Published online Jul 16, 2024. doi: 10.4253/wjge.v16.i7.413
Revised: May 22, 2024
Accepted: June 19, 2024
Published online: July 16, 2024
Processing time: 161 Days and 5.8 Hours
Routine outpatient endoscopy is performed across a variety of outpatient settings. A known risk of performing endoscopy under moderate sedation is the potential for over-sedation, requiring the use of reversal agents. More needs to be reported on rates of reversal across different outpatient settings. Our academic tertiary care center utilizes a triage tool that directs higher-risk patients to the in-hospital ambulatory procedure center (APC) for their procedure. Here, we report data on outpatient sedation reversal rates for endoscopy performed at an in-hospital APC vs at a free-standing ambulatory endoscopy digestive health center (AEC-DHC) following risk stratification with a triage tool.
To observe the effect of risk stratification using a triage tool on patient outcomes, primarily sedation reversal events.
We observed all outpatient endoscopy procedures performed at AEC-DHC and APC from April 2013 to September 2019. Procedures were stratified to their respective sites using a triage tool. We evaluated each procedure for which sedation reversal with flumazenil and naloxone was recorded. Demographics and characteristics recorded include patient age, gender, body mass index (BMI), American Society of Anesthesiologists (ASA) classification, procedure type, and reason for sedation reversal.
There were 97366 endoscopic procedures performed at AEC-DHC and 22494 at the APC during the study period. Of these, 17 patients at AEC-DHC and 9 at the APC underwent sedation reversals (0.017% vs 0.04%; P = 0.06). Demographics recorded for those requiring reversal at AEC-DHC vs APC included mean age (53.5 ± 21 vs 60.4 ± 17.42 years; P = 0.23), ASA class (1.66 ± 0.48 vs 2.22 ± 0.83; P = 0.20), BMI (27.7 ± 6.7 kg/m2vs 23.7 ± 4.03 kg/m2; P = 0.06), and female gender (64.7% vs 22%; P = 0.04). The mean doses of sedative agents and reversal drugs used at AEC-DHC vs APC were midazolam (5.9 ± 1.7 mg vs 8.9 ± 3.5 mg; P = 0.01), fentanyl (147.1 ± 49.9 μg vs 188.9 ± 74.1 μg; P = 0.10), flumazenil (0.3 ± 0.18 μg vs 0.17 ± 0.17 μg; P = 0.13) and naloxone (0.32 ± 0.10 mg vs 0.28 ± 0.12 mg; P = 0.35). Procedures at AEC-DHC requiring sedation reversal included colonoscopies (n = 6), esophagogastroduodenoscopy (EGD) (n = 9) and EGD/colonoscopies (n = 2), whereas APC procedures included EGDs (n = 2), EGD with gastrostomy tube placement (n = 1), endoscopic retrograde cholangiopancreatography (n = 2) and endoscopic ultrasound's (n = 4). The indications for sedation reversal at AEC-DHC included hypoxia (n = 13; 76%), excessive somnolence (n = 3; 18%), and hypotension (n = 1; 6%), whereas, at APC, these included hypoxia (n = 7; 78%) and hypotension (n = 2; 22%). No sedation-related deaths or long-term post-sedation reversal adverse outcomes occurred at either site.
Our study highlights the effectiveness of a triage tool used at our tertiary care hospital for risk stratification in minimizing sedation reversal events during outpatient endoscopy procedures. Using a triage tool for risk stratification, low rates of sedation reversal can be achieved in the ambulatory settings for EGD and colonoscopy.
Core Tip: Our study highlights the effectiveness of a triage tool used at our tertiary care hospital for risk stratification in minimizing sedation reversal events during outpatient endoscopy procedures. By directing higher-risk patients to appropriate settings, we achieved low rates of sedation reversal, enhancing patient safety and optimizing resource utilization in ambulatory care settings. This approach can have a significant impact on improving patient outcomes and resource allocation in similar healthcare settings.
- Citation: Walayat S, Stadmeyer P, Hameed A, Sarfaraz M, Estrada P, Benson M, Soni A, Pfau P, Hayes P, Kile B, Cruz T, Gopal D. Sedation reversal trends at outpatient ambulatory endoscopic center vs in-hospital ambulatory procedure center using a triage protocol. World J Gastrointest Endosc 2024; 16(7): 413-423
- URL: https://www.wjgnet.com/1948-5190/full/v16/i7/413.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i7.413
Endoscopy, introduced in 1868, has seen growing use by clinicians for its diagnostic and therapeutic purposes[1]. In the United States, it is estimated that approximately 20 million endoscopies are performed annually[2]. Colonoscopy ranks as the most common endoscopic procedure, followed closely by esophagogastroduodenoscopy[3]. To enhance patient comfort during these often uncomfortable procedures, sedation is frequently administered, primarily aiming to provide anxiety relief, amnesia, and pain relief, which in turn results in enhanced patient satisfaction rates and increased procedural success[4].
In the United States, the preferred method of sedation is moderate sedation, achieved using a combination of benzodiazepines and opioids[5]. However, deeper sedation using propofol is gaining popularity among physicians, particularly for more complex procedures[6]. Sedation, however, comes with its associated risks, including respiratory depression and hypotension[7]. Although endoscopy is generally considered a safe procedure, with adverse events occurring in less than 1% of cases, the risk significantly increases in patients with more severe comorbidities[8]. Therefore, there is a growing emphasis on pre-procedural assessment for endoscopic procedures[9-11]. Risk stratification has been shown to reduce mortality rates, allowing higher-risk patients to receive more meticulous care[12].
