Published online Dec 16, 2024. doi: 10.4253/wjge.v16.i12.678
Revised: September 26, 2024
Accepted: October 10, 2024
Published online: December 16, 2024
Processing time: 163 Days and 16.7 Hours
Endoscopic resection of giant gastric leiomyomas, particularly in the fundus and cardia regions, is infrequently documented and presents a significant challenge for endoscopic surgery.
Herein, a case of a 59-year-old woman with a giant gastric leiomyoma was re
Endoscopic resection of a giant leiomyoma in the cardiac fundus is feasible and suitable for skilled endoscopists.
Core Tip: Endoscopic resection of giant gastric leiomyomas, particularly in the fundus and cardia regions, is infrequently documented. We report a rare case of giant gastric leiomyoma located in the fundus and cardia. The lesion was completely resected by Endoscopic submucosal excavation and Endoscopic full-thickness resection. The lesion, ginger-shaped and measuring 8 cm × 5 cm, led to transient peritonitis post-surgery. With no cardiac complications, the patient was discharged one week after surgery. Endoscopic resection of a giant leiomyoma in the cardiac fundus is feasible and does not com
- Citation: Li P, Tang GM, Li PL, Zhang C, Wang WQ. Endoscopic resection of a giant irregular leiomyoma in fundus and cardia: A case report. World J Gastrointest Endosc 2024; 16(12): 678-685
- URL: https://www.wjgnet.com/1948-5190/full/v16/i12/678.htm
- DOI: https://dx.doi.org/10.4253/wjge.v16.i12.678
Gastric leiomyoma is a relatively common gastric submucosal tumor (SMT). Most gastric leiomyomas are asymptomatic benign tumors, often monitored without surgical intervention[1]. However, previous case reports have indicated that gastric leiomyomas, especially those in gastric antrum[2], in proximity to the gastroesophageal junction[3], or ulcerated[4], can lead to severe digestive tract hemorrhage. Traditional methods for resecting gastric leiomyomas include laparoscopic and endoscopic approaches. Laparoscopic resection may disrupt the stomach's anatomy and function, notably in the cardiac area, potentially leading to postoperative cardiac dysfunction, stricture, or inadequate closure of the cardiac sphincter[5]. With the advancement of endoscopic technology, methods such as endoscopic submucosal dissection (ESD)[6,7], endoscopic submucosal excavation (ESE)[8], endoscopic full-thickness resection (EFTR)[9,10], and submucosal tunnel endoscopic resection (STER)[10] have been attempted to resect gastric leiomyomas. However, endoscopic resection poses a significant challenge for giant leiomyomas, especially for lesions involving the cardia. Herein, a case of a giant gastric leiomyoma in a 59-year-old woman was presented, and the methods and experience of endoscopic resection of this giant gastric leiomyoma were correspondingly summarized.
A 59-year-old Chinese woman presented to the department of hepatological surgery with a complaint of right upper abdominal pain for one month and worsening for one week.
Symptoms started one month before presentation with persistent, dull pain in the right upper abdomen, without radia
The patient was in good health, denied chronic history, hepatitis, tuberculosis, and other infectious diseases, and denied any history of trauma, surgery, or blood transfusion.
The patient denied any family history of malignant tumors.
On physical examination, the vital signs were as follows: Body mass, M (B) = 68 kg; body temperature, T (B) = 36.3 °C; blood pressure, P (B) = 18.4/10.4 kPa; heart rate, 56 beats per minute; and respiratory rate, 17 breaths per minute. The patient's abdomen was observed to be flat with bilateral symmetry. Tenderness in the upper abdomen was detected upon examination. There was an absence of rebound pain and muscle rigidity, and no abdominal mass was palpable. Murphy's sign was negative, and there was no tenderness in the right lower abdomen.
Levels of serum tumor markers, including carbohydrate antigen (CA)125, CA199, CA153, carcino-embryonic antigen, and alfa-fetal protein were normal. The routine blood test results indicated a white blood cell count of 2.92 × 109/L, and a red blood cell count of 3.75 × 1012/L. The coagulation function was within normal limits.
