Published online May 16, 2024. doi: 10.4253/wjge.v16.i5.259
Revised: March 12, 2024
Accepted: April 22, 2024
Published online: May 16, 2024
Processing time: 98 Days and 23.6 Hours
Esophageal chromoendoscopy with iodine solution is important for detecting early esophageal cancer. The effect of routine treatment for lesions lightly stained with Lugol’s iodine solution is limited, and the addition of natural substances to a regular diet is becoming increasingly common. Vinegar has antitumor effects as reported in previous studies.
To evaluate whether vinegar supplementation could improve the prognosis of patients with lightly stained esophageal lesions.
This prospective single-centre trial included consecutive patients with lightly stained lesions between June 2020 and April 2022. Patients in the experimental group received increased amounts of vinegar for 6 months. The primary outcome of the study was the clinical therapeutic effect. Complications related to vinegar ingestion and adverse events were also recorded in detail.
A total of 166 patients were included in the final analysis. There was no significant difference in the baseline data between the two groups. Intention-to-treat (ITT) analysis demonstrated that the rates at which endoscopic characteristics improved were 33.72% in the experimental group and 20.00% in the conventional group (P = 0.007); and the rates at which biopsy pathology improved were 19.77% and 8.75%, respectively (P = 0.011). Additional vinegar consumption had a statistically protective effect on the rate at which endoscopic characteristics improved [hazard ratio (HR) ITT = 2.183, 95%CI: 1.183-4.028; HRper-protocol (PP) = 2.307, 95%CI: 1.202-4.426] and biopsy pathology improved (HRITT = 2.931, 95%CI: 1.212-7.089; HRPP = 3.320, 95%CI: 1.295-8.507). No statistically significant effect of increased vinegar consumption on preventing high-grade intraepithelial neoplasia or early cancer was observed (HRITT = 0.382, 95%CI: 0.079-1.846; HRPP = 0.382, 95%CI: 0.079-1.846). The subgroup analyses indicated that the overall therapeutic improvement of endoscopic characteristics and biopsy pathology seemed more obvious in older (age > 60) male patients with small lesions (lesion size ≤ 0.5 cm). Three patients in the experimental group reported acid regurgitation and heartburn. No adverse event during gastroscopy were recorded during follow-up.
A moderately increased ingestion of vinegar could not directly reduce the risk of esophageal cancer in the mucosa dysplasia population, but it improved the endoscopic characteristics and ameliorated the biopsy pathology to a certain extent. Further research is needed to verify the effect of nutritional intervention on precancerous esophageal lesions.
Core Tip: Esophageal lesions stained lightly with iodine solution may progress pathologically even though they have a relatively better prognosis. Vinegar was thought to have an antitumor effect according to previous studies. However, its effect on lesion progression is still unclear. In the present study, we reported that moderate vinegar consumption improved the prognosis of several esophageal lesions lightly stained with Lugol’s iodine solution at a tertiary referral endoscopy centre in China.
- Citation: Gao Y, Ye LS, Li X, Yu B, Liao K, Xie J, Du J, Zhang QY, Hu B. Effect of vinegar supplementation on patients with esophageal lesions lightly stained with Lugol’s iodine solution: Prospective single-centre trial. World J Gastrointest Endosc 2024; 16(5): 259-272
- URL: https://www.wjgnet.com/1948-5190/full/v16/i5/259.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i5.259
Esophageal cancer is the eighth most common malignancy worldwide with more than 600000 new cases diagnosed annually[1]. The poor prognosis and increasing incidence of esophageal cancer highlight the need for improved detection and prediction methods[2-4]. Esophageal chromoendoscopy with iodine solution is an important diagnostic method for detecting superficial esophageal cancer[5]. The esophageal mucosa is considered abnormal when there are “unstained” or “lightly stained” lesions[6]. Generally, unstained areas indicate high-grade intraepithelial neoplasia (HGIN) or early cancer, while lightly stained areas generally indicate inflammation, squamous epithelial hyperplasia, low-grade intraepithelial neoplasia (LGIN), etc.[7]. Endoscopic resection is recommended for treating HGIN and early cancer, while continuous surveillance is recommended mainly for treating LGIN and squamous hyperplasia because these conditions are thought to have relatively better prognoses[3,8]. Most authorities recommend increased endoscopic surveillance with biopsies and a healthy diet for these lightly stained lesions, but the progression of these lesions cannot be ignored[9,10].
Previous laboratory experiments and trials have demonstrated that vinegar can block the synthesis of N nitroso compounds and proline nitrosamines, which can induce cancer in the human body, making this agent capable of preventing cancer[11-14]. Retrospective clinical studies have also reported that vinegar consumption is associated with a decreased risk for esophageal cancer[15,16]. However, no prospective clinical study has verified these findings.
This prospective clinical trial was designed to evaluate whether increased vinegar consumption could improve the prognosis of patients with lightly stained esophageal lesions.
This prospective clinical trial was conducted at the Endoscopy Center of West China Hospital, Sichuan University, China. The study protocol was approved by the Biomedical Research Ethics Committee, West China Hospital of Sichuan University (No. HX-IRB-AF-03-V3.0) and was registered in the Chinese Clinical Trial Registry (No. ChiCTR1900024686).
Patients were enrolled in this study after receiving endoscopic evaluations from June 1, 2020, to April 30, 2022 , at a tertiary referral endoscopy centre in China. In accordance with the inclusion criteria, patients were selected as follows: (1) Patients aged 18–80 years; (2) underwent gastroscopy and biopsy histopathology; and (3) had esophageal lesions lightly stained with Lugol’s iodine solution. The exclusion criteria prohibited inclusion of the following patients: (1) Patients were pregnant or lactating; (2) patients who had an allergy to iodine and its derivatives; (3) patients who had a tumour requiring surgery, chemotherapy or radiotherapy; (4) patients who had reflux esophagitis; and (5) patients who refused to participate in the study or were unable to provide informed consent. All patients received a preoperative consultation with a detailed explanation of the pros and cons of different approaches, including endoscopic resection, increased vinegar intake and surveillance. Written informed consent was obtained from all patients or their legal representatives.
