Published online Dec 16, 2022. doi: 10.4253/wjge.v14.i12.739
Peer-review started: September 27, 2022
First decision: October 20, 2022
Revised: October 30, 2022
Accepted: November 9, 2022
Article in press: November 9, 2022
Published online: December 16, 2022
Processing time: 77 Days and 11.5 Hours
Non-variceal upper gastrointestinal bleeding (NVUGIB) is a common gastroenterological emergency associated with significant morbidity and mortality. Upper gastrointestinal endoscopy is currently recommended as the gold standard modality for both diagnosis and treatment, with computed tomography traditionally playing a limited role in the diagnosis of acute NVUGIB. Following the introduction of multidetector computed tomography (MDCT), this modality is emerging as a promising tool in the diagnosis of NVUGIB. However, to date, evidence concerning the role of MDCT in the NVUGIB diagnosis is still lacking. The aim of our study was to review the current evidence concerning the role of MDCT in the diagnosis of acute NVUGIB.
Core Tip: Upper gastrointestinal endoscopy is currently recommended as the first-line technique for diagnosis and treatment of non-variceal upper gastrointestinal bleeding (NVUGIB). Conversely, computed tomography has a limited role in the diagnosis of acute NVUGIB. However, following the introduction of multidetector computed tomography (MDCT), this modality is emerging as a promising tool in the diagnosis of NVUGIB. Nevertheless, to date, evidence concerning the role of MDCT in the NVUGIB diagnosis is still lacking. Our study aimed to review the current evidence concerning the role of MDCT in the diagnosis of acute NVUGIB.
- Citation: Martino A, Di Serafino M, Amitrano L, Orsini L, Pietrini L, Martino R, Menchise A, Pignata L, Romano L, Lombardi G. Role of multidetector computed tomography angiography in non-variceal upper gastrointestinal bleeding: A comprehensive review. World J Gastrointest Endosc 2022; 14(12): 739-747
- URL: https://www.wjgnet.com/1948-5190/full/v14/i12/739.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i12.739
Acute upper gastrointestinal bleeding (UGIB) is the most common gastroenterological emergency with an annual incidence of 40-150/100000 population[1-3]. It is defined as hemorrhage occurring from a source located proximal to the ligament of Treitz. Based on the etiology, it is usually classified as variceal and non-variceal upper gastrointestinal bleeding (NVUGIB), with peptic ulcers, neoplasms and Mallory-Weiss syndrome being the most common causes of NVUGIB[1,2,4].
Despite marked advances in the management of acute UGIB, its mortality rate is still high ranging from 8% to 14%[5-7], and increasing up to 40% in high-risk patients[8].
Following hemodynamic stabilization, esophagogastroduodenoscopy (EGD) is currently re
As opposed to acute lower gastrointestinal bleeding[14-16], computed tomography (CT) has currently a limited role in the diagnosis of acute UGIB and its routine adoption in the setting of acute NVUGIB is not recommended[9-11]. However, the introduction of multidetector CT (MDCT) technology has led to increased image resolution and markedly decreased scanning time, thus allowing the identification of contrast medium (CM) extravasation into the bowel lumen before contrast medium dilution. Furthermore, the ability of helical CT to detect active gastrointestinal bleeding may exceed the lower limit of 0.5 mL/min reported for mesenteric angiography and may approach the 0.2 mL/min limit of 99mTc-red blood cell scintigraphy[17]. Thus, recently, MDCT has been increasingly adopted in the diagnostic approach of most vascular diseases, and a promising role of this technique in the NVUGIB diagnosis has been suggested[18,19]. Anyway, evidence regarding the value of MDCT in NVUGIB is still limited. The aim of our study was to extensively review the current evidence with regard to the role of MDCT in the diagnosis of acute NVUGIB.
We performed a comprehensive literature search of the PubMed (MEDLINE) and EMBASE electronic databases up to July 2022, in order to identify relevant studies evaluating the role of MDCT in the diagnosis of acute NVUGIB. The medical search strategy used the terms “computed tomography”, “CT”, “computed tomography angiography”, “CTA”, “multidetector computed tomography”, “MDCT”, “non-variceal upper gastrointestinal bleeding”, and “non-variceal upper gastrointestinal haemorrhage” in various combinations, using the Boolean operators AND, OR, and NOT. Search strategy was limited to human studies and articles written in English. Meeting abstracts, individual case reports, case series (< 5 cases), review articles, position papers, editorials, commentaries, and book chapters were excluded from our review. The reference lists of pertinent identified studies and related review articles were carefully hand-searched in order to obtain any additional eligible studies.