While stratification scales are used for conditions like upper gastrointestinal bleeding, the adoption of a risk stratification scale for endoscopic procedures at a nationwide level has yet to be seen[13].
An increasing trend of performing outpatient glycemic index (GI) endoscopies in ambulatory surgery centers has been observed due to its lower cost, provider preference, and consumer demand[14]. One national survey revealed that outpatient procedures performed in ambulatory centers increased 300% from 1996 to 2006, while procedures performed in hospital outpatient departments were relatively stable. However, very little has been reported on the rates of sedation reversal across different studies[15]. We assessed a triage tool used at our academic center that is used to assess risk factors for endoscopic procedures and directs higher-risk patients from a free-standing ambulatory endoscopy digestive health center (AEC-DHC) to an in-hospital ambulatory procedure center (APC) and observed the effect of this risk stratification on sedation related complications.
We analyzed all outpatient GI procedures performed between April 2013 and September 2019. At our tertiary-care hospital, outpatient endoscopy and colonoscopy are performed in two settings: A free-standing AEC-DHC and an in-hospital APC. Patient risk assessment for endoscopic procedures is done using a triage tool, directing higher-risk patients to the APC for management. All procedures performed under conscious sedation (using Midazolam and Fentanyl) within our study period were included in the study. Procedures performed under deep sedation were excluded.
The primary outcome of the assessment was a 'sedation reversal event,' defined as any endoscopic procedure requiring flumazenil and/or naloxone to reverse sedation during or soon after the procedure in either of the two settings.
The indication for reversal of sedation was also recorded and included one of the following: (1) Hypoxia; (2) Hypotension; or (3) Excessive somnolence. For descriptive purposes of this study, 'hypoxia' was defined as oxygen saturation < 90%, 'hypotension' was identified by a systolic blood pressure reading < 90 mm of Hg or a drop in blood pressure requiring intervention and 'excessive somnolence' was characterized by difficulty arousing the patient or unresponsiveness with a brief response to rigorous stimuli.
Adverse events and outcomes, including death, were also assessed.
Further demographic details and characteristics were recorded for the patients who received sedation reversal during the procedure, including patient age, gender, body mass index (BMI), procedure type, indication for sedation reversal, and the American Society of Anesthesiologists (ASA) classification.
A triage tool developed by UW Health plays a significant role within our hospital by streamlining the allocation of patients to appropriate endoscopic procedure sites. The triage tool serves multiple purposes, including identifying higher-risk patients to be managed at the in-hospital APC rather than at the DHC, alongside categorizing patients who require monitored anesthesia care or deeper sedation with propofol and distinguishing patients with significant comorbidities whose procedure request is to be sent to a triage nurse (Figure 1).
Risk stratification using this tool assesses multiple risk factors (Figure 1). Patients meeting one or more of these risk criteria are directed toward the in-hospital care pathway.
Clinical data from April 2013 to September 2019 was extracted from a computerized endoscopy database of the hospital. Data was categorized for both the settings (AEC-DHC and APC) separately.
Continuous variables were presented as mean ± SD, while categorical variables were represented as frequency (%) and numbers (n). All analyses were conducted using STATA version 16. To evaluate differences in characteristics such as age, BMI, procedure type, indication for sedation reversal, and ASA grade between the two patient groups receiving sedation reversal, univariate analysis employed the χ2 test (for categorical data) and Student's t-test (for continuous data). The null hypothesis tested was the absence of association between variables in both groups, with statistical significance defined as a P value < 0.05.
Across both settings, 119860 endoscopic procedures were performed under conscious sedation from April 2013 to September 2019. The procedures that required deep sedation were excluded from the study. Of these, 97366 (81.23%) endoscopic procedures were performed at AEC-DHC and 22494 (18.77%) at the APC.
During the study period, 17 patients at the AEC-DHC were given flumazenil and/or naloxone, representing 0.017% of all the procedures at AEC-DHC. In contrast, nine instances of sedation reversal were recorded at the APC, making up 0.04% of the total. A difference was noted between the number of overall sedation reversal events between both settings; however, this difference was not statistically significant (P = 0.06) (Table 1).
| AEC-DHC (n = 17) | APC (n = 9) | P value | |
| Sedation reversal events | 17 (0.01) | 9 (0.04) | 0.06 |
| Age (years) | 53.5 (21) | 60.4 (17.42) | 0.23 |
| Female | 11 (66) | 2 (22) | 0.04a |
| BMI (kg/m2) | 27.7 (6.7) | 23.7 (4.03) | 0.12 |
| ASA | 1.66 (0.48) | 2.22 (0.83) | 0.20 |
Demographic details of patients requiring reversal at AEC-DHC vs APC were compared and are provided in Table 1. A gender-based analysis demonstrated that females constitute a higher percentage in the AEC-DHC group at 64.7% (n = 11), while in the APC group, females account for only 22% (n = 2) (P = 0.04). This difference was statistically significant.
No statistical difference was observed in the mean age of individuals requiring reversal (DHC vs APC: 53.5 ± 21 years vs 60.4 ± 17.42 years; P = 0.23), and the difference in the BMI of the two groups was also insignificant (27.7 ± 6.7 kg/m2vs 23.7 ± 4.03 kg/m2; P = 0.06). Similarly, the ASA class distribution for the AEC-DHC cohort displays an ASA class average of 1.66 ± 0.48, which is comparatively lower than the ASA class average of 2.22 ± 0.83 in the APC group (P = 0.20). Despite this variance, it is essential to emphasize that the difference did not reach statistical significance.
