Published online Mar 16, 2024. doi: 10.4253/wjge.v16.i3.157
Peer-review started: October 18, 2023
First decision: December 25, 2023
Revised: January 7, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 16, 2024
Processing time: 147 Days and 18.9 Hours
Conventional magnifying endoscopy with narrow-band imaging (NBI) observation of the gastric body mucosa shows dominant patterns in relation to the regular arrangement of collecting venules, subepithelial capillary network, and gastric pits.
To evaluate the effectiveness of a new one-dual (near) focus, NBI mode in the assessment of the microscopic features of gastric body mucosa compared to conventional magnification.
During 2021 and 2022, 68 patients underwent proximal gastrointestinal endoscopy using magnification endoscopic modalities subsequently applying acetic acid (AA). The GIF-190HQ series NBI system with dual focus capability was used for the investigation of gastric mucosa. At the time of the endoscopy, the gastric body mucosa of all enrolled patients was photographed using the white light endoscopy (WLE), near focus (NF), NF-NBI, AA-NF, and AA-NF-NBI modes.
The WLE, NF and NF-NBI endoscopic modes for all patients (204 images) were classified in the same order into three groups. Two images from each patient for the AA-NF and AA-NF-NBI endoscopic modes were classified in the same order. According to all three observers who completed the work independently, NF magnification was significantly superior to WLE (P < 0.01), and the NF-NBI mode was significantly superior to NF magnification (P < 0.01). After applying AA, the three observers confirmed that AA-NF-NBI was significantly superior to AA-NF (P < 0.01). Interobserver kappa values for WLE were 0.609, 0.704, and 0.598, respectively and were 0.600, 0.721, and 0.637, respectively, for NF magnification. For the NF-NBI mode, the values were 0.378, 0.471, and 0.553, respectively. For AA-NF, they were 0.453, 0.603, and 0.480, respectively, and for AA-NF-NBI, they were 0.643, 0.506, and 0.354, respectively.
When investigating gastric mucosa in microscopic detail, NF-NBI was the most powerful endoscopic mode for assessing regular arrangement of collecting venules, subepithelial capillary network, and gastric pits among the five endoscopic modalities investigated in this study. AA-NF-NBI was the most powerful endoscopic mode for analyzing crypt opening and intervening part.
Core Tip: Narrow-band imaging has enabled the analysis of gastrointestinal mucosa in microscopic detail. However, this technique gives a dark image and makes it impossible to identify the color and structural microanatomy changes of the stomach mucosa, and it is necessary to combine it with the mechanical addition on the top of the scope (conventional magnification). These additions improve the visualization of the gastric mucosa but significantly complicate the procedure. We presented a new endoscopic mode called “near focus” that achieves the same or better visualization and does not require any additional accessories.
- Citation: Kurtcehajic A, Zerem E, Bokun T, Alibegovic E, Kunosic S, Hujdurovic A, Tursunovic A, Ljuca K. Could near focus endoscopy, narrow-band imaging, and acetic acid improve the visualization of microscopic features of stomach mucosa? World J Gastrointest Endosc 2024; 16(3): 157-167
- URL: https://www.wjgnet.com/1948-5190/full/v16/i3/157.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i3.157
Currently, endoscopic platforms offer high-resolution images, image-enhanced endoscopy (IEE) techniques, and magnification, allowing for the inspection of gastric mucosa at a more detailed level. Endoscopic microscopic features (microanatomy) of the gastric body mucosa are classified into the microvascular architecture, such as the regular arrangement of collecting venules (RAC) and the regular honeycomb subepithelial capillary network (SECN). The microsurface structure is characterized by regular round gastric pits (GP), regular oval crypt opening (CO), and the intervening part (IP), which constitutes the space between the crypts. In gastric-related diseases such as Helicobacter pylori (H. pylori) infection, intestinal metaplasia, and gastric atrophy, the microanatomy has been structurally changed to an absence of venules, irregularity of capillary network, and enlarging pits and crypts[1-6].
In previous reports, white light endoscopy (WLE) has failed to assess endoscopic microanatomy. The need and wish to improve the differentiation of normal, inflammatory, and malignant lesions by gastrointestinal endoscopy has fueled research to accelerate the development of novel types of video endoscopy systems, based on new optical technologies. Electronic chromoendoscopy via narrow-band imaging (NBI) (the most useful tool in the many clinical trials) has highlighted the vascular patterns of gastric mucosa[1,3,6-8]. Three studies focused on acetic acid (AA), which enhances and determines the pathology of gastric lesions[9-11].
There has been a need for increased magnification to assess the endoscopic microscopic features in more detail. Conventional magnifying endoscopy (ME) with NBI observation of the normal gastric mucosa has been described previously; studies reported dominant patterns with RAC, SECN, and GP[2-4,6,7]. Recent, optical innovation relating to the dual focus function allows the endoscopist to select between a normal mode and a near focus (NF) mode. The NF mode is optimized for near-field observation with 45-fold magnification. Studies have reported that NF-NBI successfully replaced ME-NBI in the detection of pathological lesions in the esophagus and pharynx as well as the identification of celiac disease[12-15]. Recent studies reported the ability of the NF-NBI mode for the detection of early gastric cancer lesions[16,17]. Two recently published studies considered NF endoscopy in the evaluation of gastric atrophy, intestinal metaplasia, and H. pylori infection[18,19].
Conventional ME uses a soft rubber at the top of the scope due to the demanding manipulation in the remaining stomach can be replaced with the simple, more pragmatic, and novel endoscopic way of magnification. Therefore, in this study, we evaluated the possibility of a new one-dual (near) focus, NBI mode in the assessment of RAC, SECN, GP, and CO in the gastric body for the visualization of microscopic features of the mucosa.
The first aim of this study was to determine the clinical (endoscopic) usefulness of the NF-NBI mode in the observation of gastric microvascular architecture and microsurface structure. Secondly, by applying 1.5% AA, we compared the power of visualization of the AA-NF and AA-NF-NBI mode when assessing microsurface patterns containing CO and IP.