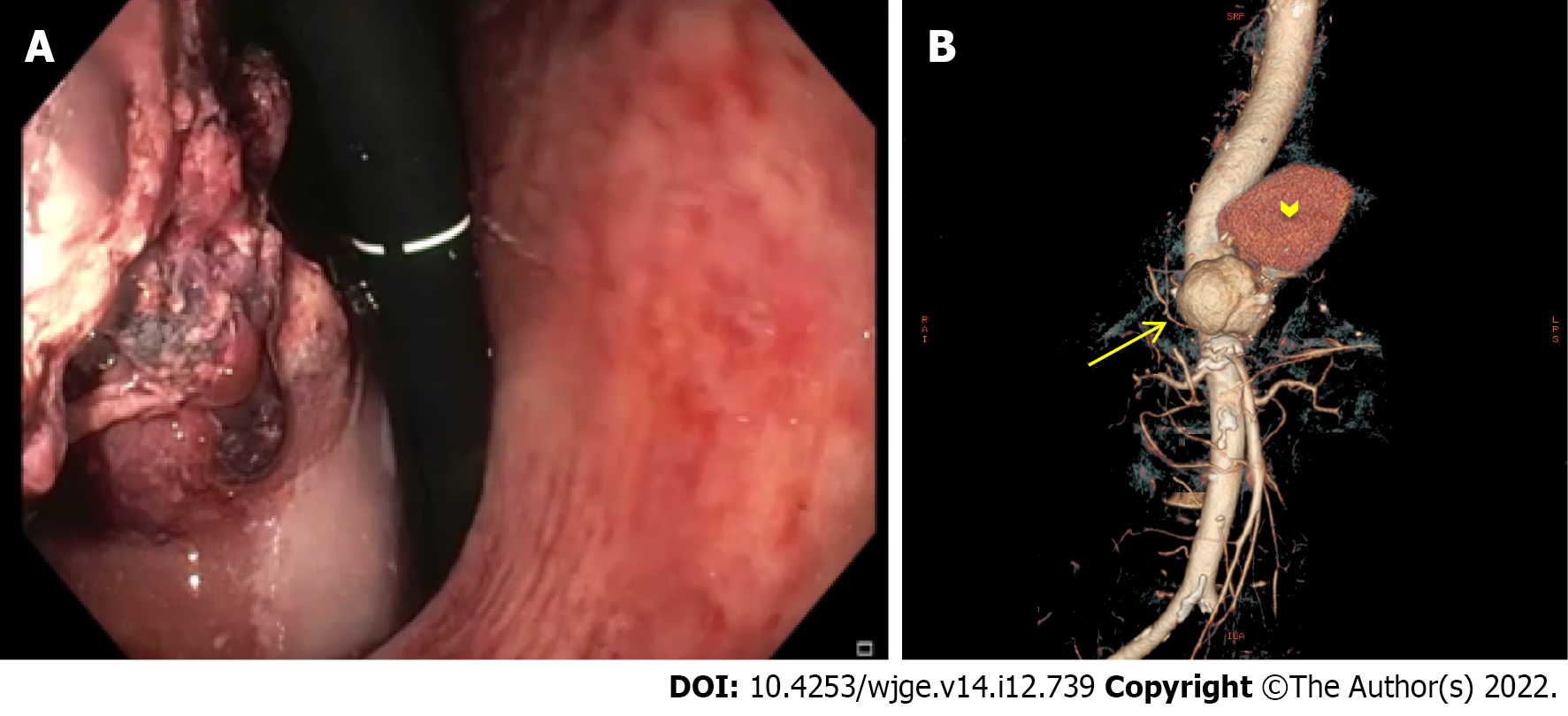
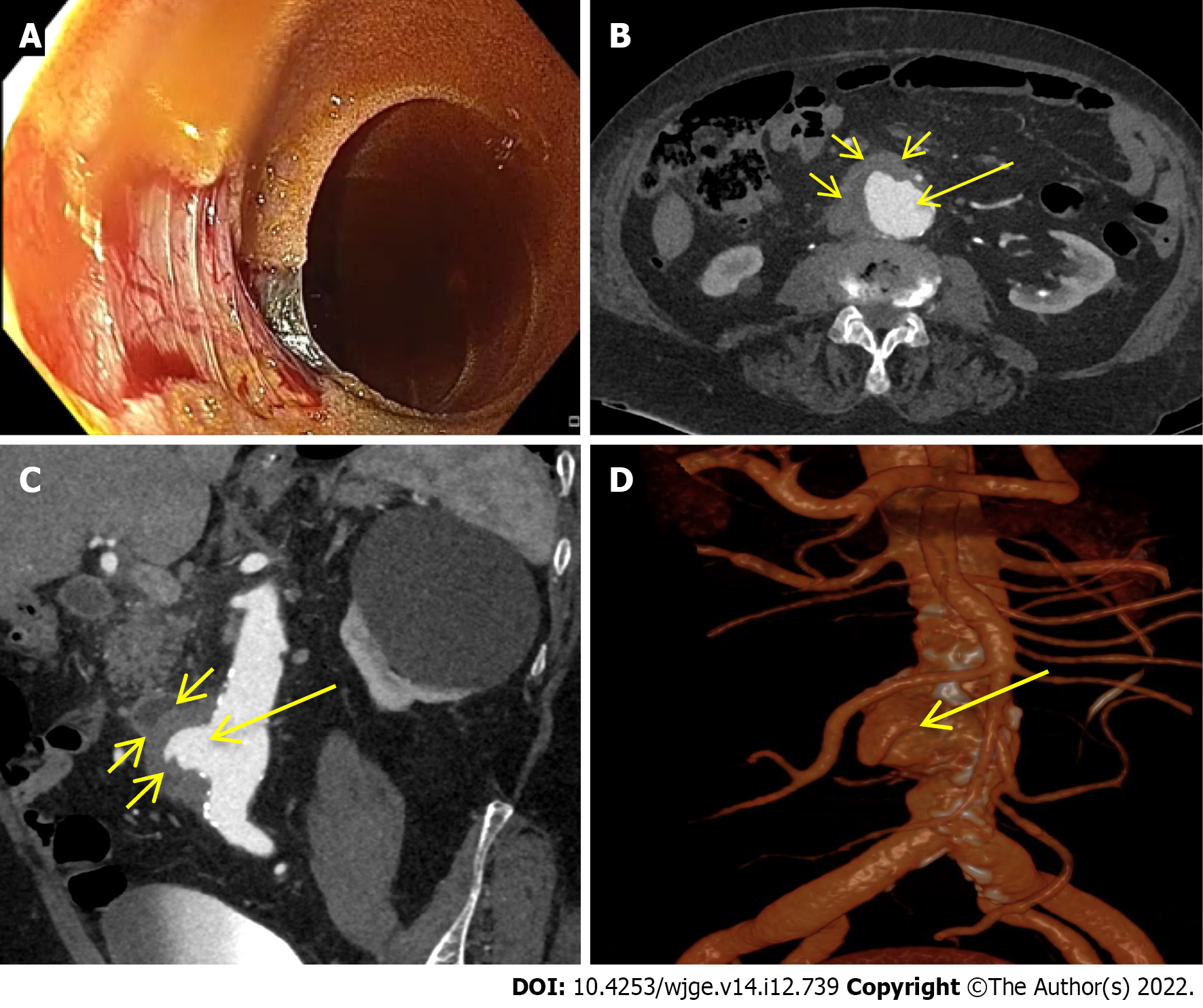
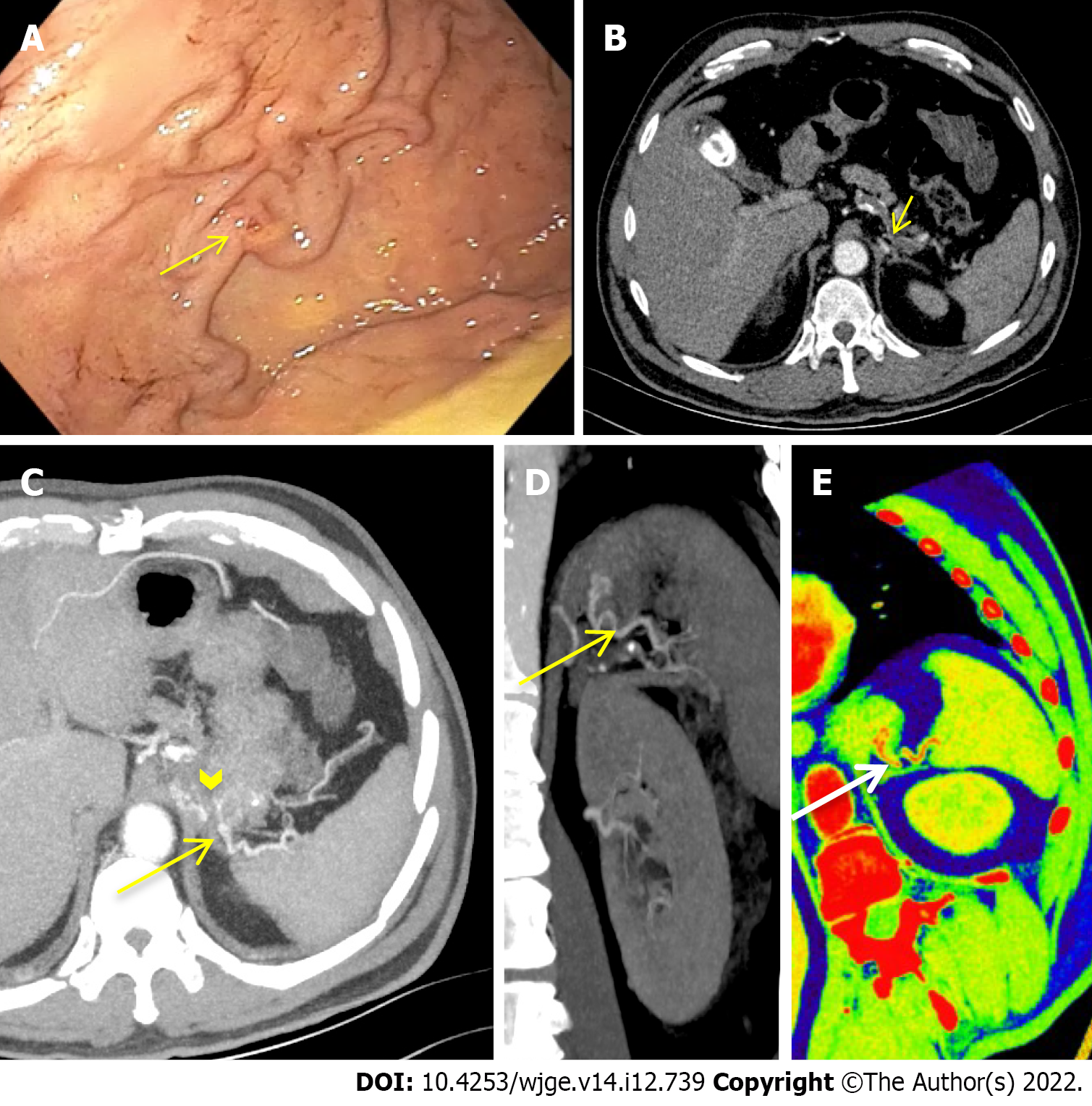
A total of 9 studies were included in our final analysis[20-28]. All but 3 prospective studies[20,24,25] were retrospective[21-23,26-28]. With the exception of one study comparing enhanced and unenhanced MDCT[26], in all of the remnant studies intravenous contrast-enhanced MDCT scan with at least an arterial phase acquisition was evaluated[20-25,27,28]. No CM was orally administered in any of the included studies. Main characteristics of the included studies in which MDCT was adopted in the diagnosis of acute NVUGIB are summarized in Table 1. Figures 1-3 show three cases of severe NVUGIB in which MDCT was performed immediately after EGD, providing bleeding etiology identification and thus guiding further treatment.
| Ref. | Study design | Patients, n | Type of CT | Inclusion criteria | Exclusion criteria | Criteria for positive CT | Reference standard | Study aim | Results |
| Yoon et al[20], 2006 | P | 7 | 4-MDCT | Patients with massive UGIB in whom endoscopic examination or hemostasis failed | - | Active GIB: Extravasation of CM with attenuation> 90 HU within bowel lumen | Angiography | Accuracy of MDCT for detection and localization of acute massive UGIB | GIB detection: TP: 4/7, FN: 2/7, FP: 1/7, TN: 0/7, GIB localization: TP: 7/7 |
| Scheffel et al[21], 2007 | R | 10 | 4-, 16-, or 64- MDCT | Patients with UGIB who underwent CT in the acute phase of hemorrhage | - | Acute GIB: Active extravasation of CM within bowel lumen; or extravasated CM with attenuation > 90 HU | Surgery, angiography, endoscopy, or pathology | Ability of MDCT to identify source and etiology of acute UGIB | GIB detection: 10/10; GIB etiology identification: 9/10 |
