Published online Mar 16, 2023. doi: 10.4253/wjge.v15.i3.122
Peer-review started: November 9, 2022
First decision: November 30, 2022
Revised: December 20, 2022
Accepted: February 9, 2023
Article in press: February 9, 2023
Published online: March 16, 2023
Processing time: 125 Days and 3.3 Hours
Endoscopic retrograde cholangiopancreatography (ERCP) is the preferred modality for drainage of the obstructed biliary tree. In patients with surgically altered anatomy, ERCP using standard techniques may not be feasible. Ente
Core Tip: Endoscopic retrograde cholangiopancreatography is the mainstay for biliary drainage in benign and malignant biliary obstruction. Surgically altered anatomy poses a significant challenge to successful endoscopic retrograde cholangiopancreatography (ERCP). Enteroscopy assisted ERCP may need to be performed in this situation with variable rates of success. On the other hand, Endoscopic ultrasound guided biliary drainage represents a potential alternative to enteroscopy assisted ERCP. In patients with benign biliary obstruction, endoscopic ultrasound (EUS) guided rendezvous is the primary option for accessing the bile duct and ensuring clinical success of ERCP. In malignant obstruction, EUS guided antegrade intervention or transmural stent placement are options. EUS-BD ensures technical and clinical success is higher than 90% in expert hands.
- Citation: Sundaram S, Kale A. Endoscopic ultrasound guided biliary drainage in surgically altered anatomy: A comprehensive review of various approaches. World J Gastrointest Endosc 2023; 15(3): 122-132
- URL: https://www.wjgnet.com/1948-5190/full/v15/i3/122.htm
- DOI: https://dx.doi.org/10.4253/wjge.v15.i3.122
Endoscopic retrograde cholangiopancreatography (ERCP) is the preferred method for biliary drainage. Although quiet successful in normal anatomy, it challenging to perform ERCP in patients with surgically altered anatomy[1]. Even with the use of single or double balloon enteroscope, when standard duodenoscope fails to reach papilla, it is technically difficult to bring papilla en-face for cannulation[1]. Cannulation using existing ERCP equipment is also challenging. Traditional alternative for biliary drainage was percutaneous transhepatic biliary drainage (PTBD) however, with development in endoscopic ultrasound (EUS) it has become possible to visualise and get access to biliary tree by various approaches using linear array echoendoscopes[2]. With better echoendoscopes with wide working channel it has become possible to perform EUS guided biliary interventions not only for malignant diseases but also for benign cases even in patients with surgically altered anatomy[3,4]. In this review we will cover role of EUS biliary drainage (EUS-BD) in patients with surgically altered anatomy (SAA), various approaches, methods, their advantages and disadvantages.
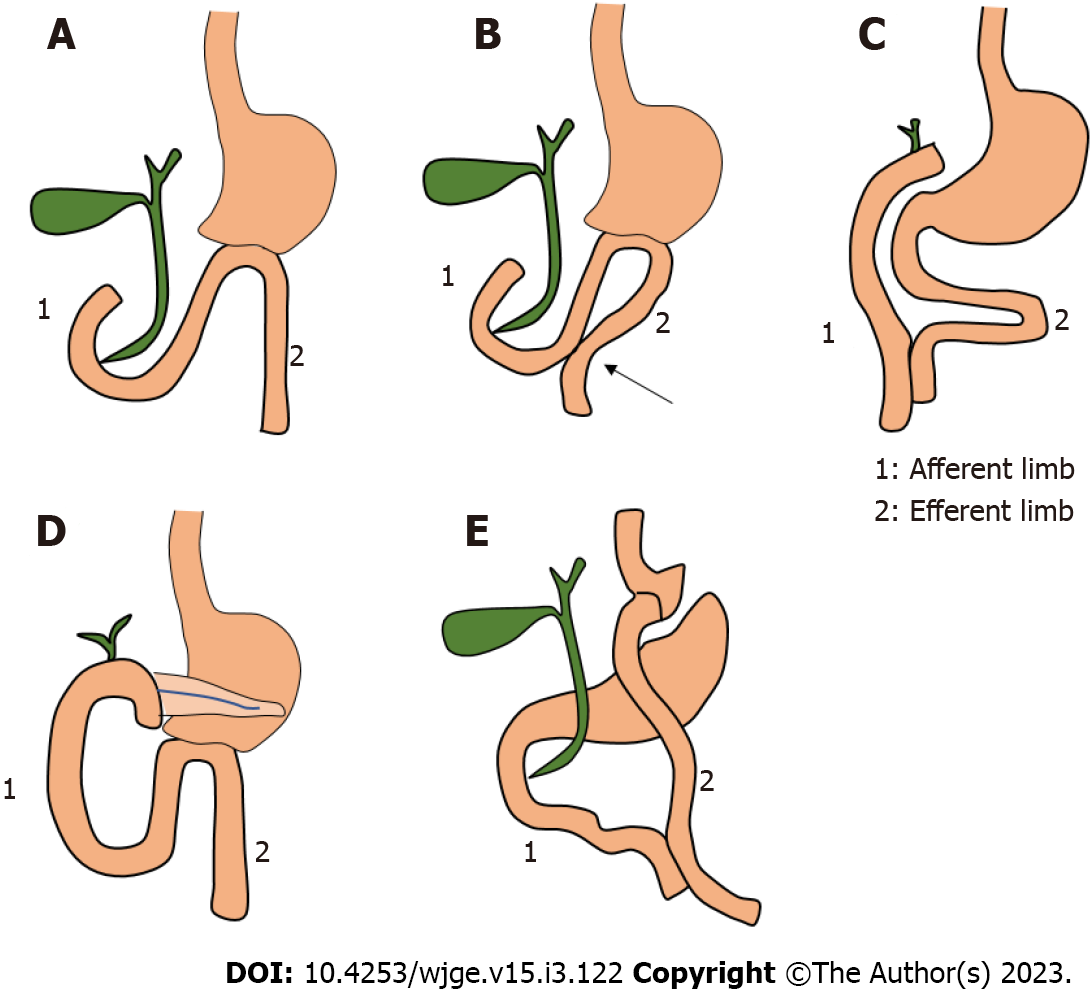
Surgically altered anatomy (SAA) can be divided into two distinct types. Type 1 when duodenum is still in continuity with gastric remnant and standard duodenoscope can be passed till Ampulla of Vater to perform ERCP. Type II is one in which stomach remnant or stomach itself is not in continuity with duodenum and there is need of enteroscope or colonoscope to reach the ampulla causing difficulties. Examples of type I include sleeve gastrectomy and Billroth I type anatomy while type II SAA includes partial gastrectomy with Billroth II reconstruction or gastrojejunostomy (GJ) without gastric resection, Whipple anatomy, Roux-en-Y gastric bypass, and Roux-en-Y heapticojejunostomy[5] (Figure 1).