The mean doses of sedatives (midazolam and fentanyl) and reversal agents used (naloxone and/or flumazenil) between the two groups are discussed in Table 2. A combination of midazolam and fentanyl was used in all the patients for sedation. However, the only statistically significant difference between the two groups was in the average doses of midazolam used (5.9 ± 1.7 mg vs 8.9 ± 3.5 mg; P = 0.01). For reversal, flumazenil was used in 8 cases (47%) and naloxone in 13 procedures (76.5%) at DHC vs 6 (66.6%) and 9 procedures (100%) APC, respectively.
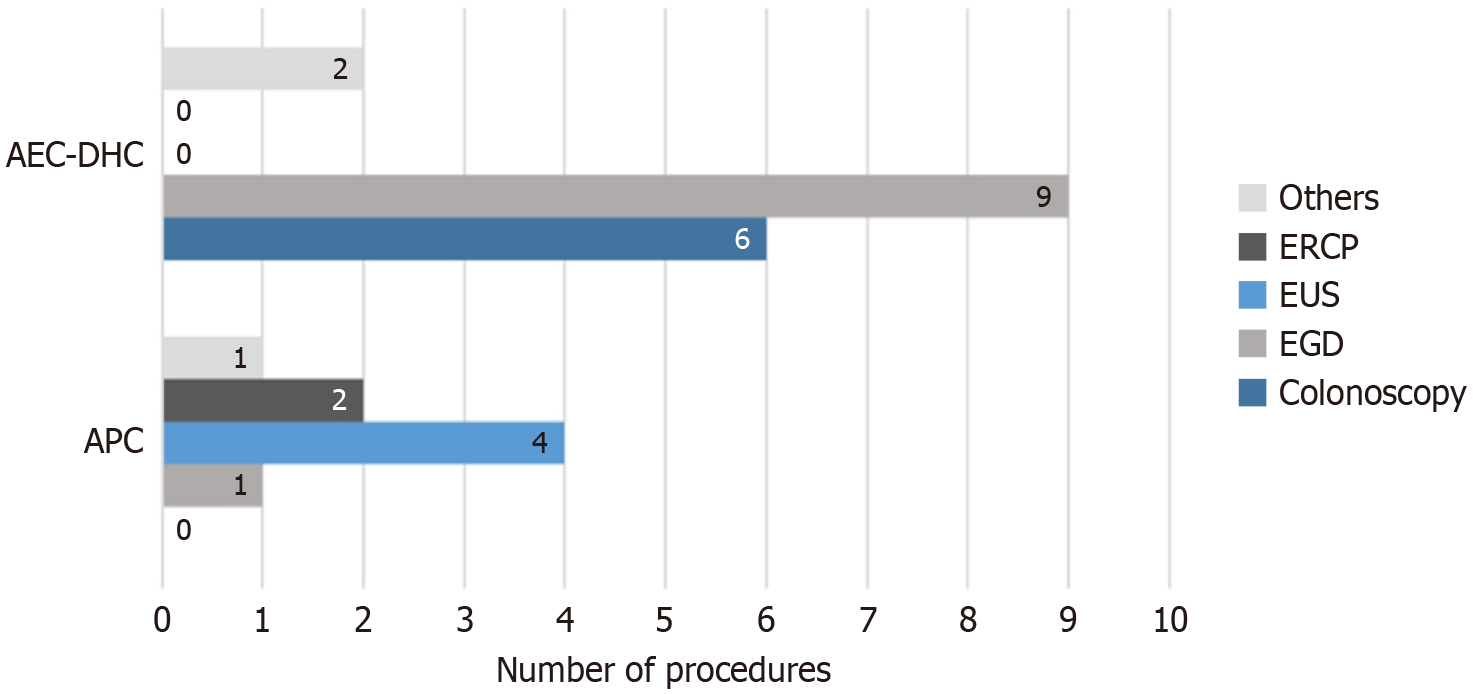
The most frequent procedure type leading to sedation reversal at the AEC-DHC was esophagogastroduodenoscopy (EGD) (n = 9), followed by colonoscopy (n = 6) and a combination of EGD/colonoscopy (n = 2). Conversely, at APC, the need for reversal was most pronounced during endoscopic ultrasound (EUS) procedures (n = 4), followed by EGD (n = 2), endoscopic retrograde cholangiopancreatography (ERCP) (n = 2), and a single case involving EGD with gastrostomy tube placement (Figure 2). These accounted for 0.03% of total ERCPs (5694 total) and 0.07% of all EUSs (5715 total) performed during the study period.