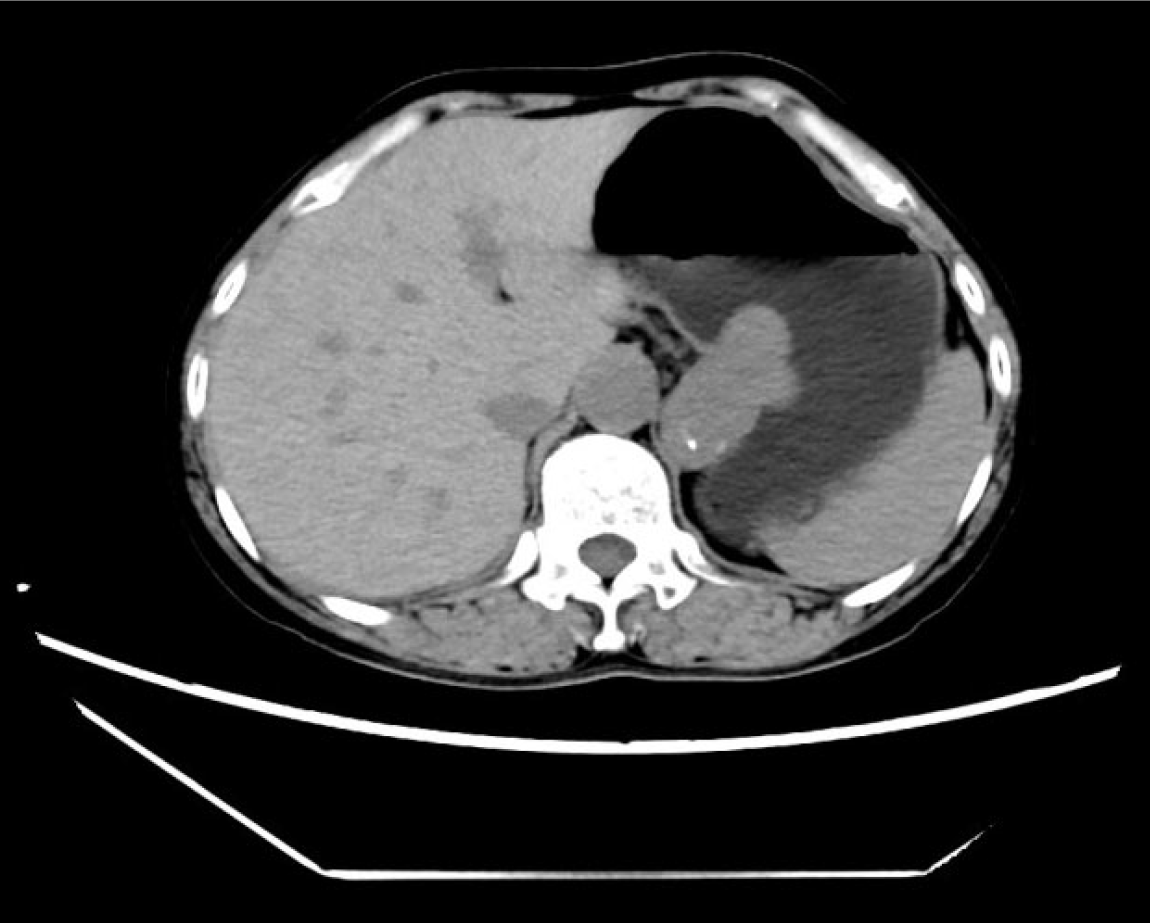
Abdominal enhanced computed tomography (CT) revealed multiple gallstones and an enlarged gallbladder, along with a submucosal irregular mass of the gastric fundus and cardia. The mass was partially protruded out of the cavity, measuring approximately 4.5 cm × 3.2 cm × 4.9 cm in size, with well-defined margins and exhibiting nodular calcifications. The CT values of the mass were about 42 HU, 52 HU, and 54 HU during the normal scan period, arterial period, and venous period, respectively. There were no obvious enlarged lymph nodes around the lesion (Figure 1). Gastroendoscopy indicated a submucosal mass in the fundus and cardia of the stomach, with a maximum length of about 5 cm and an irregular shape (Figure 2A). The patient refused endoscopic ultrasonography (EUS) and EUS guided fine needle biopsy.
Combined with the patient’s medical history, a pre-operative diagnosis of gastric SMT and gallstones with cholecystitis was made.