In this procedure the greater curvature of the stomach is resected, and the remnant stomach is kept in continuity with the small bowel. Duodenoscope can be passed through gastric sleeve to reach Ampulla of Vater and ERCP can be performed using standard accessories. In case of failed ERCP procedure, EUS guided access to bile duct is possible through duodenum and segment 2 or 3 radicals can be accessed from remnant stomach for antegrade approach[3,5,6].
In this procedure, antrectomy is performed followed by an end-to-end anastomosis between the remnant stomach and the duodenum. Since duodenum is in continuity with stomach remnant ERCP can be performed using duodenoscope from major papilla. As in sleeve gastrectomy EUS guided access to bile duct is possible through duodenum and segment 2 or 3 biliary radicals can be accessed through gastric remnant[3,5,6].
Partial gastrectomy with gastrojejunostomy is commonly performed for gastric cancer while gastrojejunostomy is performed for complications of peptic ulcer disease like gastric outlet obstruction. In both cases afferent limb of variable length is in continuity with duodenum and efferent limb is connected to small bowel. Approach to the papilla is through the afferent limb. Success of cannulation depends on length of afferent limb, angulation of anastomosis and position of papilla. EUS guided approach to biliary tree is through segment 2 or 3 biliary radicles which can be accessed through gastric remnant[3,5,6]. If there is difficulty inserting an e-ERCP scope in Billroth-II anatomy, switching to Interventional EUS without straining is a reasonable option.
In this procedure, the stomach is divided into small proximal pouch and large distal pouch which is in continuity with duodenum. Small bowel is divided into two limbs one is biliopancreatic which is formed by duodenum and proximal jejunum, while Roux limb is formed by small bowel distal to division and anastomosed with gastric pouch as gastojejunostomy (GJ). Enteroscope assisted ERCP is possible, however with a low success rate[7,8]. Papilla can be accessed in up to 84% cases with successful cannulation achieved in 94%. This rate is lower than other surgically altered anatomy[9]. EUS guided approach to biliary tree is through segment 2 or 3 biliary radicles which can be accessed through gastric remnant[3,5,6].