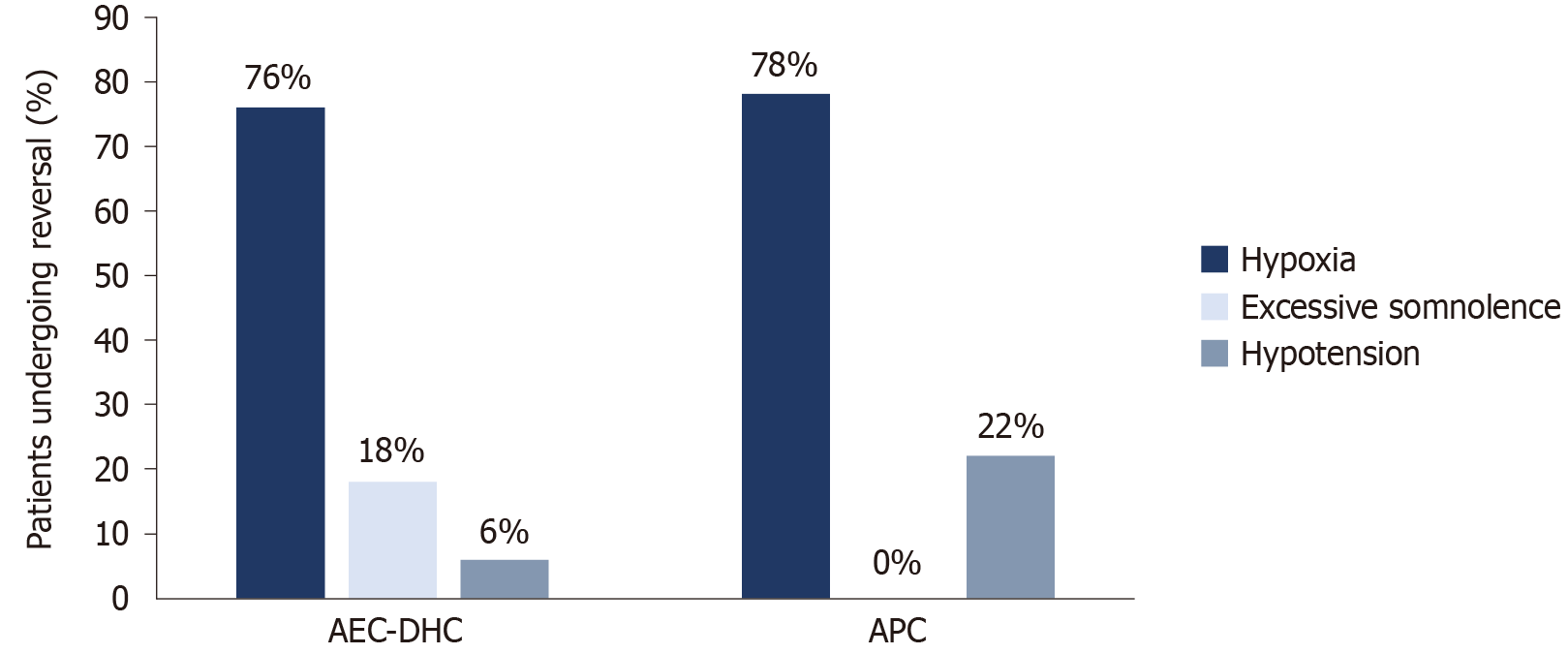
No mortalities or adverse outcomes related to sedation were documented at either site. The indications for sedation reversal at AEC-DHC contributed mainly to hypoxia (n = 13, 76% of all sedation reversal events at AEC-DHC), followed by excessive somnolence (n = 3, 18%) and hypotension (n = 1, 6%). At APC, sedation reversal indications included hypoxia (n = 7, 78%) and hypotension (n = 2, 22%) (Figure 3).
Sedation is a critical component of endoscopic procedures and aims to enhance procedural efficacy, elevate patient comfort, and contribute to the satisfaction of both patients and endoscopists. Amidst the array of available sedation techniques, conscious sedation remains the most widely employed technique, primarily owing to its superior patient tolerance and satisfaction levels, along with reduced incidence of side effects[16]. However, the administration of anesthesia comes with its risks. A study evaluating 207134 endoscopic procedures concluded that most adverse effects during the procedure were attributed to the sedative medication used[17]. Our study aimed to assess the efficacy of risk stratification in enhancing patient outcomes by evaluating two distinct patient populations classified using a triage tool.
While the risk factors associated with sedation reversal have been examined by specific studies, to our knowledge, no study has been conducted to evaluate the impact of risk stratification on patient outcomes[9-11]. The University of Wisconsin Health triage tool is used to identify well-established risk factors for endoscopy, including cardiac disease, distortion of upper airway anatomy, oxygen dependency, thrombocytopenia, morbid obesity, pregnancy, etc. (Figure 1). By identifying higher-risk patients, they are transferred to the in-hospital APC for thorough monitoring and meticulous procedural care. As we confront a growing shortage of anesthesia providers, particularly impacting timely access to surgical procedures like gastrointestinal endoscopies, the necessity for practical triage tools becomes increasingly apparent[18]. These tools are vital for stratifying patients based on risk and determining the most suitable setting (inpatient vs ambulatory) and type of sedation (such as moderate conscious sedation, deep sedation with monitored anesthesia care or general endotracheal intubation). Given resource limitations, prioritizing endoscopic procedures becomes imperative, and innovative process for risk assessment, timely triage scheduling and stratification are crucial. This approach should not only address resource utilization but also ensure patient safety and uphold the standards of quality care. Numerous researchers have advocated the need for pre-procedural stratification to reduce the risk of complications. Thus, we needed to fill this literature gap by reporting our findings.
Endoscopic procedures in severe disease states: Endoscopic procedures have been found to increase the risk of complications in patients with cardiac diseases, particularly heart failure, and shortly after a recent myocardial infarction (MI)[19]. One study found that endoscopy within one month following an MI can lead to cardiopulmonary complications in up to 1.5% of patients. In contrast, another study reported higher rates of minor cardiovascular complications in patients undergoing colonoscopy following an MI[20,21]. Pulmonary hypertension is also associated with higher rates of periprocedural complications[22,23]. The clinical value of close monitoring in these patients is undeniable. Hence, the triage tool recommends transferring patients who have experienced an MI in the last six months, heart failure, stent placement, valvular stenosis, oxygen dependency, and pulmonary hypertension to an inpatient APC for closer monitoring and care.
