiency anemia due to intestinal ulceration[14-17]. Acute intestinal schistosomiasis presents with fever, abdominal pain, and diarrhea (with or without blood). Endoscopic examination typically shows hyperemic and edematous mucosa with hemorrhage. Mucosal histology in these patients is remarkable for eosinophils and neutrophils infiltrating the intestinal mucosa. Chronic intestinal schistosomiasis may present with symptoms caused by intestinal obstruction or nonspecific abdominal pain and mass. Endoscopic features may show abnormal vascular patterns, grayish mucosal nodules indicating calcified eggs and polyps, and luminal stenosis. Histology in these patients often reveals predominance of plasma cells and lymphocytes[18-20].
Intestinal polyps, dysplasia, strictures, and inflammatory masses can result from the intense inflammation targeting the Schistosoma eggs deposited in the bowel wall[21]. These were noted in several case reports describing patients presenting with isolated colonic ulcers, large intestinal polyps in isolation or in large groups[22-26], appendiceal mass[27], mesenteric vein involvement[28], and large obstructing rectal mass[29]. These features often lead to the erroneous diagnosis of malignancy and inflammatory bowel disease (IBD) until histopathology results become available[30].
In a study done in Riyadh from 1979 to 1988, out of 216 patients with colonic schistosomiasis diagnosed by colonoscopic biopsy, the ova of S. mansoni were detected in only 11% of patients on stool examination. The most common presenting symptoms were abdominal pain or distension, and the most common endoscopic findings were patchy mucosal congestion with petechiae, and patchy erosion with ulceration seen in 28% and 10% of patients, respectively. Other less common features noted were telangiectasia and polyps. In total, 118 patients had normal colonoscopic examination, but had evidence of Schistosoma ova with or without inflammatory cells upon histopathology evaluation. The most common sites of involvement were the colon and rectum. Some patients with very little ulceration upon endoscopic examination had evidence of ova with moderate-to-severe inflammatory cell reaction on biopsy. Histologically, most patients had ova with characteristic features, but some were empty with only calcification. After treatment, most patients with colonic schistosomiasis either become asymptomatic or have reduced symptoms with improved sigmoidoscopy features. This is in stark contrast to hepatosplenic schistosomiasis patients, most of whom do not improve[28].
In a study depicting endoscopic and clinicopathologic descriptions of 46 cases of colonic schistosomiasis, 72.5% of patients had endoscopy findings consistent with chronic active schistosomiasis colitis. There were 12 patients who were misdiagnosed as IBD and ischemic colitis before a definitive diagnosis was made. The rectum and sigmoid colon were the most affected sites, accounting for approximately 63% of patients. Additionally, 17 patients had a single polyp, and hyperplastic morphology was the most common histologic subtype[31].
In another study that retrospectively evaluated the clinical features of 248 individuals with schistosomiasis, the most common sites of involvement were the sigmoid colon, rectum, and descending colon, accounting for 96% of all patients. This is consistent with the sites of involvement seen in our cases. Different degrees of yellow intestinal mucosa, yellow flat or protruding nodules, abnormal vascular patterns, and Schistosoma eggs were observed upon histologic examination. Furthermore, 64.5% of patients had either single or multiple intestinal polyps, and the incidence thereof was higher among older individuals aged more than 60 years. The most common polyp morphology was Paris type IIa. It was also noted that female patients had a higher rate of colorectal cancer when compared to a control group, hinting at an association between chronic schistosomiasis and colorectal cancer. However, the number of patients evaluated was too small to make a firm conclusion[18].
The diagnosis of intestinal schistosomiasis is often made using laboratory tests. which include serum antibody tests, Schistosoma circulating antigens (circulating anodic antigen and circulating cathodic antigen), stool microscopy for egg detection in feces, and molecular testing using polymerase chain reaction to detect Schistosoma DNA in serum or stool. In a setting where laboratory tests fail to establish the diagnosis, histopathologic examination of a biopsy specimen from rectal or intestinal mucosa demonstrates the characteristic granulomas surrounding Schistosoma eggs in up to 61% of patients[32].
Praziquantel is the drug of choice for the treatment of intestinal schistosomiasis and is typically given as a single dose[33-36]. The efficacy of this treatment was also demonstrated by a meta-analysis of several studies done in Ethiopia[34]. Oxamniquine is sometimes used for refractory S. mansoni infection, although it cannot be used in pregnancy and is less effective compared to praziquantel[35].