This surgery is performed for periampullary carcinoma and pancreatic head carcinoma. It consists of removal of the pancreatic head, distal stomach, duodenum, proximal jejunum, distal common bile duct, and gallbladder. Reconstruction is done by creating a pancreaticojejunostomy (PJ), choledochojejunostomy (CJ), and GJ. EUS guided approach to biliary tree is possible through segment 2 or 3 biliary radicles which can be accessed through gastric remnant[3,5,6].
EUS guided biliary drainage can be performed by three approaches: EUS-rendezvous (EUS-RV), transluminal and EUS-guided antegrade approaches. These procedures are performed using CO2 insufflator, under general anaesthesia or conscious sedation after administration of prophylactic antibiotics[3,5,6,10]. No previous studies have assess the comparative need based on surgical altered anatomy.
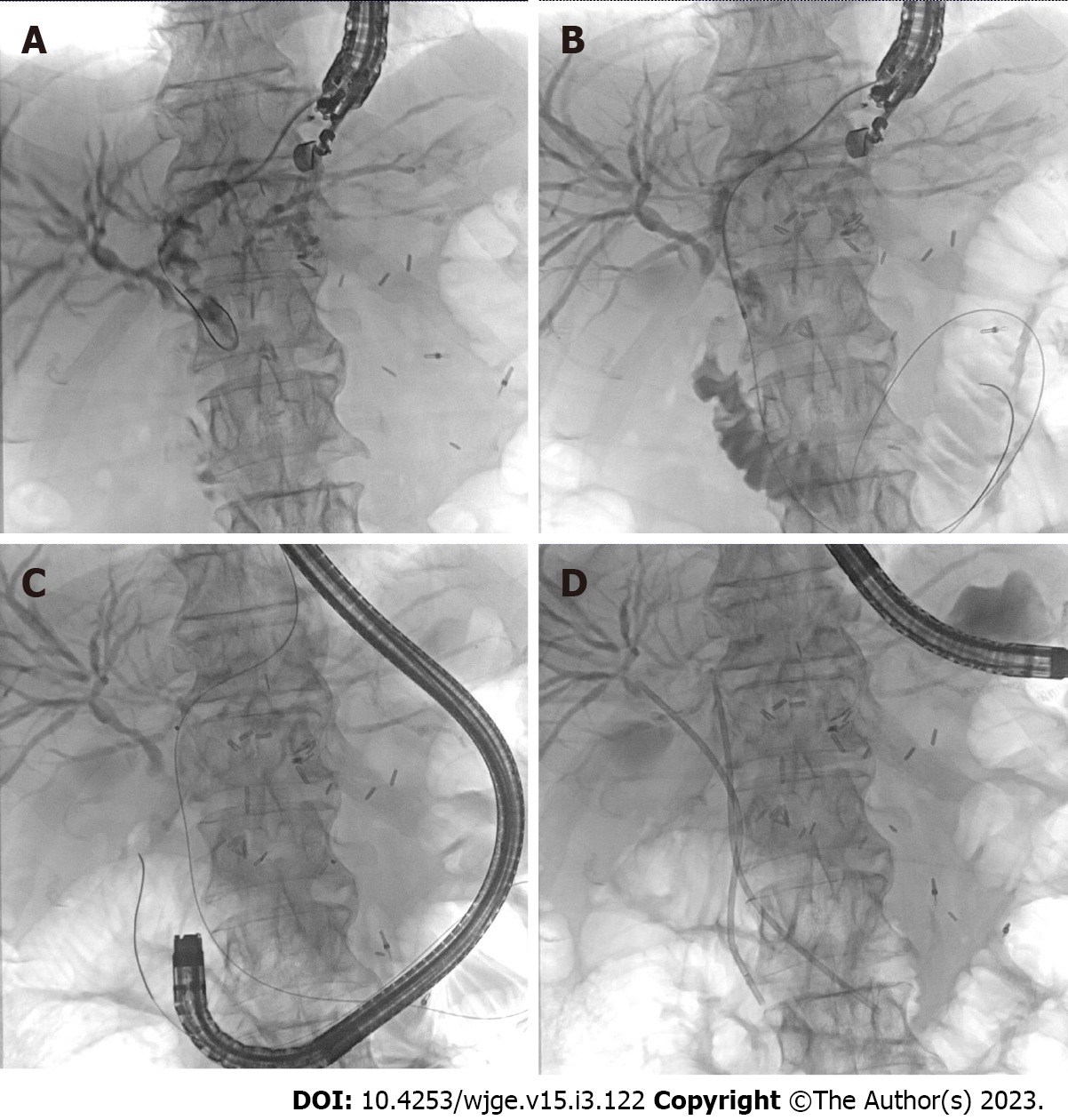
This procedure should only be attempted in the SAA cases where papilla is accessible using duodenoscope or balloon assisted enteroscope. Dilated biliary tree can be accessed using stomach from where segment 2 or 3 ducts can be accessed or dilated bile duct can be accessed from first part of duodenum (D1) as in EUS guided choledochoduodenostomy (EUS-CDS). Guidewire is then passed across dilated biliary tree through the papilla, where it is captured using duodenoscope or enteroscope after careful exchange of endoscopes to avoid slippage of guide-wire[3,5,6,10]. EUS-RV is the preferred technique in benign biliary obstruction (Figure 2).
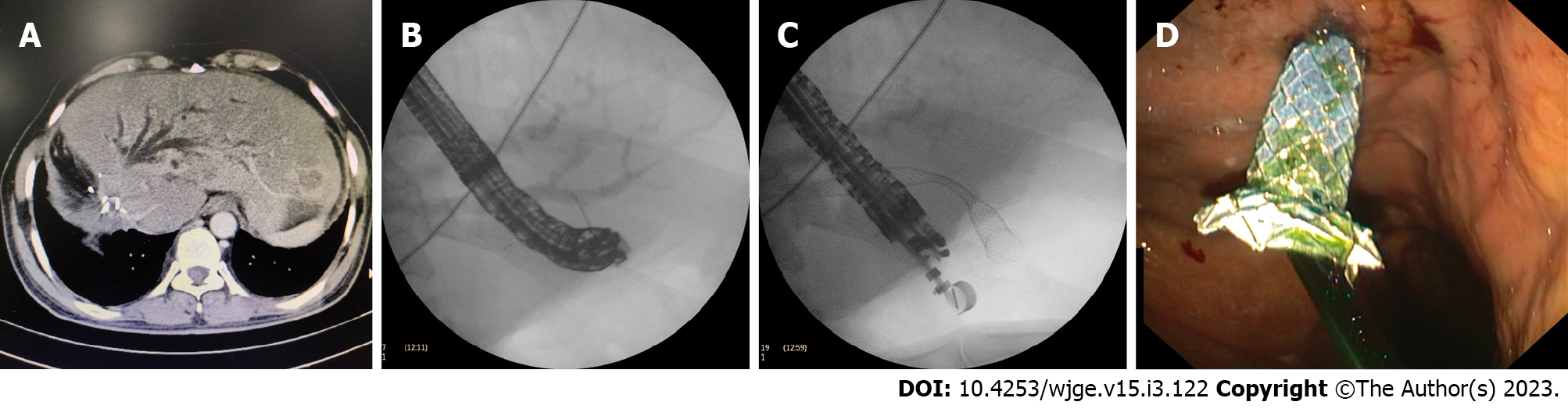
It involves creation of fistula between part of biliary tree and lumen of gastrointestinal tract. This can be between bile duct and duodenum as in EUS-CDS or segment 2 or 3 ducts and stomach or gastric remnant as in EUS-HGS. Puncture of common bile duct or segment 2 and 3 radicals is made from duodenum or stomach respectively. Guidewire is passed deep inside biliary tree. After guidewire insertion fistula is created using cystotome across which self- expandable metal stent (SEMS) can be placed (Figure 3). In cases of total gastrostomy with jejunum anastomosed to oesophagus transluminal drainage can be performed from afferent jejunal limb and creation of choledocho-jejunostomy or hepaticojejunostomy[3,5,6,10].