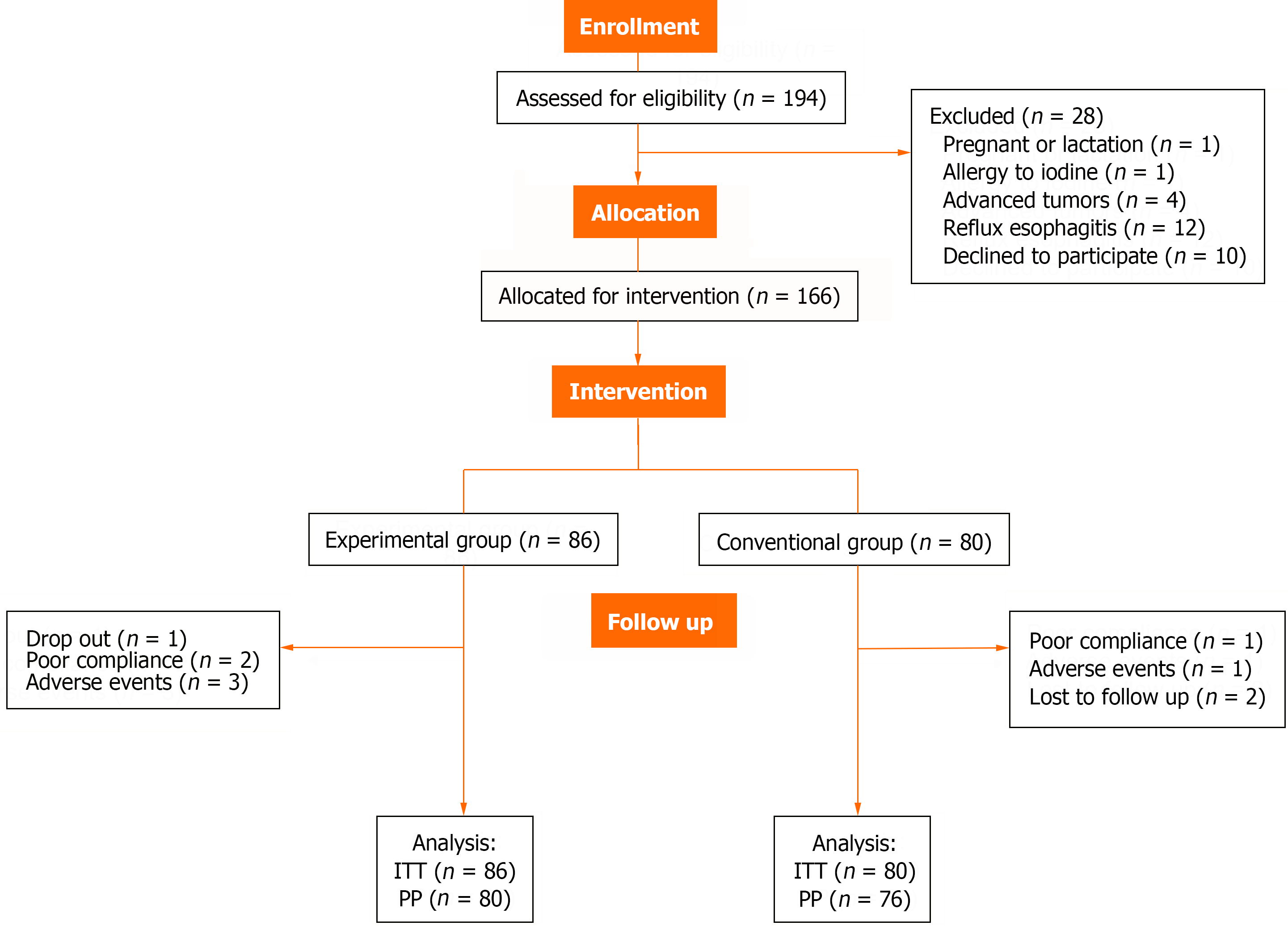
A total of 166 patients who underwent gastroscopy and had histopathological proof of lightly stained esophageal lesions were recruited for the study and assigned to two groups according to patient choice: The experimental group and the conventional group. Allocation of eligible patients was completed by two nurses who were not directly involved in the data analysis or patient enrolment. Investigators who were involved in the data analysis and endoscopists were blinded to the group assignments until all the data collection and data queries had been completed and the database was locked.
Patients assigned to the experimental group received 20 mg of 9% Baoning vinegar (Sichuan Baoning Vinegar Co., Ltd., Langzhong, China) three times a day (tid) diluted with 50 mL of warm water for 6 months. The amount of vinegar used was determined according to previous retrospective reports, which showed that the incidence rate of esophageal cancer in people who consumed ≥ 40 g/w vinegar was significantly lower than that in people who consumed 0-39 g/w vinegar[16]. The use of other drugs was stopped when the patient consumed more vinegar. Patients assigned to the conventional group received the same health education to quit smoking, stop drinking, and avoid hot and spicy food.
The primary outcome of the study was the clinical therapeutic effect, which was classified into the following four categories. Endoscopic characteristics improved is defined as a reduction of more than 50% in the maximum diameter of the lesion, or a reduction of more than 50% in the number of lesions. Endoscopic characteristics deteriorated is defined as an increase of more than 50% in the maximum diameter of the lesion or an increase of more than 50% in the number of lesions. Biopsy pathology improved is defined as less malignant pathological results than before. Biopsy pathology deteriorated is defined as more malignant pathological results than before. The incidence of lesions progressing to HGIN or early cancer in patients was also concerned. The rates were determined by both intention-to-treat (ITT)- and per-protocol (PP)-based analyses. All enrolled patients were included in the ITT analysis, but the PP analysis excluded those patients who dropped out due to side effects, loss to follow-up, or poor compliance.
During the experiment, members of the quality supervision team contacted the patients through interviews and phone calls every month to remind them to consume vinegar. The investigator recorded all complications related to vinegar therapy, such as acid regurgitation, heartburn, nausea, vomiting, taste abnormalities, abdominal pain, abdominal distension, and diarrhoea[12]. The compliance of patients with medication and incidence of complications was assessed by conducting a questionnaire at the following points: Before treatment, during treatment (1 wk, 2 wk, 3 wk, 1 month, 3 months), and after treatment (6 months). The gastroscopy and biopsy histopathology re-examinations were arranged after treatment completion and follow-up. Adverse events during gastroscopy were also recorded and were defined as bleeding, perforation or severe cardiopulmonary accidents[17]. After treatment completion, the patients were followed up monthly, hospital medical records were reviewed, and endoscopic and pathological examination results were collected. All patients were followed until December 13, 2023. The time to show treatment effect was also recorded, and deterioration of the lesion was treated with caution. A team of senior doctors conducted a review to ultimately determine the changes in the lesions and ensure the accuracy of the judgement. Two people completed the data analysis in parallel to ensure the accuracy of the data.
Based on previous studies showing that increased vinegar consumption reduces the incidence of esophageal cancer[6,15], we expected a difference in the incidence rate of esophageal cancer between health education therapy combined with increased vinegar ingestion and health education therapy alone (10% vs 25%). The model has a power of 80% and a two-sided significance level of 0.05 with an assumed 10% dropout rate. Survival analysis based on the Log-rank test was performed with a final sample size of 154 patients (77 per group). The full analysis set should be as close as possible to the ITT set. The standards and population of the PP dataset was to be finalized after data-blinding verification. The direct deletion method was used to treat missing data.