A multi-disciplinary treatment (MDT) approach was taken, involving collaboration between the departments of gastroenterology, hepatobiliary surgery, gastrointestinal surgery, oncology, and other departments. The gastrointestinal surgeons concluded that stromal tumor was more likely to be considered for the gastric fundus SMT. However, the patient's tumor was notably large and extended into the cardiac region. Laparoscopic resection might affect the cardiac function and lead to postoperative cardiac stenosis or poor closure. Endoscopic resection was thus recommended. Laparoscopic surgery was considered as an alternative in the event that endoscopic resection was not feasible. The department of hepatobiliary surgery believed that the onset of abdominal pain in the patient was related to gallstones and cholecystitis, and laparoscopic cholecystectomy was feasible. Meanwhile, the department of oncology considered it likely that the gastric fundus SMT was a stromal tumor, arguing that either endoscopic resection or laparoscopic surgery was feasible. The pathological results after resection determined the nature of the lesion and subsequent treatment. Besides, the gastroenterologist's opinion was that considering the possibility of stromal tumor, endoscopic resection could be initially performed. If endoscopic resection was not feasible for complete excision, laparoscopic surgery became the next option. Following discussions with the patient and family, endoscopic resection was finally chosen.
The patient was anesthetized by tracheal intubation, underwent laparoscopic cholecystectomy in the department of hepatobiliary surgery first, and then experienced endoscopic tumor resection. The HD-550 type gastroendoscope from SonoScape Company in China was employed for the treatment. The SMT was exposed in the fundus and cardiac of the stomach, with a maximum length of about 5 cm (Figure 2A). A disposable mucosal injection needle (Micro-Tech Co. LST, China) was used to inject 0.1% methylene blue with saline at the edge of the lesion to lift the submucosa surrounding the lesion. The mucosa was cut into the submucosa using a disposable mucosal incision knife (Micro-Tech Co. LST, China), layer by layer. This procedure exposed the tumor, which