| Jaeckle et al[22], 2008 | R | 10 | 16- or 40-MDCT | Patients with UGIB in whom endoscopic examination failed to identify the bleeding source | Serum creatinine > 250 µmol/L; or iodinated CM allergy | Active GIB: Active extravasation of CM with attenuation > 90 HU within bowel lumen; or collection of hyperdense intraluminal blood with attenuation > 90 HU | Endoscopy, angiography and/or surgery | Accuracy of MDCT for detection and localization of acute UGIB | GIB detection: TP: 9/10; FN: 1/10; GIB localization: TP: 9/10; FN: 1/10 |
| Fung et al[23], 2008 | R | 6 | 64-MDCT | Patients with UGIB who underwent angiography | - | Acute GIB: Mass, abnormal vessel, or active extravasation of CM within bowel lumen | Angiography | Accuracy of MDCT for detection of acute UGIB | TP: 6/6 |
| Frattaroli et al[24], 2009 | P | 11 (1 VUGIB) | 16-MDCT | Patients with severe acute UGIB following endoscopy | Hemodynamicinstability; non-severe, intermittent, or chronic GIB; or effective endoscopic hemosthasis | Acute GIB: Active extravasation of CM within bowel lumen | Endoscopy, angiography, surgery, or post-mortem findings | Ability of MDCT to identify UGIB site and etiology | GIB site identification: Sensitivity 100% (vs 72.7% of endoscopy); GIB etiology identification: Sensitivity 90.9% (vs 54.5% of endoscopy) |
| Sun et al[25], 2012 | P | 33 | 16-, 64-, or dual-source MDCT | Patients with acute UGIB who underwent; MDCT as the initial diagnostic examination | Iodinated CM allergy; pregnancy; or serum creatinine > 2.0 mg/dL | Active GIB: Active extravasation of CM with attenuation > 90 HU within bowel lumen; focal or segmental abnormal bowel mucosal enhancement; presence of a vascular malformation; polyp or diverticulum with abnormal enhancement; or tumor | Endoscopy, angiography, surgery, or pathology | Accuracy of MDCT for detection of active UGIB | TP: 25/33; FN: 3/33; TN: 5/33 |