In this study, the demographic and clinical characteristics of the patients are summarized as the mean ± SD or median (interquartile range) based on their distribution type. The incidence rates of therapeutic outcomes are expressed in terms of the number of patients and percentage. Qualitative variables were compared using the Chi-squared test, while Student’s t-test was used for quantitative variables. The specific test methods used are listed below the table. A Cox proportional hazard model was used to estimate the hazard ratio (HR) and 95%CI. Comparisons of clinical therapeutic effects between two groups were performed using the Log-rank test, Kaplan-Meier curves were generated, and a two-tailed P value < 0.05 was considered to indicate statistical significance. Subgroup analyses were performed by age, gender, lesion size, lesion location and biopsy characteristics[18-20]. All the statistical analyses were performed by blinded professional statisticians with IBM SPSS for Mac (version 26.0 statistical software package; Armonk, NY: IBM Corp.).
A total of 194 patients who fulfilled the inclusion criteria were enrolled in this trial, with 86 patients in the experimental group and 80 patients in the conventional group were included in the ITT analysis. Ten patients (6.0%) were excluded from the PP analysis. Three patients in the experimental group discontinued treatment because of severe acid regur
| Characteristics | Experimental group | Conventional group | P value |
| No. of participants | 86 | 80 | |
| Age, mean ± SD, yr | 59.84 ± 8.40 | 59.75 ± 9.21 | 0.9491 |
| BMI, mean ± SD, kg/m2 | 23.89 ± 3.35 | 23.49 ± 2.29 | 0.3721 |
| Sex | 0.7982 | ||
| Male | 51 (59.30) | 49 (61.25) | |
| Female | 35 (40.70) | 31 (38.75) | |
| Basic diseases | |||
| Diabetes | 9 (10.47) | 8 (10.00) | 0.9212 |
| Hypertension | 14 (16.28) | 6 (7.50) | 0.0832 |
| Coronary heart disease | 4 (4.65) | 4 (5.00) | 0.9162 |
| Family history of esophageal cancer or stomach cancer | 0.8002 | ||
| No | 67 (77.91) | 61 (76.25) | |
| Yes | 19 (22.09) | 19 (23.75) | |
| Smoking | 0.8992 | ||
| No-smoker | 46 (53.49) | 42 (52.50) | |
| Smoker | 40 (46.51) | 38 (47.50) | |
| Smoking index | 0.6182 | ||
| ≤ 200 | 15 (17.44) | 11 (13.75) | |
| 200-400 | 14 (16.28) | 13 (16.25) | |
| ≥ 400 | 11 (12.79) | 14 (17.50) | |
| Alcohol drinking | 0.7122 | ||
| No-drinker | 39 (45.35) | 34 (42.50) | |
| Drinker | 47 (54.65) | 46 (57.50) | |
| Alcohol ingestion, g/d | 0.8332 | ||
| ≤ 20 | 23 (26.74) | 20 (25.00) | |
| 20-60 | 13 (15.12) | 13 (16.25) | |
| ≥ 60 | 11 (12.79) | 13 (16.25) | |
| Prefer hot dishes/hot tea | 0.4592 | ||
| No | 50 (59.14) | 51 (63.75) | |
| Yes | 36 (41.86) | 29 (36.25) | |
| Prefer spicy food | 0.2272 | ||
| No | 76 (88.37) | 75 (93.75) | |
| Yes | 10 (11.63) | 5 (6.25) | |
| Prefer pickled dishes | 0.0952 | ||
| No | 77 (89.53) | 77 (96.25) | |
| Yes | 9 (10.47) | 3 (3.75) | |
| Multiple Lugol’s voiding lesions | 0.2082 | ||
| No | 52 (65.00) | 57 (75.00) | |
| Yes | 28 (35.00) | 19 (25.00) | |
| Lesion location | 0.1972 | ||
| Upper thoracic esophagus | 12 (13.95) | 8 (10.00) | |
| Middle thoracic esophagus | 44 (51.16) | 52 (65.00) | |
| Lower thoracic esophagus | 30 (34.88) | 20 (25.00) | |