Between September 2021 and May 2022, 68 patients underwent proximal gastrointestinal endoscopy using conventional WLE, NF magnification, and NF-NBI with the subsequent application of AA. The patients consisted of 30 males and 38 females with a mean age of 38.5 years (range: 25-65 years).
The study excluded patients who were H. pylori positive (either one serology or rapid urease test), those who had received anticoagulant therapy or drugs for chronic metabolic diseases (diabetes mellitus, hypothyroidism) and systemic inflammation disease as well as nonsteroidal anti-inflammatory drugs and anxiolytics, and patients with chronic decompensated liver and kidney diseases. The study was approved by the ethical committee of the Blue Medical Group, and signed, well-informed consent was obtained from all participants.
The endoscopic video information system, EVIS EXERA III CLV-190, was used with an Olympus high-resolution endoscope, GIF-190HQ series NBI system, with dual focus capability for the investigation of the gastric mucosa. This scope allows switching between two focus settings: “normal mode” and “near focus mode”. The “normal mode” or WLE suits normal observation at 5-100 mm and a 170° field of view, while the NF magnification of up to × 70 allows close observation of the finest mucosal surfaces at 2-6 mm. When switching to the NF mode at the simple touch of a button, the field of view will remain almost the same (160°).
NBI is based on the principle that depth of light penetration into tissues is directly proportional to the wavelength, which implies that the shorter the wavelength, the more superficial the penetration. The NBI resembles chromoendoscopy without dye, focusing on the capillaries.
AA via magnification enables vivid observation of the CO of the glandular epithelium, which has a deep brown appearance. The IP has a whitish appearance because of reversible alterations in the molecular structure of the cellular proteins that are induced by AA and lasts from several seconds to several minutes.
Two hours before the procedure all patients took 80 mg of simethicone with a small amount of water to remove gastric mucus. The procedure was performed under intravenous application of propofol. An experienced gastroenterologist (Kurtcehajic A) performed all the procedures. The whole esophagus, stomach, and duodenum were examined to exclude obvious lesions with conventional WLE, followed by the manufacturer incorporated NF-NBI mode in the scope, by applying 3 mL of AA via a single catheter. The focus area was the anterior wall or greater curvature of the upper gastric body. Biopsies were taken from the antrum and corpus mucosa, and rapid urease test was performed to evaluate H. pylori infection.
At the time of the endoscopy, the gastric body mucosa of all enrolled patients was photographed using the WLE, NF, NF-NBI, AA-NF, and AA-NF-NBI modes. Regarding the WLE, NF and NF-NBI endoscopic modalities, the regular/clear appearance of each mucosa microscopic feature, RAC, SECN, GP, and CO scored 1 point. The unclear appearance of each scored a half point, and the absence of each scored 0.
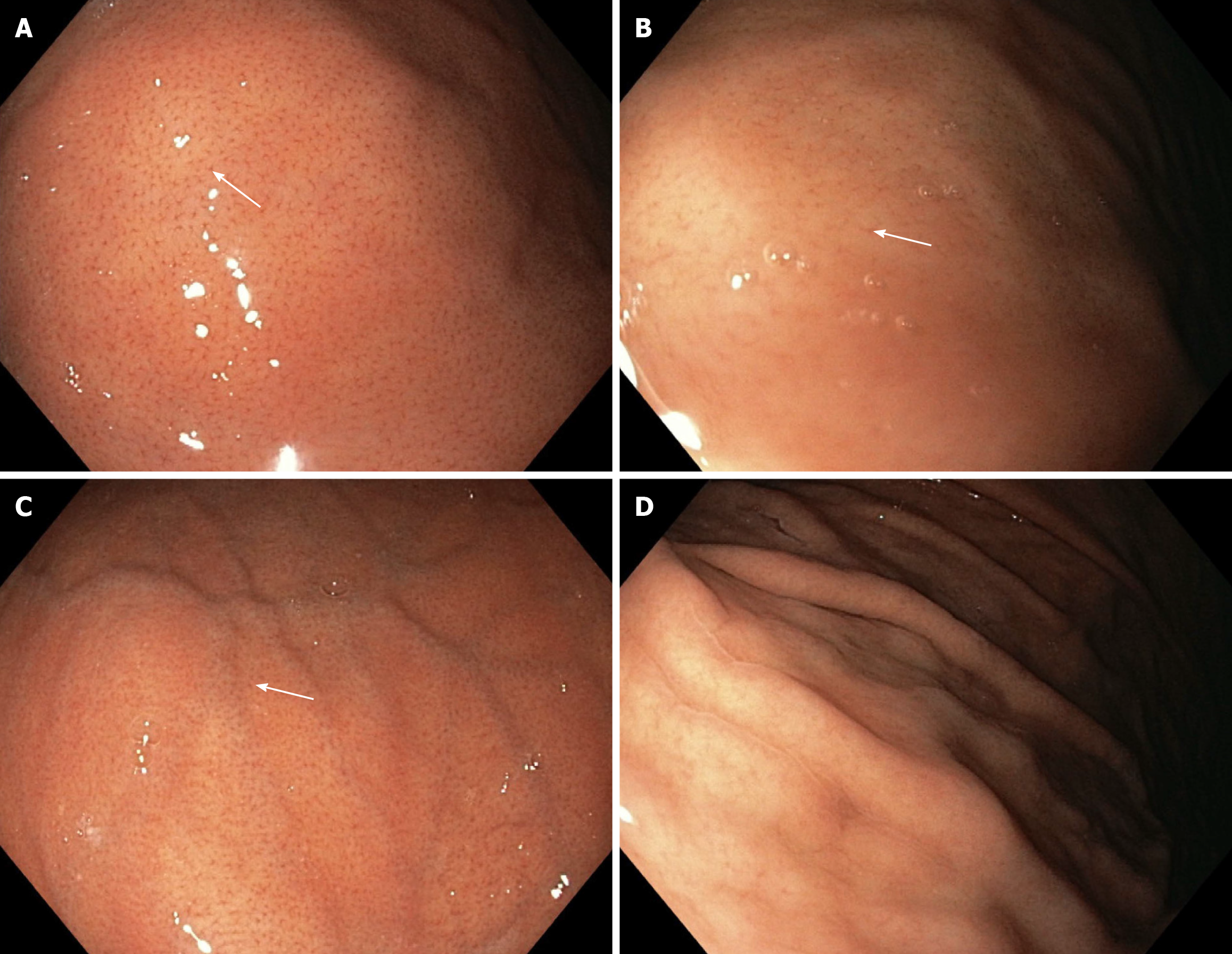
In relation to the WLE mode, the WLE-1a and WLE-2a patterns clearly show the RAC and score 1 point. WLE-2a presents a faded appearance of the RAC. The WLE-b pattern shows the RAC less clearly and scores a half point. The WLE-c pattern does not show the RAC and scores 0 (Figure 1).