Implanted electronic devices: Implanted electronic devices are at an increased risk of electromagnetic interference due to the electromagnetic field from another source on the implanted device. During endoscopic procedures, this may occur due to conduction via diathermy or radiation from imaging devices[24]. The American Society for Gastrointestinal Endoscopy (ASGE) advises caution when performing endoscopic procedures in individuals with implanted electronic devices, particularly in free-standing centers[25]. Although studies have not found an increased risk of adverse events in patients undergoing endoscopic electrosurgery with implanted devices, these studies are small-scale, and there is a notable research gap in this area[26,27]. Therefore, it is essential to take precautions in these patients (with cardiac defibrillators/pacemakers and/or implanted organ stimulators that are unable to turn off), including considering transfer to an inpatient setting.
Pregnancy: Pregnancy is another factor associated with increased risk of complications during endoscopic procedures due to fetal sensitivity to maternal hypoxia and hypotension, along with the narrow safety profile of the drugs administered[28]. Most sedatives used are classified as category B or C by the food and drug administration[29]. ASGE recommends avoiding endoscopy in the first trimester whenever possible, meticulous procedural care, and careful administration of sedatives by anesthesia providers[28].
Head and neck abnormalities/surgery: The ASA deemed airway management difficult for patients with oral, jaw, and neck abnormalities[30]. The ASA taskforce guidelines also suggest that these abnormalities, coupled with the potential for deep sedation, will increase the likelihood of adverse, sedation-related events.
Young age: Age below 18 years is another criterion for referral. While administering anesthetic agents to younger patients has been associated with increased rates of mental disorders, developmental delays, and attention deficit hyperactivity disorder, there is no evidence to suggest increased sedation-related complications in younger children[31]. Nonetheless, The royal college of emergency medicine recommends involving senior personnel in the emergency department when administering procedural sedation to children aged 1-5 years[32].
Despite the higher risk of complications reported in the conditions used to filter patients to the APC, our study's outcomes revealed no significant difference in the sedation reversal events between the two settings (P = 0.06, CI: 95%). This finding suggests that the triage tool effectively identified and successfully managed patients who are more likely to have adverse outcomes within the in-hospital setting. While most literature reports 30-day mortality rates associated with endoscopy ranging from 0.004% to 1.89%, one study specifically studied inpatient endoscopy mortality and found it to be as high as 5%-10%[33-35]. This starkly contrasts our findings, revealing zero 30-day mortality. This outcome suggests rigorous risk stratification and management protocols likely account for this discrepancy. While the existing research supports the notion that risk stratification diminishes mortality rates associated with endoscopic procedures in specific cases, such as upper gastrointestinal bleeding, a notable gap exists in the available data pertaining to the impact of pre-procedural stratification on non-urgent endoscopic procedures[13].
The impact of gender on sedation rates and complications is inconsistent. While certain studies indicate that females may metabolize midazolam more rapidly with higher clearance rates, most literature fails to show a significant gender-related effect on midazolam's efficacy[36-39]. In the case of fentanyl, one study proposes that males may necessitate a 25% higher dose[40]. Conversely, Hung et al[10] observed elevated sedation reversal in females. Our results showed that a higher proportion of female patients needed sedation reversal at AEC-DHC compared to APC. However, because we observed similar reversal rates between the two settings, it is challenging to conclude how gender influenced sedation complications. One possible interpretation is that the male population at the APC had a higher prevalence of risk factors assessed by the triage tool. This could explain the greater number of males there, and females were at a higher risk of sedation complications when risk factors were absent. However, this relationship needs to be explored further.
In our study of sedation reversal cases, only one patient (at the APC) was aged over 80 years, and none were under 18 years. Even though a higher BMI has been identified as a risk factor for sedation-related complications, and the triage tool refers patients with a high BMI (> 50 or > 45 if requiring propofol) to the APC, we did not observe any significant difference in BMI between the two groups[41]. Thus, owing to the limited number of sedation reversal events in our study and the narrow distribution of age and BMI, a comprehensive analysis of either variable was not possible.
The most frequent complications associated with procedural sedation are predominantly related to respiratory and cardiovascular depression[7]. These complications include hypoxemia arising from either hypoventilation or airway blockage, hypotension, cardiac arrhythmias, and apnea. Our findings indicate hypoxia to be the most critical reason for sedation reversal events. The ASA and the ASGE advise using pulse oximetry during all endoscopic procedures under sedation and considering supplemental oxygen during moderate sedation and for all deep sedation procedures[42,43].
In most cases in the United States, endoscopic procedures are performed under moderate sedation, achieved through a combination of benzodiazepines and narcotics[5]. The most used benzodiazepines include midazolam and diazepam. Although the efficacy of the two is comparable, midazolam is preferred by endoscopists due to its faster onset, shorter duration of action, and superior amnesia properties[44]. Among opiates, fentanyl and meperidine are the most used, and due to fentanyl's more rapid onset of action and clearance and lower rates of nausea, it is usually preferred over meperidine[45,46]. The usual dose of midazolam used is 2.5 to 5 mg, and the maximal dosage for endoscopic procedures is 6 to 7.5 mg[44,47,48]. When midazolam is used in combination with an opioid, a reduction in the dosage is required. The initial doses are 0.5 to 1 mg midazolam plus 12.5 to 75 μg fentanyl[49-51]. A dose reduction of 20% or more is recommended for patients older than 60 and those with an ASA grade of 3 or greater[52]. The average dose of midazolam and fentanyl used at AEC-DHC was 5.9 ± 1.7 mg and 147.1 ± 49.9, while at the APC, it was 8.9 ± 3.5 and 188.9 ± 74.1 respectively, with a significant increase in the dose of midazolam. The dosage of drugs used was not per the guidelines as the average dose was greater than the recommended dosage, and the dose reduction for higher ASA grade at the APC was not done.