A definitive diagnosis of colonic schistosomiasis was made in all our patients after histopathologic examination showed Schistosoma eggs. All our patients were treated with oral praziquantel, and most had improvement of their symptoms.
Our report highlights the diagnostic challenges of intestinal schistosomiasis, which can present with clinical features mimicking colon cancer, polyps, and IBD. A definitive diagnosis can be made through colonoscopy and biopsy revealing Schistosoma ova. The findings emphasize the importance of considering intestinal schistosomiasis in patients from endemic areas or with relevant travel history to such regions, who present with chronic abdominal pain, anorexia, or diarrhea, even when ova are not detected in stool examinations. Patients may be unnecessarily exposed to immunosuppressive medications or surgical resection if this condition is misdiagnosed. Therefore, it is imperative to conduct an appropriate clinical assessment and employ pertinent investigative modalities to avert incorrect diagnoses and guarantee suitable treatment.
Further case-control or prospective studies are needed to identify specific features that can differentiate colonic schistosomiasis from other gastrointestinal conditions, enabling timely and accurate diagnosis and treatment.
We would like to acknowledge the patients for providing us consent to share their case history and images.
| 1. | Hotez PJ, Kamath A. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis. 2009;3:e412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 685] [Cited by in RCA: 816] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 2. | Sady H, Al-Mekhlafi HM, Mahdy MA, Lim YA, Mahmud R, Surin J. Prevalence and associated factors of Schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis. 2013;7:e2377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Aula OP, McManus DP, Jones MK, Gordon CA. Schistosomiasis with a Focus on Africa. Trop Med Infect Dis. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 4. | Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1429] [Cited by in RCA: 1468] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 5. | Hailu FA, Department of Biology, Dilla University, College of Natural and Computational Sciences, Dilla, Ethiopia, Hailu YA, Hailu TA, Addis Ababa Kolfe Health Center, Department of Clinical Nurse, Addis Ababa, Ethiopia, Addis Ababa Medical and Business College, Addis Ababa, Ethiopia. Dental Anatomy and Physiology of Human Tooth and the Consequences of Pathogenic Microbiota on the Oral Cavity. J Clin Stud Rev Rep. 2020;2:1-7. [DOI] [Full Text] |
| 6. | Clerinx J, Van Gompel A. Schistosomiasis in travellers and migrants. Travel Med Infect Dis. 2011;9:6-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Utzinger J, Raso G, Brooker S, De Savigny D, Tanner M, Ornbjerg N, Singer BH, N'goran EK. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136:1859-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 295] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 8. | Hotez PJ, Alvarado M, Basáñez MG, Bolliger I, Bourne R, Boussinesq M, Brooker SJ, Brown AS, Buckle G, Budke CM, Carabin H, Coffeng LE, Fèvre EM, Fürst T, Halasa YA, Jasrasaria R, Johns NE, Keiser J, King CH, Lozano R, Murdoch ME, O'Hanlon S, Pion SD, Pullan RL, Ramaiah KD, Roberts T, Shepard DS, Smith JL, Stolk WA, Undurraga EA, Utzinger J, Wang M, Murray CJ, Naghavi M. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis. 2014;8:e2865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 610] [Cited by in RCA: 737] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 9. | Pearce EJ. Priming of the immune response by schistosome eggs. Parasite Immunol. 2005;27:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Strickland GT. Gastrointestinal manifestations of schistosomiasis. Gut. 1994;35:1334-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 11. | Bartley PB, Ramm GA, Jones MK, Ruddell RG, Li Y, McManus DP. A contributory role for activated hepatic stellate cells in the dynamics of Schistosoma japonicum egg-induced fibrosis. Int J Parasitol. 2006;36:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Cheever AW, Hoffmann KF, Wynn TA. Immunopathology of schistosomiasis mansoni in mice and men. Immunol Today. 2000;21:465-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Ross AG, McManus DP, Farrar J, Hunstman RJ, Gray DJ, Li YS. Neuroschistosomiasis. J Neurol. 