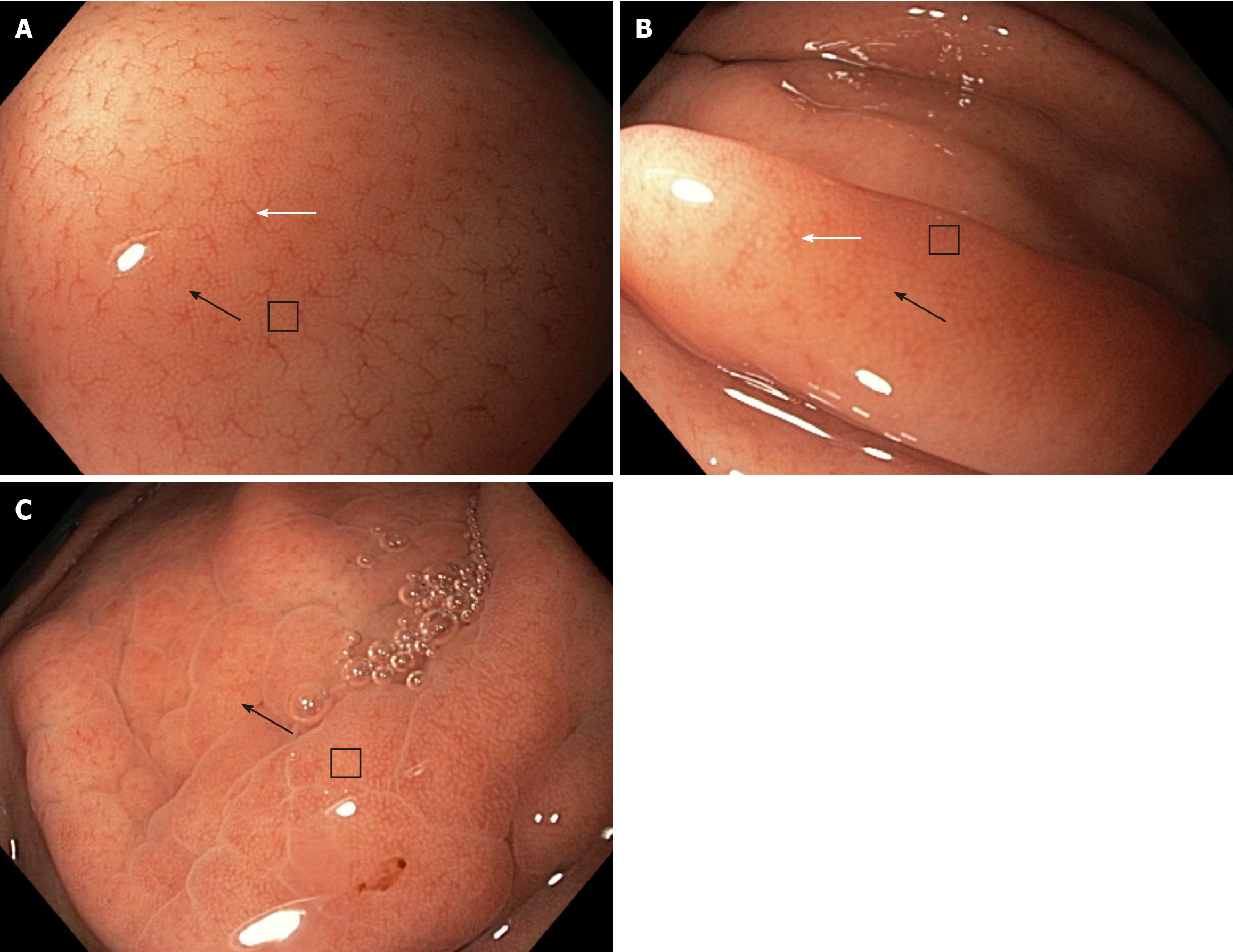
With reference to the NF magnification, the NF-a pattern clearly shows the RAC, SECN, and GP and scores 3 points. The NF-b pattern shows the RAC less clearly but clearly shows the SECN and GP and scores 2.5 points. The NF-c pattern does not show the RAC but clearly shows the SECN and GP and scores 2 points (Figure 2).