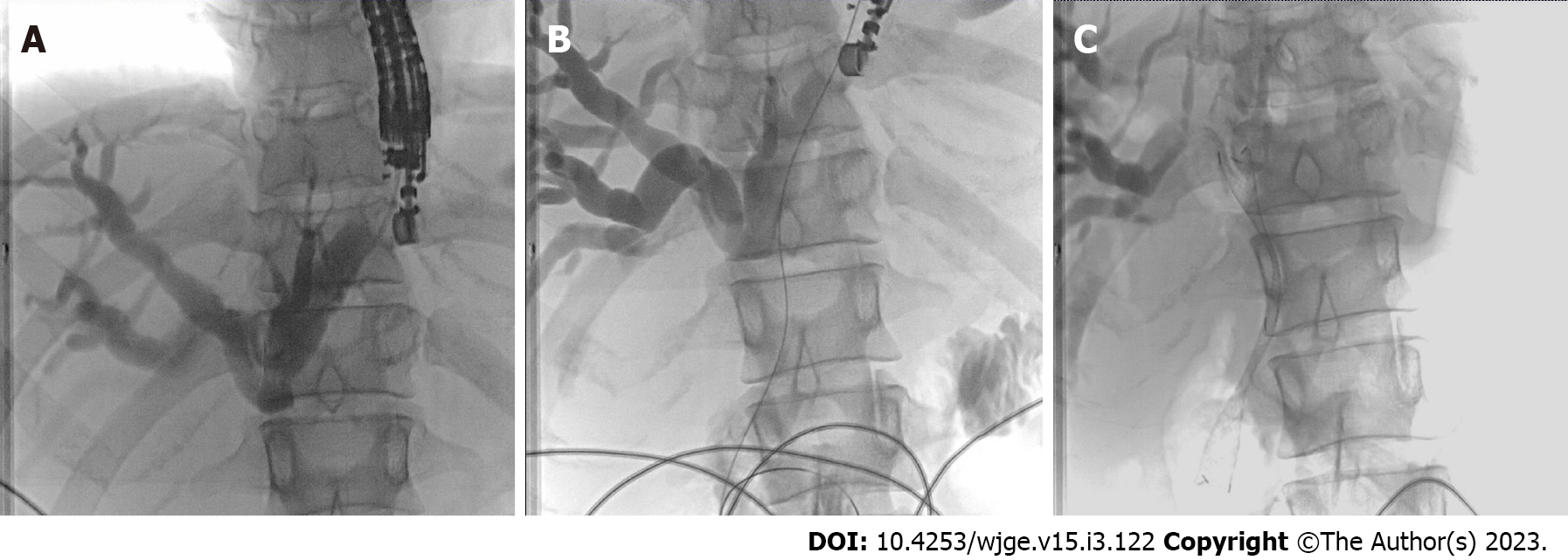
Approach to biliary tree is from segment 2 or 3 biliary radicals of left lobe of liver. Guidewire is negotiated across the stricture or anastomotic site and stent is placed across the stricture or papilla in antegrade fashion (Figure 4). In case of choledocholithiasis balloon dilatation of sphincter or anastomotic stricture in antegrade fashion can be performed and stones can be pushed into the small intestine using balloon catheter[3,5,6,10,11].
Using linear array echoendoscope gastric remnant is identified. Puncture is taken using 19G FNA needle. Contrast-saline is injected to confirm the position. Electrocautery enhanced lumen opposing metal stent (LAMS) is placed across the fistula. Balloon dilatation of the stent is carried out to 18 mm and ERCP is performed by passing the scope across the stent from gastric pouch to gastric remnant which is in continuity with duodenum. ERCP can be performed using standard duodenoscope and accessories through papilla[12]. ERCP can be performed immediately after LAMS placement or after 4 weeks once fistula is mature. If performed immediately then chances of LAMS dislodgement are high and require fixation of LAMS using sutures[5]. LAMS can be removed once biliary intervention is completed. Fistula is allowed to close by secondary intention and closure is confirmed at 8 wk by oral contrast study or endoscopy. In case of failure of fistula to close over the scope clip or suturing can be performed.
In patients with surgically altered anatomy approach to EUS guided biliary drainage depends on access to papilla. In case of sleeve gastrectomy stomach remnant is in continuity with the duodenum and ampulla is accessible. Hence in case of failed conventional ERCP, EUS guided rendezvous and transluminal procedures like EUS-CDS can be performed as in native anatomy[5]. However in cases where access to papilla is not possible or difficult e.g. Billroth II gastrectomy, Roux-en-Y reconstruction, rendezvous procedure is not possible. In these cases antegrade approaches by puncturing segment II or III duct or transluminal approaches like hepaticogastrostomy (EUS-HGS) or hepaticojejunostomy (EUS-HJ), in cases of accessible afferent limb, need to be performed. Multiple procedures can also be combined together, especially for benign indications like choledocholithiasis[3,5,6,10,11]. Table 1 gives a summary of surgically altered anatomy with approach to biliary tree and EUS guided biliary drainage procedures.
| No | Surgically altered anatomy | Approach to biliary tree | EUS biliary drainage procedure |
| 1 | Sleeve gastrectomy | From duodenum bile duct can be punctured; From Segment 2 or 3 ducts | Transmural: EUS CD, Rendezvous procedure; Transmural: EUS HGS, Antegrade drainage procedure |
| 2 | Billroth-I gastrectomy | From duodenum bile duct can be punctured; From segment 2 or 3 ducts | Transmural: EUS CD, Rendezvous procedure; Transmural: EUS HGS, Antegrade drainage procedure |
| 3 | Billroth-II gastrectomy | From segment 2 or 3 ducts | Transmural: EUS HGS, Antegrade drainage procedure |
| 4 | Roux-en-Y gastric bypass | From segment 2 or 3 ducts | Transmural: EUS HGS, Antegrade drainage procedure; EDGE procedure |
| 5 | Whipple’s procedure | From segment 2 or 3 ducts | Transmural: EUS HGS, Antegrade drainage procedure |
| 6 | Roux-en-Y hepatojejunostomy | From segment 2 or 3 ducts | Transmural: EUS HGS, Antegrade drainage procedure |
Initial studies with antegrade drainage procedures showed lower success rate of about 67% however recent studies showed shown clinical and technical success rate of more than 90%[13-21]. In a large series of EUS guided antegrade stent placement (n = 54) including patients with surgically altered anatomy, technical success was 88.7% with clinical success of 95.7%[22]. Complication rate has also reduced from 70% to 10% with increasing expertise and use of different techniques[13-23]. Mukai et al[21] had used two staged technique to tackle choledocholithiasis with > 90% clinical and technical rate. At first, EUS HGS was performed with placement of covered SEMS followed by interventions to remove stone using cholangioscope and lithotripsy devices after maturation of the fistulous tract.