| Miyaoka et al[26], 2014 | R | 330 | 64-MDCT | Patients with acute UGIB who underwent MDCT prior to urgent endoscopy | Patients who underwent other therapeutic modalities rather than urgent endoscopy due to MDCT findings | Active GIB: Extravasation of CM within bowel lumen; possible bleeding: Wall thickening; focal wall enhancement; masses, varices, and aneurysms, with or without the intraluminal high-attenuation substance | Endoscopy | Accuracy of MDCT for detection of acute UGIB origin | Enhanced MDCT: 57.8% (130/227); unenhanced MDCT: 19.4% (20/103) |
| Jono et al[28], 2019 | R | 386 | 16- or 64- MDCT | Patients with NVUGIB who underwent MDCT prior to urgent endoscopy | VUGIB; or no CT exam | UGI hemorrhage: Yes or no; UGI wall change: Concavity or hypertrophy | Endoscopy | OR of risks scores based on clinical data and CT findings for predicting mortality, rebleeding and need for endoscopic therapy in NVUGIB | UGI hemorrhage: Not significant in predicting mortality and rebleeding, but significant in predicting need for endoscopic therapy (OR 10.1 for RS and 10.70 for GBS); UGI wall change: Not significant in predicting mortality, rebleeding and need for endoscopic therapy |
| Kim et al[27], 2022 | R | 269 (53 VUGIB) | 64-MDCT | Patients with acute UGIB who underwent MDCT prior to endoscopy | Execution of endoscopy 24 h after admission; endoscopic examination failure; LGIB; acute or chronic kidney injure; or iodinated CM allergy | Active bleeding: Active extravasation of CM within bowel lumen; recent bleeding: Hemorrhagic content, suspicious hematoma, and blood clots | Endoscopy | Accuracy of MDCT for identification of