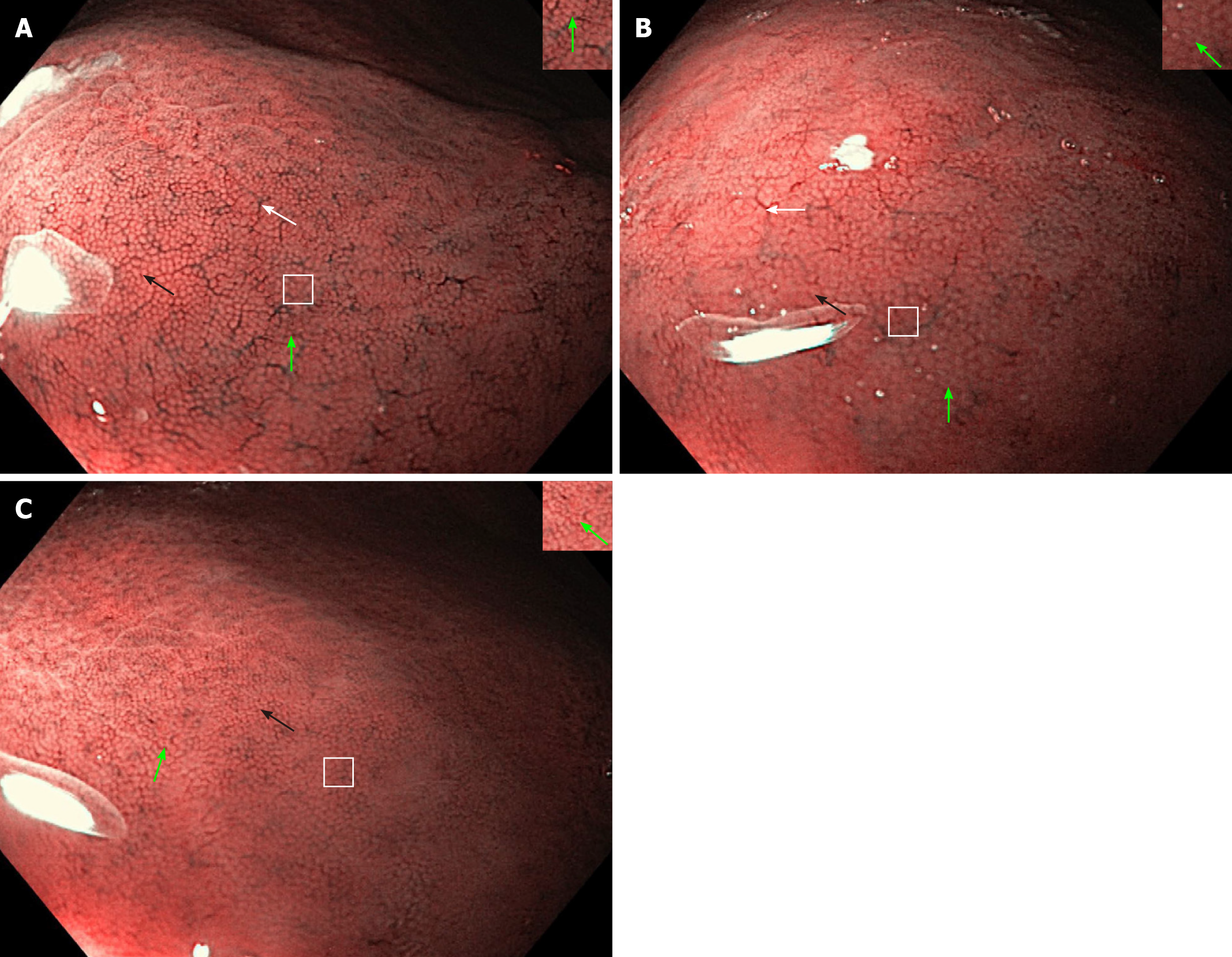
Regarding the NF-NBI endoscopic visualization, the NF-NBI-1a and NF-NBI-2a patterns clearly show the RAC, SECN, GP, and CO and score 4 points. The 2a pattern has less distribution of the RAC and a slightly enlarged GP and CO than the 1a pattern. The NF-NBI-b pattern does not show the RAC, clearly shows the SECN and GP, and shows the CO less clearly and scores 2.5 points (Figure 3).
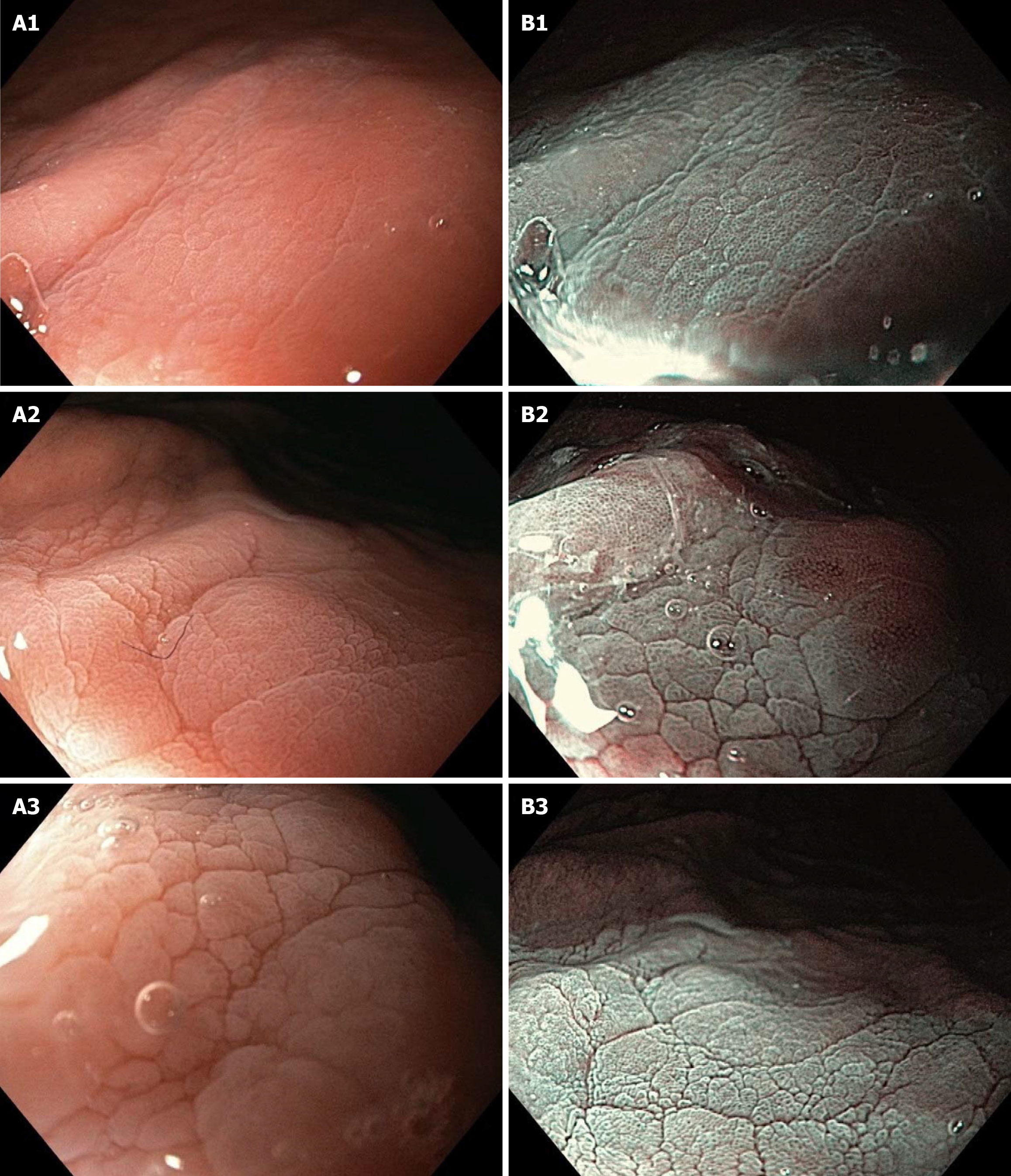
According to the scoring rules, one pattern could score the most points (4) (clear presence of all microscopic features) or the least number of points (0) (absence of all microscopic features). After enhancing the area of observation with AA, a pattern was suddenly visible on the AA-NF and AA-NF-NBI containing CO and IP. On the AA-NF mode, the pattern shows small, brown, oval CO and light white IP. On the AA-NF-NBI mode, the pattern shows small, black, oval CO and dense white IP (Figure 4). The strong contrast between the CO regarding the shape/size and the IP on these two patterns within the same patient is graded as 1 point, medium contrast is graded as 0.5 points, and low contrast is graded as 0 points. Endoscopic patterns were observed and scored by three independent endoscopists.
The differences between the scoring of each endoscopic modality for all observers were compared using the Wilcoxon Matched Pairs Test. P values < 0.05 were considered significant. The interobserver diagnostic agreement was analyzed with a kappa value. In theory, perfect disagreement has a kappa value of -1, and perfect agreement has a kappa value of +1. A value of 0 means an agreement by chance alone. As per the Landis and Koch scale, kappa values were graded as follows: 0.01-0.20 slight; 0.21-0.40 fair; 0.41-0.60 moderate; 0.61-0.80 substantial; and 0.81-1.00 almost perfect. Cohen’s suggested interpretation may be too lenient for health-related studies because it implies that a score as low as 0.41 might be acceptable[9,14,20]. Data were analyzed using SPSS 23 (IBM, United States).
After meeting the criteria of long-term epigastric discomfort and non-specific abdominal pain, the study initially included 74 patients. During the endoscopy, 4 patients (three male, one female) did not undergo NF magnification due to 1 patient having benign stenosis of the distal esophagus, 1 patient having cancer of the cardia, and 2 patients having pyloric stenosis. Two patients (one male, one female) were excluded due to severe bile reflux.