Huang et al[2] in their study showed that clinical and technical success rate of transmural drainage procedures (EUS-HG, EUS-CD, EUS-rendezvous) in patients with surgically altered anatomy is 93.3% and 84.9%. Minaga et al[23] also noted similar success rate. Complication rate was 8%-9% in both studies. Haemorrhage, cholangitis, bile leak were complications noted in both studies[2,23].
Kedia et al[24] compared laparoscopy assisted ERCP with EDGE procedure in Roux-en-Y Gastric Bypass (RYGB) and found similar technical success (EDGE 96.5% vs LA-ERCP 97.7%), number of ERCP procedures needed to achieve clinical resolution (EDGE 1.2 vs LA-ERCP 1.02) and adverse event rate (EDGE, 24%, 7/29 and LA-ERCP, 19%, 8/43). However total procedure time (73 vs 184 min) and length of hospital stay (0.8 vs 2.65 d) was significantly shorter for EDGE compared to LA-ERCP. Bukhari et al[25] in their study comparing EDGE procedure to enteroscope assisted ERCP (e-ERCP) for RYGB found that technical success rate was significantly higher in the EDGE versus the e-ERCP group (100% vs 60.0%, P < 0.001). EDGE was associated with shorter procedure time Total procedure time was significantly shorter in patients who underwent EDGE (49.8 min vs 90.7 min, P < 0.001). Resource utilisation with length of hospitalization was shorter in the EUS-GG group (1 vs 10.5 d, P = 0.02) with similar rate of adverse events. While EDGE appears to have upper hand in biliary drainage, this study had a small sample size and was retrospective in nature. Also procedures in this study was performed in expert hands making the results less generalisable. Limb length often decides success in e-ERCP, with length less than 150 cm associated with higher success[26].
An international comparative study involving 98 patients (49-EUS BD group and 49 enteroscope assisted ERCP group), technical success was achieved in 98% patients in the EUS-BD group as compared to 65.3% patients in the e-ERCP group (OR 12.48, P = 0.001) and clinical success in 88% of patients in EUS-BD group as compared to 59.1% in the e-ERCP group (OR 2.83, P = 0.03). EUS BD had significantly shorter procedural time (55 min vs 95 min, P < 0.0001). AEs occurred in the EUS-BD group (20% vs 4%, P = 0.01) which were of mild/moderate severity. Both complications in e-ERCP group were pancreatitis, while patients in EUS-BD group had cholangitis, sepsis, bleeding and pneumoperitoneum, all of which were self-limiting. Length of stay was significantly longer in the EUS-BD group (6.6 d vs 2.4 d, P < 0.0001)[16]. Based on this result EUS BD can be an alternative to enteroscope assisted ERCP in patients with surgically altered anatomy. No previous studies have assessed impact of choice of procedure on quality of life or activities of daily living.
Iwashita et al[27] in their study comparing EUS guided antegrade biliary stenting and PTBD in patients with surgically altered anatomy and malignant biliary obstruction. The technical, clinical, and internalization success rates in the EUS-ABS and PTBD groups were 97.1% vs 96.6% (P = 1.00), 97.1% vs 93.1% (P = 0.586), and 97.1% vs 75.9% (P = 0.01), respectively. The adverse event rate was 11.4% vs 27.6% (P = 0.119). No significant long-term difference was seen in time to recurrent biliary obstruction and survival[28]. EUS guided antegrade biliary stenting is evolving and comparable to PTBD with lesser adverse events in EUS guided antegrade stenting group[27].
EUS guided approach to intrahepatic biliary ducts is usually from the left lobe segment 2 or 3 intrahepatic ducts. Alternatively right intrahepatic duct can be approached through the duodenal bulb. Park et al[28] presented study of 6 patients where right intrahepatic ducts were approached under EUS guidance. Three had altered anatomy. Two underwent successful anastomotic site stricture dilatation and one patient had failed procedure.
This procedure can be performed in patients with non-Roux-en-Y surgically altered anatomy. Bilioenteric limb is distended with water instillation by upper gastrointestinal (GI) scope or placement of nasobiliary drain or through PTBD catheter. Using echoendoscope distended small bowl loop is localised. Doppler signal is applied to see avascular plane for puncture for puncture. Distance between two loops is confirmed to be less than 1cm and puncture is taken. Electrocautery enhanced lumen opposing stents is placed between two loops. This is similar to an EUS guided Gastroenterostomy where the same steps of catheter passage, distension of small bowel loop, localisation of loop and use of cautery enhanced LAMS for puncture are done. ERCP is performed by passing the therapeutic upper GI scope through the LAMS after maturation of fistulous tract. Non-electrocautery enhanced LAMS can also be used. In a previous study by Ichkhanian et al[29] involving eighteen patients, post-Whipple (10/18) and Roux-en-Y hepaticojejunostomy (6/18) were the most common anatomical alterations. Technical success rate of EUS-guided lumen-apposing metal stent (LAMS) placement was 100% and of ERCP was 94.44% (17/18). Minor adverse event in the form of abdominal pain was noted in only 1 patient. Although procedure appears promising very nearly 100% success rate further large studies are required to prove its utility. Table 2 summarises the different studies of EUS guided intervention in patients with SAA.