was then carefully peeled away from the surrounding tissue (Figure 2B). The tumor, with its irregular configuration, was found to infiltrate along the musculi propria. The surgical approach involved meticulously dissecting and separating the tumor from the surrounding musculi propria. The large blood vessels detected were promptly treated with vascular clamp (Micro-Tech Co. LST, China) for electrocoagulation. A small part of the tumor protruded out of the gastral cavity, and full-layer resection was performed with an incision of about 1cm. The lesion was finally completely peeled off, measuring approximately 8 centimeters in its greatest diameter. It had an irregular, ginger-like shape, and the resection was complete with an intact envelope (Figure 2C). The length of the wound post-operation was about 8 cm (Figure 2D). After the blood vessels at the wound surface were treated with electrocoagulation, the wound was sutured with double pouches (Figure 2E). However, the lesion could not be comple
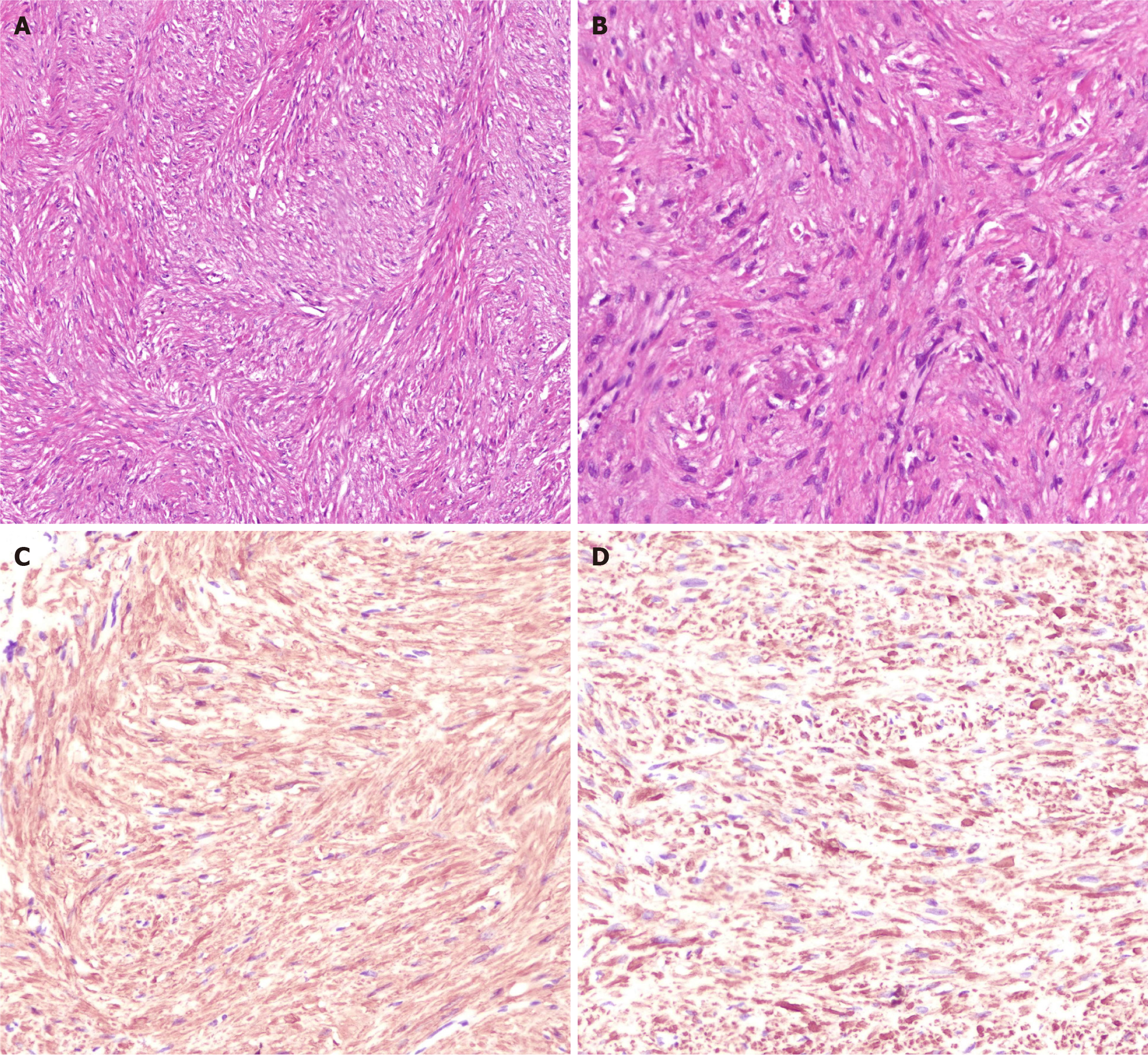
The patient experienced the onset of fever on the second post-operative day, peaking at 38.9 °C, accompanied by generalized abdominal pain and mild tenderness throughout the abdomen. There was no rebound tenderness or muscle rigidity observed. The patient was given indwelling gastric tube, gastrointestinal decompression, fasting, antibiotic anti-infection, acid inhibition, fluid rehydration, etc. After 48 hours, the temperature returned to normal, the abdominal pain and tenderness were relieved, food was resumed, antibiotics were discontinued, and the patient was discharged from hospital one week after surgery. Postoperative pathology revealed a fusiform cell tumor (fundus), which was consistent with leiomyoma in combination with morphology and immunohistochemistry. The immunohistochemical results included SMA (+), Desmin (+) and CD34 + (blood vessels), CD117 (-), DoG-1, (-), CK (-), Ki67 (about 1%) positive (Figure 3). Upon one month of follow-up, the patient had no eating obstruction or gastroesophageal reflux. After 3 months of follow-up, the wound healed well under gastroscopy, no tumor recurrence was observed, and no the complications above mentioned. The patient is still under follow-up.