Finally, 68 patients underwent proximal gastrointestinal endoscopy using the WLE, NF and NF-NBI modes to analyze the microscopic features of the gastric body mucosa. The images from all patients (204 images in total) were classified in the same order into three groups in relation to the above endoscopic modality by endoscopist AK. They were observed separately and scored by two experienced endoscopists, JF (observer I) and PJ (observer II), and one inexperienced endoscopist, OZ (observer III).
Moreover, after applying AA in the area of observation, the CO and IP were suddenly visible. Regarding the AA-NF and AA-NF-NBI endoscopic modes, 136 images (2 images per patient) were classified in the same order. The contrast between the CO and IP in the same patient was observed and graded separately by the three observers. All observers had previously passed a live course regarding NF magnification and NBI chromoendoscopy mode with AA enhancing. The course was based on the 12 endoscopic patterns that would form part of this study. Each pattern presented with ten images.
The frequency and scoring for the WLE, NF, and NF-NBI endoscopic modalities from the point of view of all three observers are shown in Table 1. According to the experienced observers (observer I and observer II), NF magnification was significantly superior to WLE (P < 0.01), and the NF-NBI mode was significantly superior to NF magnification (P < 0.01). Regarding the third inexperienced observer, NF magnification was significantly superior to WLE (P < 0.01), and the NF-NBI mode was significantly superior to the NF magnification (P < 0.01).
| Observer I | Observer II | Observer III | ||||||
| WLE | NF | NF-NBI | WLE | NF | NF-NBI | WLE | NF | NF-NBI |
| 1a (44 × 1) | a (55 × 3) | 1a (53 × 4) | 1a (47 × 1) | a (58 × 3) | 1a (60 × 4) | 1a (51 × 1) | a (56 × 3) | 1a (51 × 4) |
| 2a (2 × 1) | b (9 × 2.5) | 2a (12 × 4) | 2a (4 × 1) | b (7 × 2.5) | 2a (6 × 4) | 2a (0 × 1) | b (7 × 2.5) | 2a (12 × 4) |
| b (9 × 0.5) | c (4 × 2) | b (3 × 2.5) | b (7 × 0.5) | c (3 × 2) | b (2 × 2.5) | b (2 × 0.5) | c (5 × 2) | b (5 × 2.5) |
| c (13 × 0) | c (10 × 0) | c (15 × 0) | ||||||
| 50.5 | 195.5 | 267.5 | 54.5 | 197.5 | 269.0 | 52.0 | 195.5 | 264.5 |
The frequency and scoring for the AA-NF and AA-NF-NBI endoscopic modalities from the point of view of all three observers are shown in Table 2. According to the experienced observers (observer I and observer II), AA-NF-NBI was significantly superior to AA-NF (P < 0.01). For the third inexperienced observer, AA-NF-NBI was significantly superior to AA-NF (P < 0.01).