| Maximum diameter | 0.1342 | ||
| Lesion size ≤ 0.5 cm | 65 (75.58) | 54 (67.50) | |
| Lesion size > 0.5 cm | 21 (24.42) | 26 (32.50) | |
| Morphology | 0.3242 | ||
| Quasi circular | 31 (36.05) | 30 (37.50) | |
| Bar-type | 3 (3.49) | 7 (8.75) | |
| Irregular shape | 52 (60.47) | 43 (53.75) | |
| Biopsy pathology | 0.5062 | ||
| Squamous epithelial hyperplasia | 77 (89.53) | 74 (92.50) | |
| Low grade intraepithelial neoplasia | 9 (10.47) | 6 (7.50) | |
| Follow-up time | 28.92 ± 6.04 | 30.48 ± 5.88 | 0.09581 |
| Characteristics | Experimental group | Conventional group | P value |
| No. of participants | 80 | 76 | |
| Age, mean ± SD, yr | 59.69 ± 8.47 | 59.32 ± 9.22 | 0.7931 |
| BMI, mean ± SD, kg/m2 | 23.93 ± 3.43 | 23.49 ± 2.28 | 0.0701 |
| Sex | 0.5352 | ||
| Male | 49 (61.25) | 47 (61.84) | |
| Female | 31 (38.75) | 29 (38.16) | |
| Basic diseases | |||
| Diabetes | 9 (11.25) | 8 (10.53) | 0.5452 |
| Hypertension | 13 (16.25) | 6 (7.89) | 0.1432 |
| Coronary heart disease | 3 (3.75) | 4 (5.26) | 0.9492 |
| Family history of esophageal cancer or stomach cancer | 0.9842 | ||
| No | 62 (77.50) | 59 (77.63) | |
| Yes | 18 (22.50) | 17 (22.37) | |
| Smoking | 0.8632 | ||
| No-smoker | 41 (51.25) | 40 (52.63) | |
| Smoker | 39 (48.75) | 36 (47.37) | |
| Smoking index | 0.6872 | ||
| ≤ 200 | 14 (17.50) | 10 (13.16) | |
| 200-400 | 14 (17.50) | 13 (17.11) | |
| ≥ 400 | 11 (13.75) | 13 (17.11) | |
| Alcohol drinking | 0.8362 | ||
| No-drinker | 35 (45.75) | 32 (42.11) | |
| Drinker | 45 (56.25) | 44 (57.89) | |
| Alcohol ingestion, g/d | 0.9362 | ||
| ≤ 20 | 21 (26.25) | 19 (25.00) | |
| 20-60 | 13 (16.25) | 13 (17.11) | |
| ≥ 60 | 11 (13.75) | 12 (15.79) | |
| Prefer hot dishes/hot tea | 0.2872 | ||
| No | 46 (57.50) | 50 (65.79) | |
| Yes | 34 (42.50) | 26 (34.21) | |
| Prefer spicy food | 0.2102 | ||
| No | 70 (8.75) | 71 (93.42) | |
| Yes | 10 (12.50) | 5 (6.58) | |
| Prefer pickled dishes | 0.0872 | ||
| No | 71 (88.75) | 73 (96.05) | |
| Yes | 9 (11.25) | 3 (3.95) | |
| Multiple Lugol’s voiding lesions | 0.30182 | ||
| No | 54 (67.50) | 57 (75.00) | |
| Yes | 26 (32.50) | 19 (25.00) | |
| Lesion location | 0.1942 | ||
| Upper thoracic esophagus | 12 (15.00) | 8 (10.53) | |
| Middle thoracic esophagus | 39 (48.75) | 48 (63.16) | |
| Lower thoracic esophagus | 29 (36.25) | 20 (26.32) | |
| Maximum diameter | 0.3792 | ||
| Lesion size ≤ 0.5 cm | 60 (75.00) | 52 (68.42) | |
| Lesion size > 0.5 cm | 20 (25.00) | 24 (31.58) | |
| Morphology | 0.5182 | ||
| Quasi circular | 29 (36.25) | 28 (36.84) | |
| Bar-type | 3 (3.75) | 6 (7.89) | |
| Irregular shape | 48 (60.00) | 42 (55.26) | |
| Biopsy pathology | 0.4772 | ||
| Squamous epithelial hyperplasia | 71 (88.75) | 70 (92.11) | |
| Low grade intraepithelial neoplasia | 9 (11.25) | 6 (7.89) | |
| Follow-up time | 29.53 ± 5.73 | 31.00 ± 5.52 | 0.5141 |
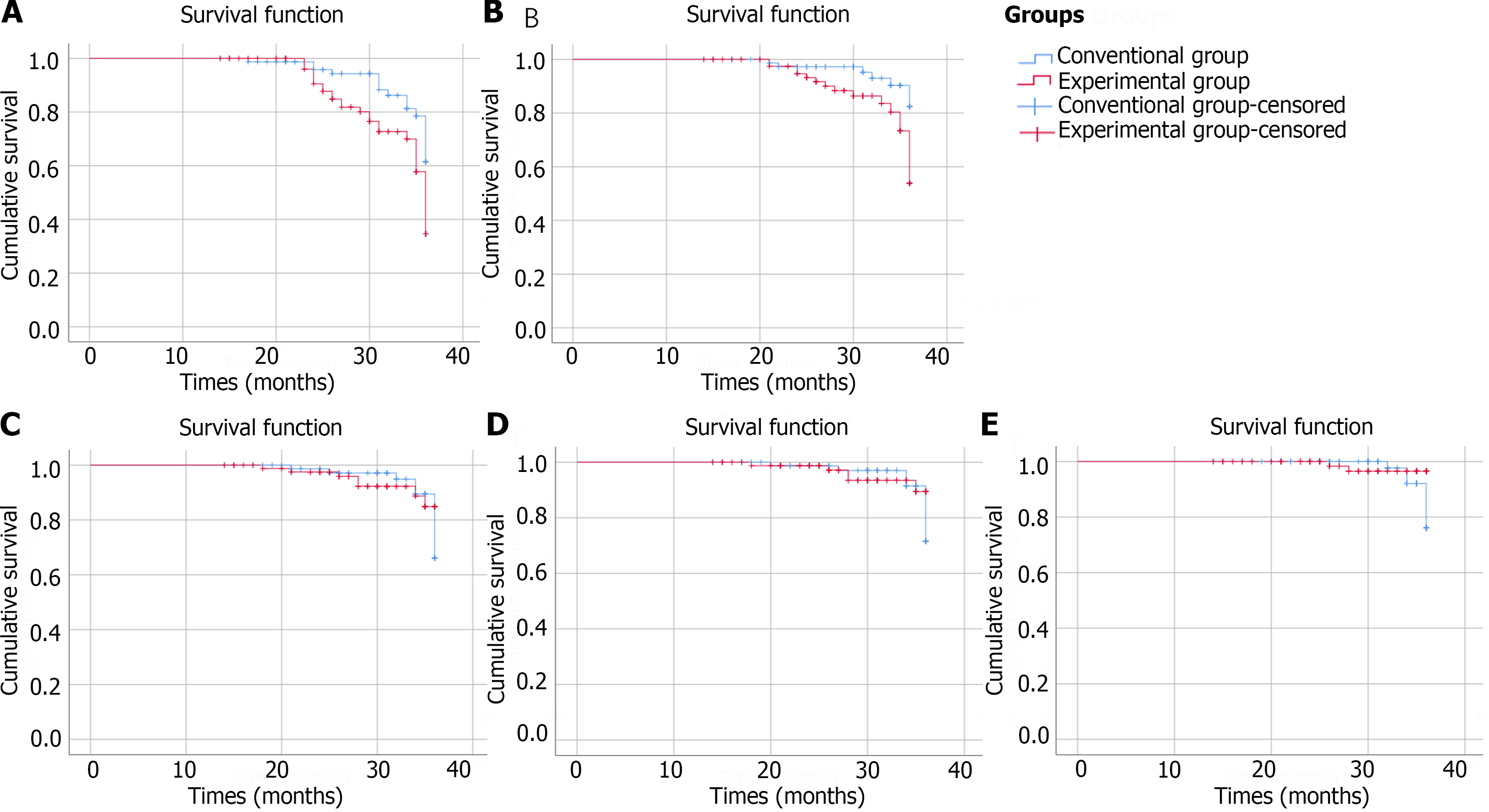
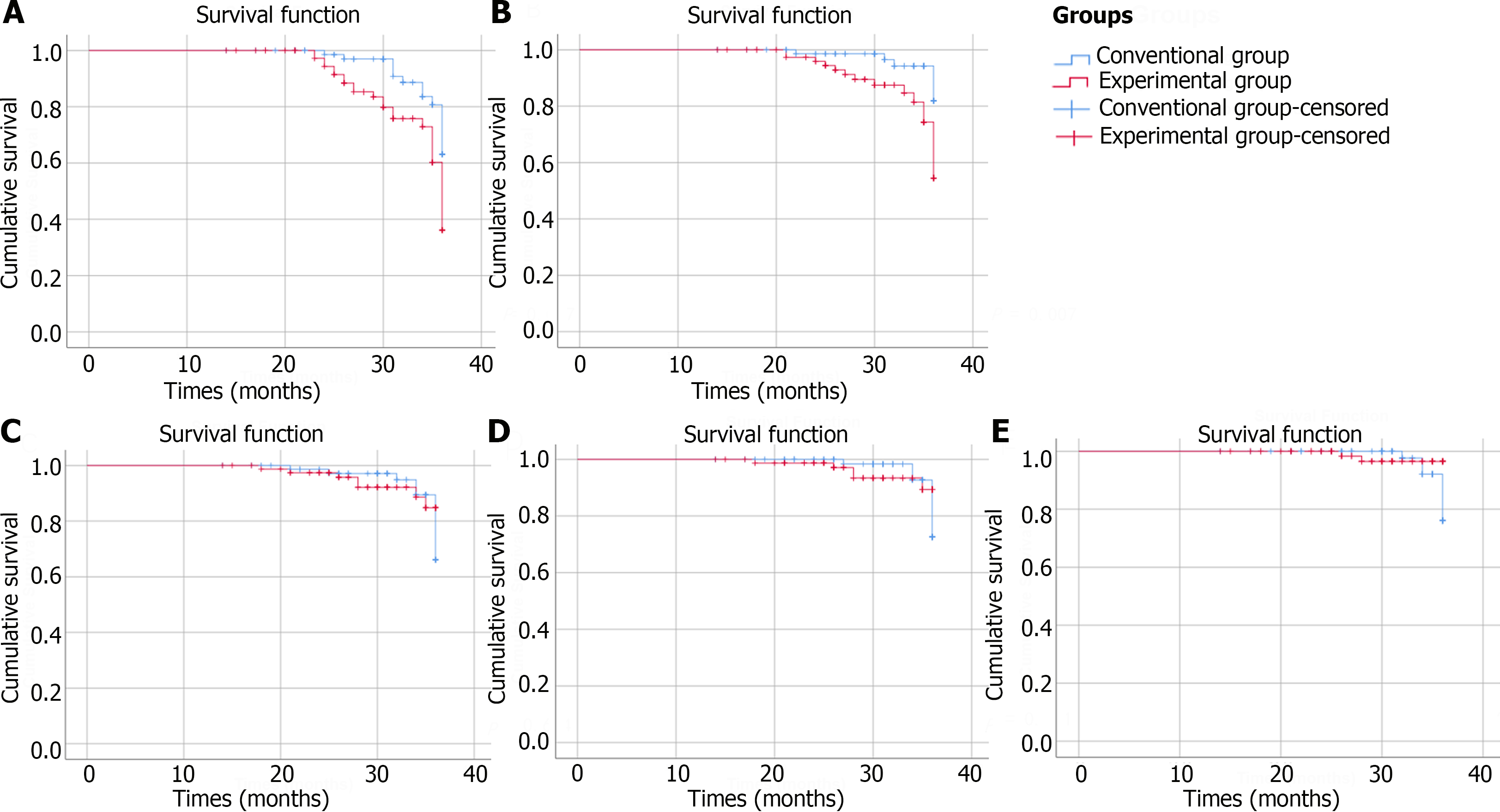