A nationwide analysis revealed that anesthesia was employed in up to 34.4% of colonoscopies, associated with a 13% risk of anesthesia-related complications[53]. Our findings indicated that 35.3% of procedures necessitating sedation reversal at the DHC were colonoscopies. One study evaluated patients by administering sedation on an 'as needed' basis, and 80% of patients underwent the procedure without sedation, resulting in an impressive satisfaction rate of 97.4%[54]. Consequently, one potential strategy to address the elevated incidence of anesthesia-related complications in colonoscopies is tailoring sedative use to individual needs.
Literature indicates ERCP to be associated with higher rates of sedation reversal, reaching up to 4%[55]. This is due to the complexity and length of the procedure, which often requires higher sedative doses than upper and lower GI endoscopies[56,57]. Only 0.03% of ERCP procedures required sedation reversal in our hospital. This discrepancy may be secondary to risk stratification, as all the ERCP cases requiring reversal were at the inpatient APC.
Owing to the retrospective nature of our study, certain limitations warrant consideration. One such limitation is our reliance on physicians for accurate record-keeping of procedures and ASA grading. The integrity of our study relies on the accuracy of record keeping; thus, it must be as precise as possible. Additionally, our ability to analyze the effectiveness of the triage tool was hindered by the relatively small number of sedation reversal events we examined. Moreover, our study was restricted by the need for more data regarding the reasoning behind referrals to the APC through the triage tool. This limitation prevented us from conducting a comprehensive analysis of the individual components of the tool itself. The tool is also used to categorize patients for deep sedation. However, our study design did not include those patients, and thus, we could not study the procedural safety and effect of stratification in those patients. Lastly our study was not designed to assess the cost effectiveness of implementing triage tool. Previous studies however have shown that sedation reversal does add cost to the procedure[58].
In conclusion, our study supports using a rigorous risk stratification tool as it may help identify high-risk patients and minimize procedure-related complications. The triage tool used in our study appeared effective for minimizing sedation-related complications and reversal events despite the large number of procedures being done at outpatient endoscopy centers. It is also important to note that with appropriate risk stratification and triage, even complex procedures like EUS/ERCP can be done under conscious sedation. This approach can have a significant impact on improving patient outcomes and resource allocation in limited healthcare settings. Our results should be interpreted with caution considering the retrospective design of our study; future large-scale randomized prospective controlled trials are needed to draw concrete conclusions.
| 1. | Ismail FW, Afzal A, Durrani R, Qureshi R, Awan S, Brown MR. Exploring Endoscopic Competence in Gastroenterology Training: A Simulation-Based Comparative Analysis of GAGES, DOPS, and ACE Assessment Tools. Adv Med Educ Pract. 2024;15:75-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022;162:621-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 582] [Article Influence: 145.5] [Reference Citation Analysis (1)] |
| 3. | Sonnenberg A, Amorosi SL, Lacey MJ, Lieberman DA. Patterns of endoscopy in the United States: analysis of data from the Centers for Medicare and Medicaid Services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Dossa F, Megetto O, Yakubu M, Zhang DDQ, Baxter NN. Sedation practices for routine gastrointestinal endoscopy: a systematic review of recommendations. BMC Gastroenterol. 2021;21:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 5. | Lässing J, Schulze A, Kwast S, Falz R, Vondran M, Schröter T, Borger M, Busse M. Effects of Custom-made Mouthguards on Cardiopulmonary Exercise Capacity. Int J Sports Med. 2021;42:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Lapidus A, Gralnek IM, Suissa A, Yassin K, Khamaysi I. Safety and efficacy of endoscopist-directed balanced propofol sedation during endoscopic retrograde cholangiopancreatography. Ann Gastroenterol. 2019;32:303-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Lee JK, Lee YJ, Cho JH, Im JP, Park CH, Jang JY, Jang BI. Updates on the Sedation for Gastrointestinal Endoscopy. Clin Endosc. 2019;52:451-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | ASGE Standards of Practice Committee; Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, Fisher L, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Dominitz JA, Cash BD. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (2)] |
| 9. | Tetzlaff JE, Maurer WG. Preprocedural Assessment for Sedation in Gastrointestinal Endoscopy. Gastrointest Endosc Clin N Am. 2016;26:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Hung A, Marshall J, Barnett S, Falchuk ZM, Sawhney M, Leffler DA. Risk Factors and Outcomes of Reversal Agent Use in Moderate Sedation During Endoscopy and Colonoscopy. J Clin Gastroenterol. 2016;50:e25-e29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Papachristou GI, Gleeson FC, Papachristou DJ, Petersen BT, Baron TH. Endoscopist administered sedation during ERCP: impact of chronic narcotic/benzodiazepine use and predictive risk of reversal agent utilization. Am J Gastroenterol. 2007;102:738-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Teheux L, Verlaat CW, Lemson J, Draaisma JMT, Fuijkschot J. Risk stratification to improve Pediatric Early Warning Systems: it is all about the context. Eur J Pediatr. 2019;178:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Robertson M, Majumdar A, Boyapati R, Chung W, Worland T, Terbah R, Wei J, Lontos S, Angus P, Vaughan R. Risk stratification in acute upper GI bleeding: comparison of the AIMS65 score with the Glasgow-Blatchford and Rockall scoring systems. Gastrointest Endosc. 2016;83:1151-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Torabi SJ, Patel RA, Birkenbeuel J, Nie J, Kasle DA, Manes RP. Ambulatory surgery centers: A 2012 to 2018 analysis on growth in number of centers, utilization, Medicare services, and Medicare reimbursements. Surgery. 2022;172:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. Natl Health Stat Report. 2009;1-25. [PubMed] |