| Observer I | Observer II | Observer III | |||
| AA-NF | AA-NF-NBI | AA-NF | AA-NF-NBI | AA-NF | AA-NF-NBI |
| 58 × 0.5 | 63 × 1 | 62 × 0.5 | 64 × 1 | 55 × 0.5 | 62 × 1 |
| 3 × 1 | 2 × 1 | 7 × 1 | |||
| 7 × 0 | 5 × 0.5 | 4 × 0 | 4 × 0.5 | 6 × 0 | 6 × 0.5 |
| 32.0 | 65.5 | 33.0 | 66.0 | 34.5 | 65.0 |
The interobserver diagnostic agreement for all five endoscopic modalities was analyzed with a kappa value. Interobserver kappa values for WLE were 0.609 for observer I and observer II, 0.704 for observer I and observer III, and 0.598 for observer II and observer III. Interobserver kappa values for NF magnification were 0.600 for observer I and observer II, 0.721 for observer I and observer III, and 0.637 for observer II and observer III. Interobserver kappa values for the NF-NBI mode were 0.378 for observer I and observer II, 0.471 for observer I and observer III, and 0.553 for observer II and observer III. Interobserver kappa values for AA-NF were 0.453 for observer I and observer II, 0.603 for observer I and observer III, and 0.480 for observer II and observer III. Interobserver kappa values for AA-NF-NBI were 0.643 for observer I and observer II, 0.506 for observer I and observer III, and 0.354 for observer II and observer III.
According to the results of our research into the investigation of gastric mucosa at a detailed microscopic level, NF-NBI was the most powerful endoscopic mode for evaluating the RAC, SECN, and GP, and AA-NF-NBI was the most powerful endoscopic mode for analyzing the CO and IP. There have been many advances in endoscopic imaging technologies. Standard definition endoscopy produces image signals with a resolution of 100000-400000 pixels. High-resolution or high-definition endoscopy produces image signals with a resolution of up to 1000000 pixels, which has the same effect as visualizing a surface at a 30-35-fold magnification. A novel IEE technique is electronic chromoendoscopy, which includes NBI, i-Scan, and flexible spectral imaging color enhancement. Over the last 15 years, NBI has been used most in clinical practice. However, it is too dark to identify the color and structural mucosal changes in organs with a large lumen, such as the stomach. Therefore, these should be combined with ME[1,6,8,14].
Over the last two decades, conventional ME was carried out with a soft rubber. Before the procedure, soft black rubber was attached at the top of the scope. In this way, the area of interest was magnified, However, the view for normal observation was reduced. Magnification with soft rubber requires skill and special training. For this reason, ME is not frequently used in Western countries[1-4,6].
Several studies using high-definition endoscopy, IEE, conventional magnifying, and histopathology have confirmed the normal appearance of gastric body mucosa with the RAC, SECN, and GP[1-3,6-8,21-25]. In our study, we successfully replaced conventional ME with the dual focus (NF magnification). Regarding our results, all three observers noted independently that the NF magnification showed a higher power of visualization than WLE (P < 0.01). NF magnification from the point of view of all three observers assessed the microscopic features of the gastric body mucosa, such as the RAC at 94%, 95%, and 92%, respectively, and the SECN and GP at 100%, in the same way as it was reported in studies powered by conventional ME[4,22,23]. The diagnostic interobserver agreement for the NF magnification showed a “substantial” level.
A retrospective study from the United Kingdom demonstrated that NF magnification improved the diagnostic yield of upper gastrointestinal mucosal lesions. However, its usefulness for gastric lesions is questionable[15]. In our study, all three observers noted independently that the NF with the NBI showed significantly more power of visualization than the NF magnification (P < 0.01). In the NF-NBI mode, from the point of view all three observers, SECN, GP, and CO were clearly seen at levels of 95.5%, 97% and 92.6%, respectively. The diagnostic interobserver agreement in relation to the NF-NBI mode from the perspective of the experienced observers (one side) and the third inexperienced observer was at the “moderate” level. The diagnostic interobserver agreement among the experienced observers was at the “fair” level. The clinical explanation could be that NF-NBI “1a” and “1b” patterns were scored the same and that the differences were qualitative (distribution of the RAC, size of GP and CO).
In the progression of gastric-related diseases, microscopic features of the stomach mucosa such as venules, capillary network, and the shape and size of gastric fossa and recess have been structurally changed. For the first time, two studies used the NF-NBI mode in the stomach for the evaluation of a tumor lesion and its margin[16,17]. The role of the NF-NBI mode has been assessed recently for atrophic gastritis, according to the Kimura-Takemoto classification and intestinal metaplasia (tubular/granular GP pattern of the corpus)[18]. This study considered the shape of the GP without analyzing the RAC, SECN, and CO. In the absence of the previously verified gastric NF-NBI magnification pattern, it could not be an appropriate definition of the pathology pattern.
In a recently published study by Fiuza et al[19], NF magnification was evaluated for the assessment of the mucosal surface pattern in H. pylori-related gastritis. This study used the NF mode without NBI chromoendoscopy and was unable to consider the appearance of the CO.
Conventional ME requires more time, skill, and special endoscopic training. The mechanical addition on the top of the scope (rubber) and the view of visualization becomes less. On the other hand, NF magnification may be easily manipulated, there is no need for the mechanical attachment, the view of visualization remains normal, and as observed in our study NF magnification with NBI chromoendoscopy showed the presence of CO.