status, location, and etiology of UGIB | Bleeding status identification: 32.9% (active bleeding); 27.4% (recent bleeding); 94.8% (no bleeding); bleeding location identification: 60.9% (esophagus), 60.6% (stomach), 50.9% (duodenum); bleeding etiology identification: 58.3% (ulcerative bleeding), 65.9% (cancerous bleeding), 56.6% (variceal bleeding) |
In 2006, Yoon et al[20] first prospectively evaluated the role of arterial phase MDCT in 7 patients admitted for acute massive NVUGIB in whom endoscopic examination or hemostasis failed. A high accuracy of MDCT for the detection and localization of the bleeding sites was showed.
Later on, in a small retrospective case series MDCT was able to detect the bleeding source in all cases and to identify the bleeding etiology in 9 out of 10 cases. Of note, CT provided a diagnosis in 6 patients after negative findings at angiography (n = 2) and endoscopy (n = 4). In the remaining 4 patients, CT was the initial imaging method providing a diagnosis in all 4, and no further diagnostic work-up was performed. Moreover, CM extravasation was detected in all patients with acute severe NVUGIB (7/10) and the identified NVUGIB etiology mainly included rare causes of massive NVUGIB (aortoduodenal fistula, n = 4 and arterial pseudoaneurysm, n = 4, and arteriobiliary fistula, n = 1), requiring non-endoscopic treatment[21].
In 2008, Jaeckle et al[22] retrospectively reported the efficacy of MDCT in 10 UGIB patients in whom upper endoscopy failed to reveal the bleeding source. In 9 out of 10 patients MDCT was able to localize the bleeding site, while active bleeding was showed in 5 cases. In the only false-negative finding, angiographic and endoscopic follow-up revealed duodenal invasion of a small pancreatic carcinoma with duodenal bleeding.