| 16. | McQuaid KR, Laine L. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest Endosc. 2008;67:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 376] [Article Influence: 20.9] [Reference Citation Analysis (1)] |
| 17. | Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc. 2001;53:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 211] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Menezes J, Zahalka C. Anesthesiologist shortage in the United States: A call for action. J Med Surg Public Health. 2024;2:100048. [DOI] [Full Text] |
| 19. | Elkafrawy AA, Ahmed M, Alomari M, Elkaryoni A, Kennedy KF, Clarkston WK, Campbell DR. Safety of gastrointestinal endoscopy in patients with acute coronary syndrome and concomitant gastrointestinal bleeding. World J Clin Cases. 2021;9:1048-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Egholm G, Thim T, Madsen M, Sørensen HT, Pedersen JB, Eggert Jensen S, Jensen LO, Kristensen SD, Bøtker HE, Maeng M. Gastroscopy-related adverse cardiac events and bleeding complications among patients treated with coronary stents and dual antiplatelet therapy. Endosc Int Open. 2016;4:E527-E533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Cappell MS. Safety and efficacy of colonoscopy after myocardial infarction: an analysis of 100 study patients and 100 control patients at two tertiary cardiac referral hospitals. Gastrointest Endosc. 2004;60:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Rajagopal S, Ruetzler K, Ghadimi K, Horn EM, Kelava M, Kudelko KT, Moreno-Duarte I, Preston I, Rose Bovino LL, Smilowitz NR, Vaidya A; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, and the Council on Cardiovascular and Stroke Nursing. Evaluation and Management of Pulmonary Hypertension in Noncardiac Surgery: A Scientific Statement From the American Heart Association. Circulation. 2023;147:1317-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 60] [Reference Citation Analysis (0)] |
| 23. | Meyer S, McLaughlin VV, Seyfarth HJ, Bull TM, Vizza CD, Gomberg-Maitland M, Preston IR, Barberà JA, Hassoun PM, Halank M, Jaïs X, Nickel N, Hoeper MM, Humbert M. Outcomes of noncardiac, nonobstetric surgery in patients with PAH: an international prospective survey. Eur Respir J. 2013;41:1302-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 24. | Samuels JM, Overbey DM, Wikiel KJ, Jones TS, Robinson TN, Jones EL. Electromagnetic interference on cardiac pacemakers and implantable cardioverter defibrillators during endoscopy as reported to the US Federal Drug Administration. Surg Endosc. 2021;35:3796-3801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Nelson G, Morris ML. Electrosurgery in the Gastrointestinal Suite: Knowledge Is Power. Gastroenterol Nurs. 2015;38:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Heller LI. Surgical electrocautery and the runaway pacemaker syndrome. Pacing Clin Electrophysiol. 1990;13:1084-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Peters RW, Gold MR. Reversible prolonged pacemaker failure due to electrocautery. J Interv Card Electrophysiol. 1998;2:343-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Reper P, van Looy K. Chest physiotherapy using intrapulmonary percussive ventilation to treat persistent atelectasis in hypoxic patients after smoke inhalation. Burns. 2013;39:192-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Sachdeva P, Patel BG, Patel BK. Drug use in pregnancy; a point to ponder! Indian J Pharm Sci. 2009;71:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Apfelbaum JL, Hagberg CA, Connis RT, Abdelmalak BB, Agarkar M, Dutton RP, Fiadjoe JE, Greif R, Klock PA, Mercier D, Myatra SN, O'Sullivan EP, Rosenblatt WH, Sorbello M, Tung A. 2022 American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. Anesthesiology. 2022;136:31-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 691] [Article Influence: 172.8] [Reference Citation Analysis (0)] |
| 31. | Ing C, Sun M, Olfson M, DiMaggio CJ, Sun LS, Wall MM, Li G. Age at Exposure to Surgery and Anesthesia in Children and Association With Mental Disorder Diagnosis. Anesth Analg. 2017;125:1988-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 32. | Kern J, Guinn A, Mehta P. Procedural sedation and analgesia in the emergency department. Emerg Med Pract. 2022;24:1-24. [PubMed] |