The current era of NF magnification endoscopic technology may be contrasted with the research of Cho et al[25] who recently used conventional ME for the evaluation of H. pylori-associated gastritis. The forthcoming studies via NF-NBI endoscopy aim to evaluate the presence/absence of RAC, the regularity/irregularity of SECN, and the shape and size of the GP and CO in relation to the H. pylori infection, intestinal metaplasia, and atrophic gastritis, etc.
In the previous studies, the focus on the CO was less. A study by Kawamura et al[26] evaluated the role of conventional ME (without NBI) by analyzing the CO as part of H. pylori-related gastritis. The whiteness of the CO was classified as the “white-edged dark spot” type, the “white” type, and the “dense white pit” type. Regarding our results, the NF-NBI mode assessed the presence of the CO as a black point, but to visualize the CO in more detail, enhancement was carried out using AA. By applying AA in the area of observation, we highlighted a unique pattern containing regular round brown CO and whiteish IP in the normal gastric body mucosa. In the AA-NF-NBI mode, the CO suddenly became black, creating a clear contrast between the CO and IP.
Our results, independently noted by all three observers, showed that AA-NF-NBI was superior to AA-NF. Our results clearly indicate the superiority of the AA-NF-NBI mode in terms of analyzing the shape and size of the CO and IP. The diagnostic interobserver agreement for the AA-NF-NBI mode among the experienced observers was at a “substantial” level. The diagnostic interobserver agreement between the experienced observers (one side) and the third inexperienced observer was at the “moderate” level and “fair” level, respectively. An explanation for this could be that there was no question about the existence of the contrast and that the discrepancy related to the grade of the contrast between the CO and IP.
One limitation of this study (as well as other studies related to this issue) was the relatively small number of patients included. Additional research (preferably randomized trials or prospective collaborative studies) is required to improve the endoscopic investigation of gastric mucosa at a detailed microscopic level and to create the conditions for a better diagnosis and treatment of these diseases.
NF-NBI was the most effective endoscopic mode for evaluating RAC, SECN, and GP. AA-NF-NBI was the most effective endoscopic mode for analyzing CO and IP. It provides a higher resolution for evaluating the relationship between the progress of gastric diseases and the existence of gastric venules, the regularity/irregularity of capillary network, and the shape and size of GP and recesses.
Narrow-band imaging (NBI) is too dark to identify the color and structural microanatomy of stomach mucosa due to large lumen. Therefore, these should be combined with magnification.
Conventional magnification endoscopy using a soft rubber at the top of the scope, which requires demanding manipulation, could be replaced with a simple, more pragmatic, and novel endoscopic magnification technique.
We evaluated the possibility of a near focus (NF) magnification, NBI mode with acetic acid (AA), in the assessment of venules, capillary network, pits, and crypts in the gastric body mucosa.
The endoscopic video information system, EVIS EXERA III CLV-190, was used with an Olympus high-resolution endoscope, GIF-190HQ series NBI system, with dual focus capability for the investigation of the gastric mucosa. At the time of the endoscopy, the gastric body mucosa of all enrolled patients was photographed using the white light endoscopy (WLE), NF, NF-NBI, AA-NF, and AA-NF-NBI modes.
From 68 patients, 204 images were classified in the same order into three groups (WLE, NF, and NF-NBI). They were observed separately and scored by two experienced endoscopists and one inexperienced endoscopist. According to all three observers independently, NF magnification was significantly superior to WLE (P < 0.01), and the NF-NBI mode was significantly superior to NF magnification (P < 0.01). Interobserver kappa values for WLE were 0.609, 0.704, and 0.598, respectively, and in the case of NF magnification, they were 0.600, 0.721, and 0.637, respectively. For the NF-NBI mode, the values were 0.378, 0.471, and 0.553, respectively. AA-NF-NBI was significantly superior to AA-NF (P < 0.01) by all three observers independently. Interobserver kappa values for the AA-NF were 0.453, 0.603, and 0.480, respectively, and for AA-NF-NBI, they were 0.643, 0.506, and 0.354, respectively.
Among the five endoscopic modalities investigated in this study, NF-NBI was the most powerful endoscopic mode for assessing venules, capillary network, and gastric pits. AA-NF-NBI was the most powerful endoscopic mode for analyzing crypts and space between crypts.
The forthcoming studies of NF-NBI and AA-NF-NBI endoscopic modalities aim to evaluate the presence/absence of venules, the regularity/irregularity of capillary network, and the shape and size of the gastric pits and crypts in relation to Helicobacter pylori infection, intestinal metaplasia, and atrophic gastritis, etc.
The authors would like to thank the staff of the Endoscopic Unit, Department of Gastroenterology and Hepatology, Blue Medical Group for the technical support.