Later on, a high MDCT accuracy for the detection of acute UGIB was reported in a small retrospective case series. Of note, MDCT criteria for acute GIB not only included the identification of active CM extravasation within bowel lumen, but also the detection of mass or pathologic vessel[23].
Subsequently, a small prospective study from Italy reported an excellent sensitivity of MDCT in identifying bleeding site and etiology (100.0% and 90.9%, respectively, compared with 72.7% and 54.5%, respectively, of endoscopy). Of note, patients in whom bleeding stopped after the operative endoscopy were not included in the study, whereas EGD failure was observed in 5 out of 11 of the included patients[24].
In 2012, Sun et al[25] prospectively evaluated the role of tri-phasic MDCT as the initial diagnostic investigation in patients with both severe and mild acute UGIB. As similarly previously reported, criteria for positive CT were not limited to the presence of active CM extravasation within bowel lumen, but also included identification of abnormal bowel mucosal enhancement, vascular malformation, abnormally enhancing polyp or diverticulum, or tumor. MDCT was shown to be a highly accurate first-line screening modality for both detection and localization of UGIB, effectively guiding further management. However, interestingly, no CM extravasation was observed in any of the included patients with mild UGIB[25].
Subsequently, the usefulness of MDCT prior to urgent endoscopy was confirmed in a similar large retrospective study. Indeed, pre-operative MDCT showed a diagnostic accuracy for the bleeding origin detection of 57.8% (130 of 227 patients) and 19.4% (20 of 103 patients) for the enhanced and unenhanced MDCT groups, respectively, among expert radiologists. To be mentioned, the authors excluded from their study patients in whom other therapeutic modalities, such as angiography or surgery, were performed rather than urgent endoscopy due to MDCT results. Finally, the average time needed for endoscopic detection of bleeding origin in the MDCT-positive group was significantly faster (88.1 s) than that in the MDCT-negative group (155.8 s) among patients who underwent the enhanced MDCT scan (P ≤ 0.05)[26].
Conversely, a recent large retrospective study showed that MDCT prior to endoscopy has a sig
Intriguingly, Jono et al[28] compared CT findings with two well validated clinical scores to predict mortality, rebleeding and need for endoscopic therapy in NVUGIB patients. In all patients CT was performed prior to upper endoscopy. Although upper gastrointestinal (UGI) hemorrhage and UGI wall findings on CT scan were not significant in predicting mortality and rebleeding, the first CT finding better predicted the need for endoscopic therapy than both clinical Rockall score (adjusted odds ratio 10.10) and Glasgow Blatchford score (adjusted odds ratio 10.70)[28].
EGD is currently recommended as the first-line modality for both diagnosis and treatment of NVUGIB, with MDCT playing only a limited role in the diagnosis of NVUGIB[9-11]. However, endoscopy may fail to identify the source of UGIB, especially in case of massive hemorrhage. Furthermore, although rare, various unusual cause of UGIB may not be properly diagnosed by endoscopy and require solely endovascular or surgical treatment[29-31]. MDCT has been suggested to be a promising non-invasive, fast and widely available diagnostic tool in the diagnosis of NVUGIB, with reported high diagnostic accuracy for both detection and localization of bleeding, especially among patients with severe hemorrhage[32]. Moreover, MDCT is capable to identify the bleeding etiology, representing the gold standard diagnostic modality for most of the unusual causes of NVUGIB. Finally, as opposed to endoscopy, MDCT is capable to accurately evaluate the bleeding lesion, providing information to extraluminal abnormalities, feeding and draining vessels, and its anatomical relationship to surrounding structures. Thus, MDCT has the potential to stratify patients who need earlier treatment and to assist clinicians in planning further safe, effective and tailored treatment, whether it is endoscopic, endovascular, and/or surgical.
In our opinion, MDCT angiography plays a primary role in NVUGIB patients in whom endoscopic examination fails to identify and/or to properly treat the bleeding lesion. Furthermore, in case of uncertain etiologic diagnosis at endoscopy, MDCT should be performed before treatment. Finally, across referral centers, MDCT angiography may play a role as first-line diagnostic modality in NVUGIB, especially among patients admitted for severe bleeding. Indeed, it may easily identify the bleeding status, addressing the timing of treatment, and provide an etiological diagnosis of the bleeding lesion, thereby strictly directing further safe and effective management. Finally, in case of failure of endoscopic hemosthasis, emergent endovascular or surgical treatment could be directly, safely and effectively performed by the pre-alerted interventional radiologist or surgeon. However, further large prospective studies in high-volume referral centers are needed to clarify the role of MDCT in NVUGIB, especially as first-line diagnostic tool in patients affected by severe acute NVUGIB. High morbidity and mortality still associated with acute NVUGIB justify active research in this field.