2012;259:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Lambertucci JR, Voieta I, Barbosa AJ. Colonic polyps in hepatosplenic schistosomiasis mansoni. Rev Soc Bras Med Trop. 2005;38:80-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Gryseels B. The epidemiology of schistosomiasis in Burundi and its consequences for control. Trans R Soc Trop Med Hyg. 1991;85:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Stephenson L. The impact of schistosomiasis on human nutrition. Parasitology. 1993;107 Suppl:S107-S123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Stothard JR, Sousa-Figueiredo JC, Betson M, Bustinduy A, Reinhard-Rupp J. Schistosomiasis in African infants and preschool children: let them now be treated! Trends Parasitol. 2013;29:197-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Qin X, Liu CY, Xiong YL, Bai T, Zhang L, Hou XH, Song J. The clinical features of chronic intestinal schistosomiasis-related intestinal lesions. BMC Gastroenterol. 2021;21:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Ye C, Tan S, Jiang L, Li M, Sun P, Shen L, Luo H. Endoscopic characteristics and causes of misdiagnosis of intestinal schistosomiasis. Mol Med Rep. 2013;8:1089-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Crum NF, Chun HM, Favata MA, Hale BR. Gastrointestinal Schistosomiasis japonicum infections in immigrants from the Island of Leyte, Philippines. J Travel Med. 2003;10:131-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Lamyman MJ, Noble DJ, Narang S, Dehalvi N. Small bowel obstruction secondary to intestinal schistosomiasis. Trans R Soc Trop Med Hyg. 2006;100:885-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Koulali H, Zazour A, Khannoussi W, Kharrasse G, Ismaili Z. Colonic schistosomiasis: A case report. World J Gastrointest Endosc. 2022;14:789-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 23. | Akere A, Oluwasola AO, Fakoya TO, Lawan A. Schistosomiasis presenting as colonic polypoid masses in a Nigerian patient. Ann Ib Postgrad Med. 2017;15:61-64. [PubMed] |
| 24. | Zhu XL, Song JZ, Yu WY, Hua LQ, Zhang ML. Intestinal schistosomiasis masquerading as intestinal polyps. BMC Infect Dis. 2021;21:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Issa I, Osman M, Aftimos G. Schistosomiasis manifesting as a colon polyp: a case report. J Med Case Rep. 2014;8:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Shuja A, Guan J, Harris C, Alkhasawneh A, Malespin M, De Melo S. Intestinal Schistosomiasis: A Rare Cause of Abdominal Pain and Weight loss. Cureus. 2018;10:e2086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Nwabuoku SE, Mukoro GD, Daniyan M, Dauda MM, Khalid L, Aliyu HO, Shittu SM, Gana SG, Suraj A, Idokoko E, Okeke C, Iji L, Audu JA. Appendiceal Schistosomiasis Presenting as an Appendiceal Mass. J West Afr Coll Surg. 2022;12:100-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 28. | Mohamed AR, al Karawi M, Yasawy MI. Schistosomal colonic disease. Gut. 1990;31:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Tantoushian L, Diamond S, Serrano A, Salvo R, Carlson B. S1983 Atypical Presentation of Chronic Schistosomiasis as a Large Obstructing Rectal Mass. Am J Gastroenterol. 2022;117:e1365-e1365. [DOI] [Full Text] |
| 30. | Birhanu Y, Asefa M, Sultan A, Debes J. S1802 Colononic Schistosomiasis. Am J Gastroenterol. 2020;115:S932-S932. [DOI] [Full Text] |
| 31. | Cao J, Liu WJ, Xu XY, Zou XP. Endoscopic findings and clinicopathologic characteristics of colonic schistosomiasis: a report of 46 cases. World J Gastroenterol. 2010;16:723-727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 32. | Harries AD, Fryatt R, Walker J, Chiodini PL, Bryceson AD. Schistosomiasis in expatriates returning to Britain from the tropics: a controlled study. Lancet. 1986;1:86-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Fukushige M, Chase-Topping M, Woolhouse MEJ, Mutapi F. Efficacy of praziquantel has been maintained over four decades (from 1977 to 2018): A systematic review and meta-analysis of factors influence its efficacy. PLoS Negl Trop Dis. 2021;15:e0009189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Hailegebriel T, Nibret E, Munshea A. Efficacy of Praziquantel for the Treatment of Human Schistosomiasis in Ethiopia: A Systematic Review and Meta-Analysis. J Trop Med. 2021;2021:2625255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 35. | Sayed AA, Simeonov A, Thomas CJ, Inglese J, Austin CP, Williams DL. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat Med. 2008;14:407-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Cioli D, Pica-Mattoccia L, Basso A, Guidi A. Schistosomiasis control: praziquantel forever? Mol Biochem Parasitol. 2014;195:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 300] [Article Influence: 25.0] [Reference Citation Analysis (0)] |