ITT analysis demonstrated that the incidence rates of endoscopic characteristics improved were 33.72% in the experimental group and 20.00% in the conventional group (P = 0.007). PP analysis indicated that 32.50% of the patients in the experimental group and 18.42% of the patients in the conventional group achieved endoscopic improved (P = 0.007). ITT analysis demonstrated that the incidence rates of biopsy pathology improved were 19.77% in the experimental group and 8.75% in the conventional group (P = 0.011). PP analysis indicated that the percentage of patients whose biopsy pathology improved was 20.00% in the experimental group and 7.89% in the conventional group (P = 0.007). Both ITT and PP analyses revealed no significant differences in the incidence rates of endoscopic characteristics deteriorate or biopsy pathology deteriorate between the experimental group and conventional group. Figures 2 and 3 depict the Kaplan-Meier survival curves for each group in the ITT analysis or PP analysis, respectively, considering the time interval before progression or improvement of each patient.
An increase in vinegar consumption had a statistically protective effect on the rate of improvement of endoscopic characteristics (HRITT = 2.183, 95%CI: 1.183-4.028; HRPP = 2.307, 95%CI: 1.202-4.426), and biopsy pathology improved (HRITT = 2.931, 95%CI: 1.212-7.089; HRPP = 3.320, 95%CI: 1.295-8.507). However, no statistically significant protective effect of increasing vinegar consumption on preventing the risk of developing HGIN or early cancer was observed (HRITT = 0.382, 95%CI: 0.079-1.846; HRPP = 0.382, 95%CI: 0.079-1.846). After adjusting for age, gender, lesion size, lesion location and biopsy characteristics, an impact of vinegar consumption on decreasing cancer risk was not observed (Tables 3 and 4).
| Therapeutic effect | HR (95%CI) | Multivariable adjusted HR (95%CI)1 |
| Endoscopic characteristics improved | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 2.183 (1.183, 4.028)a | 2.515 (1.318, 4.800)b |
| Endoscopic characteristics deteriorate | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 0.791 (0.305, 2.048) | 0.976 (0.920, 1.035) |
| Biopsy pathology improved | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 2.931 (1.212, 7.089)a | 2.710 (1.066, 6.891)a |
| Biopsy pathology deteriorate | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 0.690 (0.230, 2.069) | 0.983 (0.911, 1.060) |
| HGIN or early cancer | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 0.382 (0.079, 1.846) | 0.833 (0.125, 5.560) |
| Therapeutic effect | HR (95%CI) | Multivariable adjusted HR (95%CI)1 |
| Endoscopic characteristics improved | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 2.307 (1.202, 4.426)a | 2.545 (1.282, 5.052)b |
| Endoscopic characteristics deteriorate | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 0.793 (0.306, 2.055) | 0.979 (0.923, 1.037) |
| Biopsy pathology improved | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 3.320 (1.295, 8.507)a | 3.186 (1.179, 8.605)a |
| Biopsy pathology deteriorate | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 0.795 (0.259, 2.440) | 0.969 (0.896, 1.048) |
| HGIN or early cancer | ||
| Conventional group | 1.00 | 1.00 |
| Experimental group | 0.382 (0.079, 1.846) | 0.750 (0.140, 4.015) |
The subgroup analyses (Tables 5 and 6) indicated that the overall therapeutic improvement in endoscopic characteristics and biopsy pathology seemed more obvious in older (age > 60) male patients with small lesions (lesion size ≤ 0.5 cm).