We are grateful to Velia De Magistris for English editing.
| 1. | Laine L, Yang H, Chang SC, Datto C. Trends for incidence of hospitalization and death due to GI complications in the United States from 2001 to 2009. Am J Gastroenterol. 2012;107:1190-5; quiz 1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (1)] |
| 2. | Wuerth BA, Rockey DC. Changing Epidemiology of Upper Gastrointestinal Hemorrhage in the Last Decade: A Nationwide Analysis. Dig Dis Sci. 2018;63:1286-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 3. | Lee EW, Laberge JM. Differential diagnosis of gastrointestinal bleeding. Tech Vasc Interv Radiol. 2004;7:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 445] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 5. | Sanders DS, Perry MJ, Jones SG, McFarlane E, Johnson AG, Gleeson DC, Lobo AJ. Effectiveness of an upper-gastrointestinal haemorrhage unit: a prospective analysis of 900 consecutive cases using the Rockall score as a method of risk standardisation. Eur J Gastroenterol Hepatol. 2004;16:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | van Leerdam ME, Vreeburg EM, Rauws EA, Geraedts AA, Tijssen JG, Reitsma JB, Tytgat GN. Acute upper GI bleeding: did anything change? Am J Gastroenterol. 2003;98:1494-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 371] [Article Influence: 16.1] [Reference Citation Analysis (37)] |
| 7. | Nahon S, Hagège H, Latrive JP, Rosa I, Nalet B, Bour B, Faroux R, Gower P, Arpurt JP, Denis J, Henrion J, Rémy AJ, Pariente A; Groupe des Hémorragies Digestives Hautes de l’ANGH. Epidemiological and prognostic factors involved in upper gastrointestinal bleeding: results of a French prospective multicenter study. Endoscopy. 2012;44:998-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Walker TG. Acute gastrointestinal hemorrhage. Tech Vasc Interv Radiol. 2009;12:80-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Laine L, Barkun AN, Saltzman JR, Martel M, Leontiadis GI. ACG Clinical Guideline: Upper Gastrointestinal and Ulcer Bleeding. Am J Gastroenterol. 2021;116:899-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 347] [Article Influence: 69.4] [Reference Citation Analysis (37)] |
| 10. | Gralnek IM, Stanley AJ, Morris AJ, Camus M, Lau J, Lanas A, Laursen SB, Radaelli F, Papanikolaou IS, Cúrdia Gonçalves T, Dinis-Ribeiro M, Awadie H, Braun G, de Groot N, Udd M, Sanchez-Yague A, Neeman Z, van Hooft JE. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2021. Endoscopy. 2021;53:300-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 316] [Article Influence: 63.2] [Reference Citation Analysis (1)] |
| 11. | Sung JJ, Chiu PW, Chan FKL, Lau JY, Goh KL, Ho LH, Jung HY, Sollano JD, Gotoda T, Reddy N, Singh R, Sugano K, Wu KC, Wu CY, Bjorkman DJ, Jensen DM, Kuipers EJ, Lanas A. Asia-Pacific working group consensus on non-variceal upper gastrointestinal bleeding: an update 2018. Gut. 2018;67:1757-1768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 12. | Lieberman D. Gastrointestinal bleeding: initial management. Gastroenterol Clin North Am. 1993;22:723-736. [PubMed] |
| 13. | Vreeburg EM, Snel P, de Bruijne JW, Bartelsman JF, Rauws EA, Tytgat GN. Acute upper gastrointestinal bleeding in the Amsterdam area: incidence, diagnosis, and clinical outcome. Am J Gastroenterol. 1997;92:236-243. [PubMed] |
| 14. | Triantafyllou K, Gkolfakis P, Gralnek IM, Oakland K, Manes G, Radaelli F, Awadie H, Camus Duboc M, Christodoulou D, Fedorov E, Guy RJ, Hollenbach M, Ibrahim M, Neeman Z, Regge D, Rodriguez de Santiago E, Tham TC, Thelin-Schmidt P, van Hooft JE. Diagnosis and management of acute lower gastrointestinal bleeding: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53:850-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 15. | Strate LL, Gralnek IM. ACG Clinical Guideline: Management of Patients With Acute Lower Gastrointestinal Bleeding. Am J Gastroenterol. 2016;111:459-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 312] [Article Influence: 31.2] [Reference Citation Analysis (2)] |
| 16. | Oakland K, Chadwick G, East JE, Guy R, Humphries A, Jairath V, McPherson S, Metzner M, Morris AJ, Murphy MF, Tham T, Uberoi R, Veitch AM, Wheeler J, Regan C, Hoare J. Diagnosis and management of acute lower gastrointestinal bleeding: guidelines from the British Society of Gastroenterology. Gut. 2019;68:776-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 219] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 17. | Kuhle WG, Sheiman RG. Detection of active colonic hemorrhage with use of helical CT: findings in a swine model. Radiology. 2003;228:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Napoli A, Fleischmann D, Chan FP, Catalano C, Hellinger JC, Passariello R, Rubin GD. Computed tomography angiography: state-of-the-art imaging using multidetector-row technology. J Comput Assist Tomogr. 2004;28 Suppl 1:S32-S45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Wu LM, Xu JR, Yin Y, Qu XH. Usefulness of CT angiography in diagnosing acute gastrointestinal bleeding: a meta-analysis. World J Gastroenterol. 2010;16:3957-3963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 113] [Cited by in RCA: 110] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Yoon W, Jeong YY, Shin SS, Lim HS, Song SG, Jang NG, Kim JK, Kang HK. Acute massive gastrointestinal bleeding: detection and localization with arterial phase multi-detector row helical CT. Radiology. 2006;239:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Scheffel H, Pfammatter T, Wildi S, Bauerfeind P, Marincek B, Alkadhi H. Acute gastrointestinal bleeding: detection of source and etiology with multi-detector-row CT. Eur Radiol. 2007;17:1555-1565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Jaeckle T, Stuber G, Hoffmann MH, Jeltsch M, Schmitz BL, Aschoff AJ. Detection and localization of acute upper and lower gastrointestinal (GI) bleeding with arterial phase multi-detector row helical CT. Eur Radiol. 2008;18:1406-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Fung HS, Kwok PC, Lau S, Wong WK, Chan SCH. 64-slice multi-detector computed tomography for detection of acute gastrointestinal bleeding. J HK Coll Radiol. 2008;11:13-18. |
| 24. | Frattaroli FM, Casciani E, Spoletini D, Polettini E, Nunziale A, Bertini L, Vestri A, Gualdi G, Pappalardo G. Prospective study comparing multi-detector row CT and endoscopy in acute gastrointestinal bleeding. World J Surg. 2009;33:2209-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Sun H, Jin Z, Li X, Qian J, Yu J, Zhu F, Zhu H. Detection and localization of active gastrointestinal bleeding with multidetector row computed tomography angiography: a 5-year prospective study in one medical center. J Clin Gastroenterol. 2012;46:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Miyaoka Y, Amano Y, Ueno S, Izumi D, Mikami H, Yazaki T, Okimoto E, Sonoyama T, Ito S, Fujishiro H, Kohge N, Imaoka T. Role of enhanced multi-detector-row computed tomography before urgent endoscopy in acute upper gastrointestinal bleeding. J Gastroenterol Hepatol. 2014;29:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Kim D, Kim JH, Ko DR, Min IK, Choi A, Beom JH. Usefulness of contrast-enhanced multi-detector computed tomography in identifying upper gastrointestinal bleeding: A retrospective study of patients admitted to the emergency department. PLoS One. 2022;17:e0266622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 28. | Jono F, Iida H, Fujita K, Kaai M, Kanoshima K, Ohkuma K, Nonaka T, Ida T, Kusakabe A, Nakamura A, Koyama S, Nakajima A, Inamori M. Comparison of computed tomography findings with clinical risks factors for endoscopic therapy in upper gastrointestinal bleeding cases. J Clin Biochem Nutr. 2019;65:138-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Parikh K, Ali MA, Wong RC. Unusual Causes of Upper Gastrointestinal Bleeding. Gastrointest Endosc Clin N Am. 2015;25:583-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Munteanu L, Iancu I, Breazu C, Cioltean C, Brânzilă S, Odainii A, Furda P, Bocşe H, Herdean A, Bartoş D, Bartoş A. Rare Causes of Gastrointestinal Bleeding: Focus on Pancreatic Pathology and Visceral Artery Aneurysms. Chirurgia (Bucur). 2021;116:S5-S15. [PubMed] |
| 31. | Di Serafino M, Iacobellis F, Schillirò ML, Dell'Aversano Orabona G, Martino A, Bennato R, Borzelli A, Oliva G, D'Errico C, Pezzullo F, Barbuto L, Ronza R, Ponticiello G, Corvino F, Giurazza F, Lombardi G, Niola R, Romano L. The Role of CT-Angiography in the Acute Gastrointestinal Bleeding: A Pictorial Essay of Active and Obscure Findings. Tomography. 2022;8:2369-2402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 32. | British Society of Gastroenterology Endoscopy Committee. Non-variceal upper gastrointestinal haemorrhage: guidelines. Gut. 2002;51 Suppl 4:iv1-iv6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 90] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Associazione Italiana Gastroenterologi ed endoscopisti digestivi Ospedalieri; Società Italiana Endoscopia Digestiva.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elsayed MO, United Kingdom; Tatlıparmak AC, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Chen YL