The majority of gastric leiomyomas do not present with distinctive clinical symptoms and are usually identified coincidentally during gastroscopy or CT scans conducted for different medical indications. Herein, the patient was admitted to hospital with abdominal pain, mainly in the right upper abdomen, which was primarily caused by gallstones and cholecystitis. Gastric leiomyomas are often mistaken for stromal tumors or neuroendocrine tumors pre-operatively. While CT scans are beneficial, their diagnostic value is somewhat limited[11-13]. In this report, preoperative CT examination also considered the possibility of stromal tumor, and the judgment of tumor size was inaccurate. The size, shape, texture, and manifestations of the tumor could be observed under ordinary white light endoscopy. However, considering the position of the tumor in the submucosal layer, the intramucosal situation could not be observed. Thus, only a part of the tumor in the stomach cavity could be hereby observed under white light endoscopy, with a maximum diameter of only 5 cm, which was significantly different from the size of the actual resected lesion. Additionally, EUS is helpful to judge the origin, invasion, hardness, and blood supply of the tumor, providing certain value in the diagnosis of gastric SMT, while ultrasound-guided fine needle aspiration biopsy aids in assessing the quality of the lesion before surgery[14,15]. Preoperative EUS examination was also recommended to the patient in this case, but the patient declined EUS and EUS-guided fine needle biopsy due to cost. This led to a preoperative presumption of a gastrointestinal stromal tumor, man
Endoscopic treatment and laparoscopic surgery are the main methods for gastric SMT resection. With the rapid development of endoscopic technology, the indications for endoscopic treatment of gastric SMT are no longer limited by the size and location of the lesion. As long as complete endoscopic resection can be ensured, the procedure is considered feasible[16]. Laparoscopic resection is also a commonly-used surgical method for the treatment of gastric SMT. However, SMT involving the cardiac or gastroesophageal junction can possibly cause gastroesophageal junction malformation or lower esophageal sphincter injury, thereby resulting in eating obstruction or gastroesophageal reflux[17]. Endoscopic surgery for gastric SMTs offers advantages over traditional methods, including less trauma, minimal cardiac impact, lower cost, and quicker postoperative recovery. In order to provide the best treatment for the patient, an MDT was arranged before surgery. Gastrointestinal surgeons, digestive endoscopies, and oncologists all agreed that the patient's tumor was highly suspected to be a stromal tumor, given its large size, necessitating resection. Gastrointestinal surgeons considered that the lesion involved the cardia and was thus not suitable for laparoscopic resection. Digestive endoscopists believed that endoscopic resection was feasible. Given the patient's concurrent gallstones and cholecystitis, a decision was made to perform a laparoscopic cholecystectomy and endoscopic tumor resection in a single procedure. This approach aimed to minimize the number of anesthesia exposures and shorten the hospital stay. The methods of endoscopic resection of SMT include ESD, ESE, STER, and EFTR. Among them, STER is particularly suitable for locations such as the esophagus or the gastroesophageal junction, where it is relatively easy to create a submucosal tunnel[18-20]. Given that the majority of the tumor was situated in the fundus, the STER method was not deemed appropriate. Gastric perforation is inevitable for tumors growing outside the cavity, which can readily lead to pneumoperitoneum and intraperitoneal infection. To minimize the perforation size, the dissection along the surface of the tumor was carried out as far as possible. Once the perforation occurred, titanium clips were utilized to reduce its size without compromising the surgical procedure. Carbon dioxide was used during the operation, and once the abdominal gas increased, the puncture was discharged at the McGregor point in time. After surgery, an indwelling gastric tube was used for gastrointestinal decompression, and antibiotics were administered to guard against abdominal infections. Considering that most of the lesions were located in the stomach cavity, the ESE + EFTR method was proposed. Specifically, the lesion was first removed by ESE, such as the lesion protruding from the serosal layer to perform EFTR. Firstly, the inverted gastroendoscope method was utilized to resect the mucosa and submucosa at the top of the lesion, exposing the lesion and stripping along the edge of the lesion. During the operation, the lesion was surprisingly observed to be much larger than it was expected before surgery, and its shape was irregular, branching into the cardia. The branching lesion was continued to peel off, and the protrusion location of the base of the lesion was finally exposed. EFTR was performed, and the lesion was completely resected in about 3 hours with the wound length of about 8 cm. The wound suture time was about 1 hour. A multi-centre study from Japan retrospectively analyzed 117 patients with endoscopic resection of gastric SMT. The lesion diameter was 8-40 mm (20 ± 7.2 mm), the complete resection rate was 99%, and the mean lesion resection and wound closure time were 58 ± 38 (range, 12-254) minutes and 31 ± 41 (range, 3-330) minutes. Besides, the total excision accounted for 44%, requiring abdominal puncture exhaust accounted for 12%. Among them, 3 cases needed to be transferred to laparoscopic surgery due to luminal collapse, uncontrolled bleeding, and difficulty in closing the wound[21]. Shichijo et al[22] reported 46 patients with gastric SMT resection by EFTR, involving tumor sizes ranging from 11-28 mm (average 18.8 ± 4.5mm) and lesion resection time spanning from 22-125 minutes (average 54 ± 26minutes). The wound suture time was 12-186 min (average 33 ± 28). Different from the above reports, the lesion in this case was larger, reaching 8cm in diameter, so the resection time was also longer, reaching 3 hours. Bleeding is one of the main complications of endoscopic surgery, and the key to prevention is to pre-coagulate the large blood vessels. In the case study, thick blood vessels were also encountered during the resection. The operation was then conducted slowly and meticulously, with preemptive electrocoagulation treatment applied using a vascular clamp to manage the vessels. The intraoperative bleeding was about 50 mL. Fortunately, the bleeding was controlled. Intraperitoneal gas increased after full layer resection, while intraperitoneal pressure decreased after Mcburney point puncture. After the wound was sutured, the challenge of removing the tumor ensued, complicated by its large size which could not be extracted per os in one go, leading to significant operational difficulties. An electric snare was used to cut the lesion into small pieces with a diameter of 2-3 cm, and the lesion was removed orally. The removal took nearly 2 hours. The lesion was removed and reconstructed according to the incision margin. The size of the lesion was 8 cm × 5 cm, the texture was hard, and part of the envelope was destroyed. Fortunately, the final pathology was leiomyoma, and there was generally no possibility of abdominal implantation and metastasis. Following surgery, the patient presented with peritonitis such as fever, abdominal pain, abdominal tenderness, etc. Considering full-layer incision and prolonged operation time, stomach contents had entered the abdominal cavity, leading to intraperitoneal infection. This was promptly managed with antibiotic therapy. Postoperatively, there were no instances of gastric obstruction or gastroesophageal reflux, and no occurrences of gastric or intraperitoneal bleeding were reported.