| Subgroup | Number (n) | Rate (%) | HR (95%CI) | P value |
| Male (n = 100) | 0.008 | |||
| Conventional group (n = 49) | 7 | 14.29 | 1.00 | |
| Experimental group (n = 51) | 19 | 37.25 | 3.232 (1.353, 7.719) | |
| Female (n = 66) | 0.542 | |||
| Conventional group (n = 31) | 9 | 29.03 | 1.00 | |
| Experimental group (n = 35) | 10 | 28.57 | 1.324 (0.537, 3.263) | |
| Age > 60 (n = 82) | 0.008 | |||
| Conventional group (n = 42) | 7 | 16.67 | 1.00 | |
| Experimental group (n = 40) | 15 | 37.50 | 3.425 (1.380, 8.497) | |
| Age ≤ 60 (n = 84) | ||||
| Conventional group (n = 38) | 9 | 23.68 | 1.00 | 0.395 |
| Experimental group (n = 46) | 14 | 30.43 | 1.438 (0.622, 3.323) | |
| Lesion size ≤ 0.5 cm (n = 119) | 0.010 | |||
| Conventional group (n = 54) | 10 | 18.52 | 1.00 | |
| Experimental group (n = 65) | 24 | 36.92 | 2.708 (1.263, 5.806) | |
| Lesion size > 0.5 cm (n = 47) | 0.794 | |||
| Conventional group (n = 26) | 6 | 23.08 | 1.00 | |
| Experimental group (n = 21) | 5 | 23.81 | 1.181 (0.339, 4.119) | |
| Lesion in upper thoracic esophagus (n = 20) | 0.123 | |||
| Conventional group (n = 8) | 2 | 25.00 | 1.00 | |
| Experimental group (n = 12) | 5 | 41.67 | 3.686 (0.701, 19.366) | |
| Lesion in middle thoracic esophagus (n = 96) | 0.245 | |||
| Conventional group (n = 52) | 12 | 23.08 | 1.00 | |
| Experimental group (n = 44) | 13 | 29.55 | 1.596 (0.726, 3.506) | |
| Lesion in lower thoracic esophagus (n = 50) | 0.060 | |||
| Conventional group (n = 20) | 2 | 10.00 | 1.00 | |
| Experimental group (n = 30) | 11 | 36.67 | 4.265 (0.943, 19.298) | |
| Undergone esophageal ESD previously (n = 65) | 0.078 | |||
| Conventional group (n = 29) | 5 | 17.24 | 1.00 | |
| Experimental group (n = 36) | 12 | 33.3 | 2.386 (0.908, 6.267) | |
| Not undergone esophageal ESD previously (n = 101) | 0.076 | |||
| Conventional group (n = 51) | 11 | 21.57 | 1.00 | |
| Experimental group (n = 50) | 17 | 34.00 | 2.100 (0.924, 4.772) | |
| Lesion biopsy: Squamous epithelial hyperplasia (n = 151) | 0.009 | |||
| Conventional group (n = 74) | 14 | 18.92 | 1.00 | |
| Experimental group (n = 77) | 26 | 33.77 | 2.380 (1.238, 4.575) | |
| Lesion biopsy: Low grade intraepithelial neoplasia (n = 15) | 0.814 | |||
| Conventional group (n = 6) | 2 | 33.3 | 1.00 | |
| Experimental group (n = 9) | 3 | 33.3 | 1.241 (0.206, 7.479) |
| Subgroup | Number (n) | Rate (%) | HR (95%CI) | P value |
| Male (n = 100) | 0.043 | |||
| Conventional group (n = 49) | 4 | 8.16 | 1.00 | |
| Experimental group (n = 51) | 11 | 21.57 | 3.285 (1.040, 10.375) | |
| Female (n = 66) | 0.227 | |||
| Conventional group (n = 31) | 3 | 9.68 | 1.00 | |
| Experimental group (n = 35) | 6 | 17.14 | 2.356 (0.587, 9.449) | |
| Age > 60 (n = 82) | 0.035 | |||
| Conventional group (n = 42) | 4 | 9.52 | 1.00 | |
| Experimental group (n = 40) | 9 | 22.50 | 3.609 (1.091, 11.933) | |
| Age ≤ 60 (n = 84) | 0.187 | |||
| Conventional group (n = 38) | 3 | 7.89 | 1.00 | |
| Experimental group (n = 46) | 8 | 17.39 | 2.444 (0.648, 9.216) | |
| Lesion size ≤ 0.5 cm (n = 119) | 0.042 | |||
| Conventional group (n = 54) | 3 | 5.56 | 1.00 | |
| Experimental group (n = 65) | 14 | 21.54 | 3.199 (1.042, 9.820) | |
| Lesion size > 0.5cm (n = 47) | 0.214 | |||
| Conventional group (n = 26) | 4 | 15.38 | 1.00 | |
| Experimental group (n = 21) | 3 | 14.29 | 2.626 (0.573, 12.033) | |
| Lesion in upper thoracic esophagus (n = 20) | 0.440 | |||
| Conventional group (n = 8) | 1 | 12.50 | 1.00 | |
| Experimental group (n = 12) | 2 | 16.67 | 2.610 (0.229, 29.702) | |
| Lesion in middle thoracic esophagus (n = 96) | 0.108 | |||
| Conventional group (n = 52) | 5 | 9.62 | 1.00 | |
| Experimental group (n = 44) | 8 | 18.18 | 2.510 (0.817, 7.708) | |
| Lesion in lower thoracic esophagus (n = 50) | 0.112 | |||
| Conventional group (n = 20) | 1 | 5.00 | 1.00 | |
| Experimental group (n = 30) | 7 | 23.33 | 5.485 (0.673, 44.705) | |
| Undergone esophageal ESD previously (n = 65) | 0.088 | |||
| Conventional group (n = 29) | 2 | 6.90 | 1.00 | |
| Experimental group (n = 36) | 5 | 13.89 | 4.462 (0.799, 24.917) | |
| Not undergone esophageal ESD previously (n = 101) | 0.135 | |||
| Conventional group (n = 51) | 5 | 9.80 | 1.00 | |
| Experimental group (n = 50) | 12 | 24.00 | 2.198 (0.783, 6.171) | |
| Lesion biopsy: Squamous epithelial hyperplasia (n = 151) | 0.056 | |||
| Conventional group (n = 74) | 5 | 6.76 | 1.00 | |
| Experimental group (n = 77) | 11 | 14.29 | 2.820 (0.974, 8.161) | |
| Lesion biopsy: Low grade intraepithelial neoplasia (n = 15) | 0.342 | |||
| Conventional group (n = 6) | 2 | 33.33 | 1.00 | |
| Experimental group (n = 9) | 6 | 66.67 | 2.184 (0.436, 10.936) |
Only 3 patients in the experimental group experienced adverse events related to vinegar therapy; 2 patients experienced severe acid regurgitation and heartburn, and 1 patient claimed taste abnormalities. These patients experienced symptom relief after the cessation of vinegar ingestion, and the symptoms may have been related to mucosal stimulation in the mouth, pharynx, and esophagus caused by vinegar. No severe adverse events during gastroscopy were recorded during reexamination.
This prospective clinical trial was designed to explore the influence of increased vinegar consumption on the prognosis of patients with lightly stained esophageal lesions. Our study revealed that increased vinegar consumption did not reduce the risk of esophageal cancer in the esophageal mucosa dysplasia population, but it improved the endoscopic characteristics of a considerable number of patients with early lesions of the esophageal mucosa according to the ITT analysis (33.72% vs 20.00%, P = 0.007), and biopsy pathology improved (19.77% vs 8.75%, P = 0.011).