| 1. | Kim JW. Usefulness of Narrow-Band Imaging in Endoscopic Submucosal Dissection of the Stomach. Clin Endosc. 2018;51:527-533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 2. | Yao K. Clinical Application of Magnifying Endoscopy with Narrow-Band Imaging in the Stomach. Clin Endosc. 2015;48:481-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 3. | Li HY, Ge ZZ, Fujishiro M, Li XB. Current clinical applications of magnifying endoscopy with narrow band imaging in the stomach. Diagn Ther Endosc. 2012;2012:271914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Qi Q, Guo C, Ji R, Li Z, Zuo X, Li Y. Diagnostic Performance of Magnifying Endoscopy for Helicobacter pylori Infection: A Meta-Analysis. PLoS One. 2016;11:e0168201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Glover B, Teare J, Patel N. A systematic review of the role of non-magnified endoscopy for the assessment of H. pylori infection. Endosc Int Open. 2020;8:E105-E114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 6. | Ji R, Li YQ. Diagnosing Helicobacter pylori infection in vivo by novel endoscopic techniques. World J Gastroenterol. 2014;20:9314-9320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Okubo M, Tahara T, Shibata T, Nakamura M, Kamiya Y, Yoshioka D, Maeda Y, Yonemura J, Ishizuka T, Arisawa T, Hirata I. Usefulness of magnifying narrow-band imaging endoscopy in the Helicobacter pylori-related chronic gastritis. Digestion. 2011;83:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Jang JY. The Past, Present, and Future of Image-Enhanced Endoscopy. Clin Endosc. 2015;48:466-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Shibagaki K, Amano Y, Ishimura N, Taniguchi H, Fujita H, Adachi S, Kakehi E, Fujita R, Kobayashi K, Kinoshita Y. Diagnostic accuracy of magnification endoscopy with acetic acid enhancement and narrow-band imaging in gastric mucosal neoplasms. Endoscopy. 2016;48:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Kadowaki S, Tanaka K, Toyoda H, Kosaka R, Imoto I, Hamada Y, Katsurahara M, Inoue H, Aoki M, Noda T, Yamada T, Takei Y, Katayama N. Ease of early gastric cancer demarcation recognition: a comparison of four magnifying endoscopy methods. J Gastroenterol Hepatol. 2009;24:1625-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Sha J, Wang P, Zhu B, Zhu M, Li X, Gao F. Acetic Acid Enhanced Narrow Band Imaging for the Diagnosis of Gastric Intestinal Metaplasia. PLoS One. 2017;12:e0170957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Singh R, Shahzad MA, Tam W, Goda K, Yu LH, Fujishiro M, Uedo N, Ruszkiewicz A. Preliminary feasibility study using a novel narrow-band imaging system with dual focus magnification capability in Barrett's esophagus: is the time ripe to abandon random biopsies? Dig Endosc. 2013;25 Suppl 2:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Goda K, Dobashi A, Yoshimura N, Aihara H, Kato M, Sumiyama K, Toyoizumi H, Kato T, Saijo H, Ikegami M, Tajiri H. Dual-focus versus conventional magnification endoscopy for the diagnosis of superficial squamous neoplasms in the pharynx and esophagus: a randomized trial. Endoscopy. 2016;48:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Gulati S, Emmanuel A, Ong M, Pavlidis P, Patel M, El-Menabawey T, Vackova Z, Dubois P, Murino A, Martinek J, Sethi A, Neumann H, Haji A, Hayee B. Near-focus narrow-band imaging classification of villous atrophy in suspected celiac disease: development and international validation. Gastrointest Endosc. 2021;94:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Bond A, Burkitt MD, Cox T, Smart HL, Probert C, Haslam N, Sarkar S. Dual-focus Magnification, High-Definition Endoscopy Improves Pathology Detection in Direct-to-Test Diagnostic Upper Gastrointestinal Endoscopy. J Gastrointestin Liver Dis. 2017;26:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Kim JW, Jung Y, Jang JY, Kim GH, Bang BW, Park JC, Choi HS, Cho JH; Research Group for Endoscopic Instruments and Stents of Korean Society of Gastrointestinal Endoscopy. Narrowband imaging with near-focus magnification for discriminating the gastric tumor margin before endoscopic resection: A prospective randomized multicenter trial. J Gastroenterol Hepatol. 2020;35:1930-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Kakushima N, Yoshida N, Doyama H, Yano T, Horimatsu T, Uedo N, Yamamoto Y, Kanzaki H, Hori S, Yao K, Oda I, Tanabe S, Yokoi C, Ohata K, Yoshimura K, Ishikawa H, Muto M. Near-focus magnification and second-generation narrow-band imaging for early gastric cancer in a randomized trial. J Gastroenterol. 2020;55:1127-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Na HK, Choi KD, Park YS, Kim HJ, Ahn JY, Lee JH, Jung KW, Kim DH, Song HJ, Lee GH, Jung HY. Endoscopic scoring system for gastric atrophy and intestinal metaplasia: correlation with OLGA and OLGIM staging: a single-center prospective pilot study in Korea. Scand J Gastroenterol. 2022;57:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 19. | Fiuza F, Maluf-Filho F, Ide E, Furuya CK Jr, Fylyk SN, Ruas JN, Stabach L, Araujo GA, Matuguma SE, Uemura RS, Sakai CM, Yamazaki K, Ueda SS, Sakai P, Martins BC. Association between mucosal surface pattern under near focus technology and Helicobacter pylori infection. World J Gastrointest Endosc. 2021;13:518-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 20. | McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276-282. [PubMed] |
| 21. | Garcés-Durán R, García-Rodríguez A, Córdova H, Cuatrecasas M, Ginès À, González-Suárez B, Araujo I, Llach J, Fernández-Esparrach G. Association between a regular arrangement of collecting venules and absence of Helicobacter pylori infection in a European population. Gastrointest Endosc. 2019;90:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Yagi K, Nakamura A, Sekine A. Comparison between magnifying endoscopy and histological, culture and urease test findings from the gastric mucosa of the corpus. Endoscopy. 2002;34:376-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Anagnostopoulos GK, Yao K, Kaye P, Fogden E, Fortun P, Shonde A, Foley S, Sunil S, Atherton JJ, Hawkey C, Ragunath K. High-resolution magnification endoscopy can reliably identify normal gastric mucosa, Helicobacter pylori-associated gastritis, and gastric atrophy. Endoscopy. 2007;39:202-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 24. | Tahara T, Shibata T, Nakamura M, Yoshioka D, Okubo M, Arisawa T, Hirata I. Gastric mucosal pattern by using magnifying narrow-band imaging endoscopy clearly distinguishes histological and serological severity of chronic gastritis. Gastrointest Endosc. 2009;70:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 25. | Cho JH, Jeon SR, Jin SY, Park S. Standard vs magnifying narrow-band imaging endoscopy for diagnosis of Helicobacter pylori infection and gastric precancerous conditions. World J Gastroenterol. 2021;27:2238-2250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 26. | Kawamura M, Sekine H, Abe S, Shibuya D, Kato K, Masuda T. Clinical significance of white gastric crypt openings observed via magnifying endoscopy. World J Gastroenterol. 2013;19:9392-9398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Bosnia and Herzegovina
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu J, China S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zheng XM