In summary, the resection and extraction of an 8 cm ginger-like gastric leiomyoma were hereby successfully completed using gastroscopy in 6 hours. The main lessons learned include: (1) Giant leiomyomas that have areas of calcification can be challenging to differentiate from stromal tumors pre-operatively; (2) Preoperative evaluation of the size and shape of the tumor by gastroscopy and CT may vary significantly from the actual situation; (3) Leveraging the transparent cap and the gravitational pull on the tumor during the stripping procedure allows for full exposure of the tumor; (4) Gastric leiomyoma may present irregular branching growth. In such cases, the dissection should still be carried out at the base of the tumor. It is important to avoid severing the tumor body, ensuring that the tumor remains intact; (5) Giant gastric leiomyoma is rich in nourishing blood vessels and prone to bleeding during the operation. Therefore, the operation should be performed carefully. Upon bleeding, it is crucial to precisely expose the blood vessels and achieve hemostasis in a timely manner; (6) Full-layer excision should be carried out as far as possible at the end. After any perforation, it is important to limit the amount of gas insufflated, to monitor for increased abdominal pressure, and to perform a timely puncture to release the gas if necessary; (7) When the tumor cannot be removed at one time, the wound should be sutured first, and then the tumor can be cut into small pieces and removed from mouth. This can reduce the possibility of tumor implantation and metastasis in the abdominal cavity; (8) Postoperatively, a gastric tube should be left in place for gastrointestinal decompression, and the patient should be placed on appropriate fasting. During this period, it is important to monitor for complications such as peritonitis, bleeding, cardiac obstruction, and gastroesophageal reflux; and (9) Compared to laparoscopy, endoscopic resection of giant gastric leiomyoma involving the cardiac induces less trauma, and it is easier to preserve the anatomical structure and functional integrity of the cardia. The main disadvan
Endoscopic resection of a giant leiomyoma in the cardiac fundus is feasible and does not affect cardiac function after operation, making it suitable for skilled endoscopists.