It has been reported that the progression of esophageal mucosa generally progresses through the stages of normal epithelium, mild atypical hyperplasia, moderate atypical hyperplasia, severe atypical hyperplasia, carcinoma in situ, invasive carcinoma, etc[21]. Although esophageal dysplasia does not require immediate endoscopic resection, surveil
In recent years, the use of natural food additives for the prevention and treatment of diseases has increased. Previous nutritional intervention cohort studies have shown that appropriate doses of vitamin and mineral supplements may have a preventive effect on chronic diseases caused by malignant tumours[25,26]. However, in a study on the effect of multivitamin and mineral nutrition intervention on the mortality of upper digestive tract tumours in a population with severe esophageal squamous epithelial hyperplasia in China that had been followed up for 35 years after a 6-year intervention period, no effect of multivitamin or mineral nutrition on the mortality of upper digestive tract tumours in the population was observed[27]. Another prospective study showed that the Qilian Shupi Granule had a pathological reversal effect on mild and moderate atypical hyperplasia of the esophageal squamous epithelium, but the number of patients studied was relatively small[28]. Dietary intervention for esophageal lesions lightly stained with Lugol’s iodine solution is still worth studying.
Both grain vinegar and fruit vinegar, which are fermented by traditional methods, possess a variety of physiological functions, such as antibacterial, anti-infection, antioxidative, blood glucose control, lipid metabolism regulation, weight loss, and anticancer activities[12]. Several grain vinegars, such as Shanxi aged vinegar and Japanese black vinegar, strongly inhibit the growth of several types of cancer cells in vivo or in vitro[29,30]. Polyphenols (such as resveratrol) in some fruits have anticancer effects; thus, the long-term ingestion of fruit vinegar may also have a positive anticancer effect in humans[31,32]. According to the results of epidemiological investigations, the incidence of esophageal cancer in Linzhou (Henan, China) is negatively correlated with grain vinegar consumption[16]. In theory, supplementation with vinegar has a positive effect on the prevention of upper gastrointestinal tumours in the general population. However, as a consumable substance, vinegar may have a notable short-term health promoting effect, but its impact on the long-term risk of disease is highly controversial. In this study, it was also observed that vinegar supplementation could improve endoscopic morphology and biopsy pathology, but there was no clear statistical significance in preventing the risk of disease progression to HGIN/early cancer after analysis. This may be because the population has already experienced esophageal squamous cell hyperplasia. Supplying vinegar to such patients cannot promote the reversion of proliferative esophageal squamous epithelial cells to a normal morphology; it delays the progression of lesions to a certain extent but cannot prevent the occurrence of cancer. Based on the above results, we speculate that the therapeutic effect of vinegar on patients may be related to the fact that vinegar can improve the proliferation and differentiation of esophageal mucosal cells, regulate the body’s immunity, eliminate free radicals, and improve esophageal blood flow. In addition, the consumption of vinegar in patients with reflux esophagitis increases the risk of acid regurgitation and heartburn, so it is not recommended for these patients.
This clinical trial has several limitations. First, randomization was not performed because of patient cooperation, and selection bias may have resulted. Second, the study was limited by its single-centre nature, which also led to some data bias. Third, a larger sample size and further evaluation could more strongly support the research conclusion. The number of patients with HGIN or early cancer who developed in the experimental group within 2 years was lower than that in the conventional group, indicating that the intervention measures may have been effective and that the absolute value of occurrence may have been reduced. However, the statistical test showed no difference, which could be caused by an insufficient sample size and insufficient follow-up observation time.
This study showed that increased ingestion of vinegar could not directly reduce the risk of esophageal cancer in patients with esophageal mucosa dysplasia. A considerable number of patients benefit from vinegar ingestion, which results in improved endoscopic morphology and pathology. Due to the many limitations of this trial, including the lack of randomization, the results should be interpreted with caution, and further studies are needed.
| 1. | Dobashi A, Li DK, Mavrogenis G, Visrodia KH, Bazerbachi F. Endoscopic Management of Esophageal Cancer. Thorac Surg Clin. 2022;32:479-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 2. | Wei MT, Friedland S. Early Esophageal Cancer: What the Gastroenterologist Needs to Know. Gastroenterol Clin North Am. 2021;50:791-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Iriarte F, Su S, Petrov RV, Bakhos CT, Abbas AE. Surgical Management of Early Esophageal Cancer. Surg Clin North Am. 2021;101:427-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Watanabe M, Otake R, Kozuki R, Toihata T, Takahashi K, Okamura A, Imamura Y. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 5. | Gotoda T, Kanzaki H, Okamoto Y, Obayashi Y, Baba Y, Hamada K, Sakae H, Abe M, Iwamuro M, Kawano S, Kawahara Y, Okada H. Tolerability and efficacy of the concentration of iodine solution during esophageal chromoendoscopy: a double-blind randomized controlled trial. Gastrointest Endosc. 2020;91:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Katada C, Yokoyama T, Yano T, Kaneko K, Oda I, Shimizu Y, Doyama H, Koike T, Takizawa K, Hirao M, Okada H, Yoshii T, Konishi K, Yamanouchi T, Tsuda T, Omori T, Kobayashi N, Shimoda T, Ochiai A, Amanuma Y, Ohashi S, Matsuda T, Ishikawa H, Yokoyama A, Muto M. Alcohol Consumption and Multiple Dysplastic Lesions Increase Risk of Squamous Cell Carcinoma in the Esophagus, Head, and Neck. Gastroenterology. 2016;151:860-869.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 7. | Hashimoto CL, Iriya K, Baba ER, Navarro-Rodriguez T, Zerbini MC, Eisig JN, Barbuti R, Chinzon D, Moraes-Filho JP. Lugol's dye spray chromoendoscopy establishes early diagnosis of esophageal cancer in patients with primary head and neck cancer. Am J Gastroenterol. 