| Serial No. | Ref. | EUS BD procedure | Surgically altered anatomy | Indication | No. of cases | Success rate (Technical and clinical) | Complications |
| 1 | Weilert et al[13], 2011 | Antegrade approach | RY gastric bypass | Choledocholithiasis (CDL) | 6 | TS-67%; CS-NA | Liver hematoma- 1 case |
| 2 | Iwashita et al[14], 2013 | Antegrade approach | RY gastrojejunostomy, Whipple’s | CDL, Malignant biliary obstruction (MBO) | 6 | TS-100%; CS-NA | Mild pancreatitis-2 |
| 3 | Itoi et al[15], 2014 | Antegrade approach | RY, Gastric bypass, Billroth reconstruction | CDL, MBO | 5 | TS-60%; CS-NA | Nil |
| 4 | Khashab et al[16], 2016 | Antegrade approach | RY reconstruction, RYGB, Whipple, B-II | CDL, MBO | 49 | TS-98%; CS-88% | 20% |
| 5 | Miranda-García et al[17], 2016 | Antegrade approach | Biliary enteric anastomosis (details N/A) | Anastomotic stricture | 7 | TS-57%; CA-100% | 70% Bleeding, stent migration |
| 6 | Iwashita et al[18], 2016 | Antegrade approach | GR with RY-19; GR with BII-3; GR with jejunal interposition-2; PD-4; BDR with HJ-1 | CDL | 29 | 79% | 17% Bile peritonitis, cholecystitis, elevated CRP |
| 7 | James et al[19], 2018 | Antegrade approach | RYGB, RY, B-II reconstruction, Whipple | Benign biliary stricture | 20 | TS-95%; CS-95% | 15% Abdominal pain, mild pancreatitis, mild cholangitis |
| 8 | Hosmer et al[20], 2018 | Antegrade approach | RYGB, RY | CDL | 9 | TS-100%; CS-NA | 11% Cholangitis |
| 9 | Mukai et al[21], 2019 | Antegrade approach | RY, RYGB, Whipple, B-II | Benign biliary stricture, CDL | 48 | TS-91.9%; CS-91.9% | 8.1% Biliary peritonitis |
| 10 | Huang et al[2], 2020 | Transmural drainage; EUV RV-8; EUS-HG = 14; EUS-CD-11 | Billroth I, Billroth II, RYGB, RYHJ Roux-en-Y choledochojejunostomy | MBO | 33 | TS-93.3%; CS-84.9% | 9.09% Haemorrhage, cholangitis |
| 11 | Minaga et al[23], 2020 | Transmural stenting -24; Antegrade stenting-2; Combination of transmural and antegrade-14 | Gastrectomy with RY, Billroth-II, Pancreaticoduodenectomy, RYHJ | MBO | 40 | TS-100%; CS-95% | 15% Bile leak, biliary peritonitis, pneumoperitoneum |
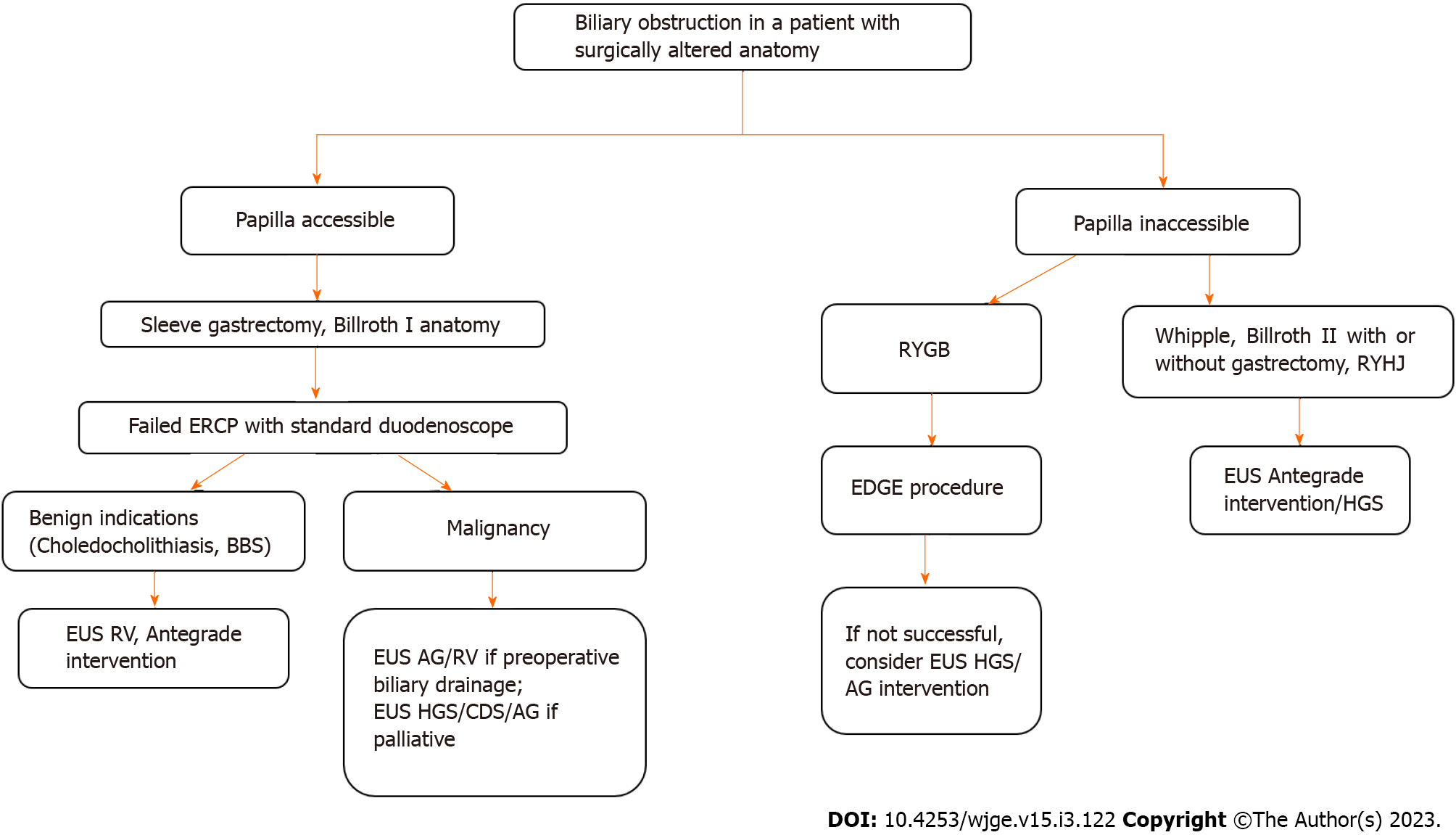
Figure 5 describes algorithm for EUS guided management of biliary obstructions in patients with surgically altered anatomy. Choice of biliary drainage procedure in patients with SAA depends on surgical procedure performed, expertise and equipments available at the center, interventional radiology and surgical back up available. In sleeve gastrectomy and Billroth I reconstruction where duodenum is continuity with gastric remnant and papilla is accessible to standard duodenoscope, ERCP can be attempted as in native anatomy. In case of failed ERCP, if EUS guided approach is planned then it depends on procedure indication. For benign indications EUS-RV and antegrade approaches can be attempted to pass guidewire across the papilla and further procedure can be completed with duodenoscope. In case of malignant distal biliary obstruction where preoperative biliary drainage is required EUS-RV and EUS-antegrade approaches and stenting can be performed which doesn’t significantly alter anatomy and allows surgical resection of tumour along with stent. In cases where palliative biliary drainage is planned EUS-RV or EUS-antegrade approach or EUS-CDS or EUS-HGS can be utilised depending. In case of inaccessible papilla with RYGB, EDGE procedure can be used with success. If EDGE procedure is not feasible then EUS-HGS is the option. For Whipple’s, Billroth II reconstruction, Roux-en-Y hepaticojejunostomy, EUS guided antegrade interventions, EUS-HGS guided interventions can be performed for both benign and malignant biliary indications. For malignant hilar obstructions with surgically altered anatomy multiple procedures may be required including percutaneous biliary drainage to drain right side hepatic ducts.
EUS guided biliary interventions are feasible in surgically altered anatomy for benign as well as malignant indications. EUS-BD equals PTBD and scores over enteroscope assisted ERCP in terms of success rate in patients with biliary obstruction and surgically altered anatomy. With advent of newer devices like LAMS these techniques will develop further and has potential to be ‘primary modality’ for biliary drainage in patients with biliary obstruction and surgically altered anatomy.