| 33. | Thompson AM, Wright DJ, Murray W, Ritchie GL, Burton HD, Stonebridge PA. Analysis of 153 deaths after upper gastrointestinal endoscopy: room for improvement? Surg Endosc. 2004;18:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Chatelanat O, Spahr L, Bichard P, Bochatay L, Goossens N, Bastid C, Frossard JL. Evaluation of 30-day mortality in patients undergoing gastrointestinal endoscopy in a tertiary hospital: a 3-year retrospective survey. BMJ Open Gastroenterol. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 35. | Ilagan-Ying YC, Almeida MN, Kahler-Quesada A, Ying L, Hughes ML, Do A, Hung KW. Increased Mortality in Patients Undergoing Inpatient Endoscopy During the Early COVID-19 Pandemic. Dig Dis Sci. 2022;67:5053-5062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Tsunoda SM, Velez RL, von Moltke LL, Greenblatt DJ. Differentiation of intestinal and hepatic cytochrome P450 3A activity with use of midazolam as an in vivo probe: effect of ketoconazole. Clin Pharmacol Ther. 1999;66:461-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 204] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA, O'Mara EM Jr, Hall SD. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 285] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Greif R, Laciny S, Mokhtarani M, Doufas AG, Bakhshandeh M, Dorfer L, Sessler DI. Transcutaneous electrical stimulation of an auricular acupuncture point decreases anesthetic requirement. Anesthesiology. 2002;96:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Holazo AA, Winkler MB, Patel IH. Effects of age, gender and oral contraceptives on intramuscular midazolam pharmacokinetics. J Clin Pharmacol. 1988;28:1040-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Gourlay GK, Kowalski SR, Plummer JL, Cousins MJ, Armstrong PJ. Fentanyl blood concentration-analgesic response relationship in the treatment of postoperative pain. Anesth Analg. 1988;67:329-337. [PubMed] |
| 41. | Kilic ET, Sayar S, Kahraman R, Ozdil K. The effects of obesity on sedation-related outcomes of advanced endoscopic procedures. North Clin Istanb. 2019;6:321-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1324] [Article Influence: 55.2] [Reference Citation Analysis (1)] |
| 43. | ASGE Ensuring Safety in the Gastrointestinal Endoscopy Unit Task Force, Calderwood AH, Chapman FJ, Cohen J, Cohen LB, Collins J, Day LW, Early DS. Guidelines for safety in the gastrointestinal endoscopy unit. Gastrointest Endosc. 2014;79:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 44. | American Association for Study of Liver Diseases; American College of Gastroenterology; American Gastroenterological Association Institute; American Society for Gastrointestinal Endoscopy; Society for Gastroenterology Nurses and Associates, Vargo JJ, DeLegge MH, Feld AD, Gerstenberger PD, Kwo PY, Lightdale JR, Nuccio S, Rex DK, Schiller LR. Multisociety sedation curriculum for gastrointestinal endoscopy. Gastrointest Endosc. 2012;76:e1-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 45. | Hayee B, Dunn J, Loganayagam A, Wong M, Saxena V, Rowbotham D, McNair A. Midazolam with meperidine or fentanyl for colonoscopy: results of a randomized trial. Gastrointest Endosc. 2009;69:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD Jr; AGA Institute. AGA Institute review of endoscopic sedation. Gastroenterology. 2007;133:675-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 320] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 47. | Waring JP, Baron TH, Hirota WK, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Faigel DO; American Society for Gastrointestinal Endoscopy, Standards of Practice Committee. Guidelines for conscious sedation and monitoring during gastrointestinal endoscopy. Gastrointest Endosc. 2003;58:317-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 225] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 48. | Riphaus A, Gstettenbauer T, Frenz MB, Wehrmann T. Quality of psychomotor recovery after propofol sedation for routine endoscopy: a randomized and controlled study. Endoscopy. 2006;38:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Laluna L, Allen ML, Dimarino AJ Jr. The comparison of midazolam and topical lidocaine spray versus the combination of midazolam, meperidine, and topical lidocaine spray to sedate patients for upper endoscopy. Gastrointest Endosc. 2001;53:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Zakko SF, Seifert HA, Gross JB. A comparison of midazolam and diazepam for conscious sedation during colonoscopy in a prospective double-blind study. Gastrointest Endosc. 1999;49:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Sipe BW, Rex DK, Latinovich D, Overley C, Kinser K, Bratcher L, Kareken D. Propofol versus midazolam/meperidine for outpatient colonoscopy: administration by nurses supervised by endoscopists. Gastrointest Endosc. 2002;55:815-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Corôa MCP, Mendes PFS, Baia-da-Silva DC, Souza-Monteiro D, Ferreira MKM, Braga GLC, Damasceno TV, Perdigão JM, Lima RR. What Is Known about Midazolam? A Bibliometric Approach of the Literature. Healthcare (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Wernli KJ, Brenner AT, Rutter CM, Inadomi JM. Risks Associated With Anesthesia Services During Colonoscopy. Gastroenterology. 2016;150:888-94; quiz e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 54. | Abraham N, Barkun A, Larocque M, Fallone C, Mayrand S, Baffis V, Cohen A, Daly D, Daoud H, Joseph L. Predicting which patients can undergo upper endoscopy comfortably without conscious sedation. Gastrointest Endosc. 2002;56:180-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Zakeri N, Coda S, Webster S, Howson W, Thillainayagam AV. Risk factors for endoscopic sedation reversal events: a five-year retrospective study. Frontline Gastroenterol. 2015;6:270-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 56. | Jurell KR, O'Connor KW, Slack J, Fraiz J, Shaar CJ, Kent L, Callon R. Effect of supplemental oxygen on cardiopulmonary changes during gastrointestinal endoscopy. Gastrointest Endosc. 1994;40:665-670. [PubMed] |
| 57. | Xu L, Li Y, Zheng H, Wang R. Optimizing perioperative anesthesia strategies for safety and high-quality during painless gastrointestinal endoscopy diagnosis and treatment. APS. 2024;2:21. [DOI] [Full Text] |
| 58. | Saunders R, Davis JA, Kranke P, Weissbrod R, Whitaker DK, Lightdale JR. Clinical and economic burden of procedural sedation-related adverse events and their outcomes: analysis from five countries. Ther Clin Risk Manag. 2018;14:393-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/