2005;100:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Mönig S, Chevallay M, Niclauss N, Zilli T, Fang W, Bansal A, Hoeppner J. Early esophageal cancer: the significance of surgery, endoscopy, and chemoradiation. Ann N Y Acad Sci. 2018;1434:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Taylor PR, Abnet CC, Dawsey SM. Squamous dysplasia--the precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:540-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Shimizu M, Ban S, Odze RD. Squamous dysplasia and other precursor lesions related to esophageal squamous cell carcinoma. Gastroenterol Clin North Am. 2007;36:797-811, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Ousaaid D, Mechchate H, Laaroussi H, Hano C, Bakour M, El Ghouizi A, Conte R, Lyoussi B, El Arabi I. Fruits Vinegar: Quality Characteristics, Phytochemistry, and Functionality. Molecules. 2021;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Chen H, Chen T, Giudici P, Chen F. Vinegar Functions on Health: Constituents, Sources, and Formation Mechanisms. Compr Rev Food Sci Food Saf. 2016;15:1124-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Ali Z, Wang Z, Amir RM, Younas S, Wali A, Adowa N, Ayim I. Potential Uses of Vinegar as a Medicine and Related in vivo Mechanisms. Int J Vitam Nutr Res. 2016;86:127-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Guo XK, Wang TJ, Gu JF. Effect of esophageal cancer- and stomach cancer-preventing vinegar on N-nitrosoproline formation in the human body. World J Gastroenterol. 1997;3:269-270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Feng ZD, An ZZ. Preliminary study on the chemical composition of Baoning vinegar and the anti-cancer effect of trace elements in the environment of Baoning vinegar factory. Sichuan Huanjing. 1984;4:1-9. |
| 16. | Xibib S, Meilan H, Moller H, Evans HS, Dixin D, Wenjie D, Jianbang L. Risk factors for oesophageal cancer in Linzhou, China: a case-control study. Asian Pac J Cancer Prev. 2003;4:119-124. [PubMed] |
| 17. | Levy I, Gralnek IM. Complications of diagnostic colonoscopy, upper endoscopy, and enteroscopy. Best Pract Res Clin Gastroenterol. 2016;30:705-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | Stahl M, Oliveira J; ESMO Guidelines Working Group. Esophageal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:32-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, Denlinger CS, Enzinger PC, Fanta P, Farjah F, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Leong S, Ly QP, Matkowskyj KA, McNamara M, Mulcahy MF, Paluri RK, Park H, Perry KA, Pimiento J, Poultsides GA, Roses R, Strong VE, Wiesner G, Willett CG, Wright CD, McMillian NR, Pluchino LA. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 720] [Article Influence: 102.9] [Reference Citation Analysis (0)] |
| 20. | Chang J, Zhao X, Wang Y, Liu T, Zhong C, Lao Y, Zhang S, Liao H, Bai F, Lin D, Wu C. Genomic alterations driving precancerous to cancerous lesions in esophageal cancer development. Cancer Cell. 2023;41:2038-2050.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Liu X, Zhang M, Ying S, Zhang C, Lin R, Zheng J, Zhang G, Tian D, Guo Y, Du C, Chen Y, Chen S, Su X, Ji J, Deng W, Li X, Qiu S, Yan R, Xu Z, Wang Y, Cui J, Zhuang S, Yu H, Zheng Q, Marom M, Sheng S, Hu S, Li R, Su M. Genetic Alterations in Esophageal Tissues From Squamous Dysplasia to Carcinoma. Gastroenterology. 2017;153:166-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 145] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 22. | Codipilly DC, Qin Y, Dawsey SM, Kisiel J, Topazian M, Ahlquist D, Iyer PG. Screening for esophageal squamous cell carcinoma: recent advances. Gastrointest Endosc. 2018;88:413-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 204] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 23. | Feng X, Hua ZL, Qian DF, Zhou Q, Shi AW, Wei WW, Zhou JY. [Efficacy of esophageal cancer screening program on population at high risk: a survey carried out in people aged 40-69 years in Yangzhong, Jiangsu province]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:908-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Li H, Cheng JL, Dong NN, Cui CY, Diao TY, Zhou XR. Effects of endoscopic mucosal resection in patients with low-grade intraepithelial dysplasia of esophageal squamous cells. Dig Surg. 2013;30:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Middha P, Weinstein SJ, Männistö S, Albanes D, Mondul AM. β-Carotene Supplementation and Lung Cancer Incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: The Role of Tar and Nicotine. Nicotine Tob Res. 2019;21:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 26. | Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, Johnson LL, Gail MH, Dong ZW, Yu B, Mark SD, Taylor PR. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | Yang H, Wang XK, Zhang JY, Fan JH, Qiao YL. Multivitamins and Minerals Supplementation and the Long-term Risk of Upper Gastrointestinal Cancer Mortalityin Esophageal Squamous Severe Dysplasia Population:a 35-year Follow-up Study in Linxian Dysplasia Nutrition Intervention Trial. Zhongguo Zhongliu. 2021;30:192-198. [DOI] [Full Text] |
| 28. | Yang JY, Kong LB. Therapeutic effect of Qilian Shupi Granule on atypical hyperplasia of esophageal squamous epithelium. Yixue Xinxi. 2011;24:2. [DOI] [Full Text] |
| 29. | Seki T, Morimura S, Shigematsu T, Maeda H, Kida K. Antitumor activity of rice-shochu post-distillation slurry and vinegar produced from the post-distillation slurry via oral administration in a mouse model. Biofactors. 2004;22:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Zhang Q, Li ZL, Zhang Y, Wang K, Zhang M, Chen PD, Yao WF, Tang YP, Wu JH, Zhang L. Effect of the vinegar-process on chemical compositions and biological activities of Euphorbia kansui: A review. J Ethnopharmacol. 2020;252:112557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Zamora-Ros R, Knaze V, Rothwell JA, Hémon B, Moskal A, Overvad K, Tjønneland A, Kyrø C, Fagherazzi G, Boutron-Ruault MC, Touillaud M, Katzke V, Kühn T, Boeing H, Förster J, Trichopoulou A, Valanou E, Peppa E, Palli D, Agnoli C, Ricceri F, Tumino R, de Magistris MS, Peeters PH, Bueno-de-Mesquita HB, Engeset D, Skeie G, Hjartåker A, Menéndez V, Agudo A, Molina-Montes E, Huerta JM, Barricarte A, Amiano P, Sonestedt E, Nilsson LM, Landberg R, Key TJ, Khaw KT, Wareham NJ, Lu Y, Slimani N, Romieu I, Riboli E, Scalbert A. Dietary polyphenol intake in Europe: the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Nutr. 2016;55:1359-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 312] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 32. | Kyrø C, Zamora-Ros R, Scalbert A, Tjønneland A, Dossus L, Johansen C, Bidstrup PE, Weiderpass E, Christensen J, Ward H, Aune D, Riboli E, His M, Clavel-Chapelon F, Baglietto L, Katzke V, Kühn T, Boeing H, Floegel A, Overvad K, Lasheras C, Travier N, Sánchez MJ, Amiano P, Chirlaque MD, Ardanaz E, Khaw KT, Wareham N, Perez-Cornago A, Trichopoulou A, Lagiou P, Vasilopoulou E, Masala G, Grioni S, Berrino F, Tumino R, Sacerdote C, Mattiello A, Bueno-de-Mesquita HB, Peeters PH, van Gils C, Borgquist S, Butt S, Zeleniuch-Jacquotte A, Sund M, Hjartåker A, Skeie G, Olsen A, Romieu I. Pre-diagnostic polyphenol intake and breast cancer survival: the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Breast Cancer Res Treat. 2015;154:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade B
P-Reviewer: Tangsuwanaruk T, Thailand S-Editor: Li L L-Editor: A P-Editor: Cai YX