| 1. | Park K, Ahn JY, Na HK, Jung KW, Lee JH, Kim DH, Choi KD, Song HJ, Lee GH, Jung HY. Natural history of gastric leiomyoma. Surg Endosc. 2024;38:2726-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 2. | García Martínez A, Fernández Olvera D, Moreno García AM. Gastric leiomyoma as an atypical cause of upper gastrointestinal bleeding. Rev Esp Enferm Dig. 2023;115:342. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | White A, Sikora J, Thannoun A, Soliman B. Gastric Leiomyoma Near the Gastroesophageal Junction Causing Massive Gastrointestinal Bleeding. Cureus. 2023;15:e48374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Ramai D, Tan QT, Nigar S, Ofori E, Etienne D, Reddy M. Ulcerated gastric leiomyoma causing massive upper gastrointestinal bleeding: A case report. Mol Clin Oncol. 2018;8:671-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Lee JS, Kim JJ, Park SM. Laparoscopic gastric wedge resection and prophylactic antireflux surgery for a submucosal tumor of gastroesophageal junction. J Gastric Cancer. 2011;11:131-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Sanahuja A, Segura MJ, Azorín MC, Merino V, Fernández C, Moles JR, Peña A. Endoscopic submucosal dissection with an SB Knife® for the treatment of subcardial gastric leiomyoma. Rev Esp Enferm Dig. 2021;113:794-796. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Wang Z, Hou G. Endoscopic submucosal dissection of endobronchial leiomyoma with a hybrid knife in an adolescent patient: a case report. Front Oncol. 2023;13:1288044. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Liu J, Tan Y, Liu D, Li C, Le M, Zhou H. Factors predicting technical difficulties during endoscopic submucosal excavation for gastric submucosal tumor. J Int Med Res. 2021;49:3000605211029808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, Liu JZ. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 10. | ASGE Technology Committee; Aslanian HR, Sethi A, Bhutani MS, Goodman AJ, Krishnan K, Lichtenstein DR, Melson J, Navaneethan U, Pannala R, Parsi MA, Schulman AR, Sullivan SA, Thosani N, Trikudanathan G, Trindade AJ, Watson RR, Maple JT. ASGE guideline for endoscopic full-thickness resection and submucosal tunnel endoscopic resection. VideoGIE. 2019;4:343-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 11. | Zhao L, Xue H, Sun Z, Chen Y, Yu H, Mao S. Clinical and CT Imaging Differences Between Gastric Schwannoma and Gastric Leiomyoma. J Coll Physicians Surg Pak. 2023;33:369-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Wang Q, Wang L, Qi X, Liu X, Xie Q, Wang Y, Shi G. Computed tomography features of gastric leiomyoma versus gastric stromal tumor: a case-control study with propensity score matching. J Int Med Res. 2023;51:3000605231171025. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Wang J, Zhou X, Xu F, Ao W, Hu H. Value of CT Imaging in the Differentiation of Gastric Leiomyoma From Gastric Stromal Tumor. Can Assoc Radiol J. 2021;72:444-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Lu Y, Zhuo X, Zhong Q, Sun J, Li C, Zhi M. Endoscopic ultrasonography is useful for predicting perforation in the endoscopic resection of gastric submucosal tumors originating from the muscularis propria: a retrospective case-control study. Ultrasonography. 2023;42:78-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Jacobson BC, Bhatt A, Greer KB, Lee LS, Park WG, Sauer BG, Shami VM. ACG Clinical Guideline: Diagnosis and Management of Gastrointestinal Subepithelial Lesions. Am J Gastroenterol. 2023;118:46-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 102] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 16. | Zhou P, Zhong Y, Li Q. [Chinese Consensus on Endoscopic Diagnosis and Management of Gastrointestinal Submucosal Tumor(Version 2018)]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:841-852. [PubMed] [DOI] [Full Text] |
| 17. | Tarcoveanu E, Bradea C, Dimofte G, Ferariu D, Vasilescu A. Laparoscopic wedge resection of gastric leiomyoma. JSLS. 2006;10:368-374. [PubMed] |
| 18. | Chiu PWY, Yip HC, Chan SM, Ng SKK, Teoh AYB, Ng EKW. Endoscopic full-thickness resection (EFTR) compared to submucosal tunnel endoscopic resection (STER) for treatment of gastric gastrointestinal stromal tumors. Endosc Int Open. 2023;11:E179-E186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 19. | Estevinho MM, Pinho R, Rodrigues J, Fernandes S, Correia J, Mesquita P, Freitas T. Effective submucosal tunneling endoscopic resection (STER) of a giant esophageal leiomyoma. Rev Esp Enferm Dig. 2023;115:746-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Dalal I, Andalib I. Advances in endoscopic resection: a review of endoscopic submucosal dissection (ESD), endoscopic full thickness resection (EFTR) and submucosal tunneling endoscopic resection (STER). Transl Gastroenterol Hepatol. 2022;7:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Shichijo S, Abe N, Takeuchi H, Ohata K, Minato Y, Hashiguchi K, Hirasawa K, Kayaba S, Shinkai H, Kobara H, Yamashina T, Ishida T, Chiba H, Ono H, Mori H, Uedo N. Endoscopic resection for gastric submucosal tumors: Japanese multicenter retrospective study. Dig Endosc. 2023;35:206-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Shichijo S, Uedo N, Sawada A, Hirasawa K, Takeuchi H, Abe N, Miyaoka M, Yao K, Dobashi A, Sumiyama K, Ishida T, Morita Y, Ono H. Endoscopic full-thickness resection for gastric submucosal tumors: Japanese multicenter prospective study. Dig Endosc. 2024;36:811-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/