| 1. | Lee A, Shah JN. Endoscopic approach to the bile duct in the patient with surgically altered anatomy. Gastrointest Endosc Clin N Am. 2013;23:483-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 2. | Huang P, Zhang H, Zhang XF, Lv W, Fan Z. Application and Value of Endoscopic Ultrasonography Guided Biliary Interventional Therapy in Patients With Biliary Obstruction and Surgically Altered Anatomy. Surg Laparosc Endosc Percutan Tech. 2020;30:454-458. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Nakai Y, Kogure H, Isayama H, Koike K. Endoscopic Ultrasound-Guided Biliary Drainage for Benign Biliary Diseases. Clin Endosc. 2019;52:212-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 4. | Law R, Baron TH. Endoscopic ultrasound-guided biliary interventions: an update on recent developments. Curr Opin Gastroenterol. 2016;32:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Jovani M, Ichkhanian Y, Vosoughi K, Khashab MA. EUS-guided biliary drainage for postsurgical anatomy. Endosc Ultrasound. 2019;8:S57-S66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Katanuma A, Hayashi T, Kin T, Toyonaga H, Honta S, Chikugo K, Ueki H, Ishii T, Takahashi K. Interventional endoscopic ultrasonography in patients with surgically altered anatomy: Techniques and literature review. Dig Endosc. 2020;32:263-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Skinner M, Popa D, Neumann H, Wilcox CM, Mönkemüller K. ERCP with the overtube-assisted enteroscopy technique: a systematic review. Endoscopy. 2014;46:560-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 161] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 8. | Shah RJ, Smolkin M, Yen R, Ross A, Kozarek RA, Howell DA, Bakis G, Jonnalagadda SS, Al-Lehibi AA, Hardy A, Morgan DR, Sethi A, Stevens PD, Akerman PA, Thakkar SJ, Brauer BC. A multicenter, U.S. experience of single-balloon, double-balloon, and rotational overtube-assisted enteroscopy ERCP in patients with surgically altered pancreaticobiliary anatomy (with video). Gastrointest Endosc. 2013;77:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 9. | Khara HS, Parvataneni S, Park S, Choi J, Kothari TH, Kothari ST. Review of ERCP Techniques in Roux-en-Y Gastric Bypass Patients: Highlight on the Novel EUS-Directed Transgastric ERCP (EGDE) Technique. Curr Gastroenterol Rep. 2021;23:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Khashab MA, Valeshabad AK, Modayil R, Widmer J, Saxena P, Idrees M, Iqbal S, Kalloo AN, Stavropoulos SN. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: rendezvous versus direct transluminal techniques (with videos). Gastrointest Endosc. 2013;78:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Martin A, Kistler CA, Wrobel P, Yang JF, Siddiqui AA. Endoscopic ultrasound-guided pancreaticobiliary intervention in patients with surgically altered anatomy and inaccessible papillae: A review of current literature. Endosc Ultrasound. 2016;5:149-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kedia P, Kumta NA, Widmer J, Sundararajan S, Cerefice M, Gaidhane M, Sharaiha R, Kahaleh M. Endoscopic ultrasound-directed transgastric ERCP (EDGE) for Roux-en-Y anatomy: a novel technique. Endoscopy. 2015;47:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Weilert F, Binmoeller KF, Marson F, Bhat Y, Shah JN. Endoscopic ultrasound-guided anterograde treatment of biliary stones following gastric bypass. Endoscopy. 2011;43:1105-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Iwashita T, Yasuda I, Doi S, Uemura S, Mabuchi M, Okuno M, Mukai T, Itoi T, Moriwaki H. Endoscopic ultrasound-guided antegrade treatments for biliary disorders in patients with surgically altered anatomy. Dig Dis Sci. 2013;58:2417-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Itoi T, Sofuni A, Tsuchiya T, Ijima M, Iwashita T. Endoscopic ultrasonography-guided transhepatic antegrade stone removal in patients with surgically altered anatomy: case series and technical review (with videos). J Hepatobiliary Pancreat Sci. 2014;21:E86-E93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Khashab MA, El Zein MH, Sharzehi K, Marson FP, Haluszka O, Small AJ, Nakai Y, Park DH, Kunda R, Teoh AY, Peñas I, Perez-Miranda M, Kumbhari V, Van der Merwe S, Artifon EL, Ross AS. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: an international comparative study. Endosc Int Open. 2016;4:E1322-E1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Miranda-García P, Gonzalez JM, Tellechea JI, Culetto A, Barthet M. EUS hepaticogastrostomy for bilioenteric anastomotic strictures: a permanent access for repeated ambulatory dilations? Endosc Int Open. 2016;4:E461-E465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Iwashita T, Nakai Y, Hara K, Isayama H, Itoi T, Park DH. Endoscopic ultrasound-guided antegrade treatment of bile duct stone in patients with surgically altered anatomy: a multicenter retrospective cohort study. J Hepatobiliary Pancreat Sci. 2016;23:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | James TW, Fan YC, Baron TH. EUS-guided hepaticoenterostomy as a portal to allow definitive antegrade treatment of benign biliary diseases in patients with surgically altered anatomy. Gastrointest Endosc. 2018;88:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 20. | Hosmer A, Abdelfatah MM, Law R, Baron TH. Endoscopic ultrasound-guided hepaticogastrostomy and antegrade clearance of biliary lithiasis in patients with surgically-altered anatomy. Endosc Int Open. 2018;6:E127-E130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Mukai S, Itoi T, Sofuni A, Tsuchiya T, Tanaka R, Tonozuka R, Honjo M, Fujita M, Yamamoto K, Nagakawa Y. EUS-guided antegrade intervention for benign biliary diseases in patients with surgically altered anatomy (with videos). Gastrointest Endosc. 2019;89:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 22. | Sundaram S, Mane K, Patil P, Rathod R, Jain AK, Tyagi U, Mehta S. Endoscopic Ultrasound-Guided Antegrade Stent Placement in Patients with Failed ERCP as a Modality of Preoperative and Palliative Biliary Drainage [published online ahead of print, 2022 Aug 10]. Dig Dis Sci. 2022;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Minaga K, Takenaka M, Ogura T, Tamura T, Kuroda T, Kaku T, Uenoyama Y, Noguchi C, Nishikiori H, Imai H, Sagami R, Fujimori N, Higuchi K, Kudo M, Chiba Y, Kitano M. Endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction with surgically altered anatomy: a multicenter prospective registration study. Therap Adv Gastroenterol. 2020;13:1756284820930964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 24. | Kedia P, Tarnasky PR, Nieto J, Steele SL, Siddiqui A, Xu MM, Tyberg A, Gaidhane M, Kahaleh M. EUS-directed Transgastric ERCP (EDGE) Versus Laparoscopy-assisted ERCP (LA-ERCP) for Roux-en-Y Gastric Bypass (RYGB) Anatomy: A Multicenter Early Comparative Experience of Clinical Outcomes. J Clin Gastroenterol. 2019;53:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Bukhari M, Kowalski T, Nieto J, Kunda R, Ahuja NK, Irani S, Shah A, Loren D, Brewer O, Sanaei O, Chen YI, Ngamruengphong S, Kumbhari V, Singh V, Aridi HD, Khashab MA. An international, multicenter, comparative trial of EUS-guided gastrogastrostomy-assisted ERCP versus enteroscopy-assisted ERCP in patients with Roux-en-Y gastric bypass anatomy. Gastrointest Endosc. 2018;88:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 26. | Schreiner MA, Chang L, Gluck M, Irani S, Gan SI, Brandabur JJ, Thirlby R, Moonka R, Kozarek RA, Ross AS. Laparoscopy-assisted versus balloon enteroscopy-assisted ERCP in bariatric post-Roux-en-Y gastric bypass patients. Gastrointest Endosc. 2012;75:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Iwashita T, Uemura S, Mita N, Iwasa Y, Ichikawa H, Mukai T, Yasuda I, Shimizu M. Endoscopic ultrasound guided-antegrade biliary stenting vs percutaneous transhepatic biliary stenting for unresectable distal malignant biliary obstruction in patients with surgically altered anatomy. J Hepatobiliary Pancreat Sci. 2020;27:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (3)] |
| 28. | Park SJ, Choi JH, Park DH, Lee SS, Seo DW, Lee SK, Kim MH. Expanding indication: EUS-guided hepaticoduodenostomy for isolated right intrahepatic duct obstruction (with video). Gastrointest Endosc. 2013;78:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Ichkhanian Y, Yang J, James TW, Baron TH, Irani S, Nasr J, Sharaiha RZ, Law R, Wannhoff A, Khashab MA. EUS-directed transenteric ERCP in non-Roux-en-Y gastric bypass surgical anatomy patients (with video). Gastrointest Endosc. 2020;91:1188-1194.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Masuda S, Japan; Sugimoto M, Japan; Tellez-Avila F, United States; Zhang JW, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH