Published online Nov 16, 2022. doi: 10.4253/wjge.v14.i11.672
Peer-review started: August 19, 2022
First decision: September 2, 2022
Revised: September 19, 2022
Accepted: October 31, 2022
Article in press: October 31, 2022
Published online: November 16, 2022
Processing time: 86 Days and 21.3 Hours
Ensuring colonoscopy procedure quality is vital to the success of screening and surveillance programmes for bowel cancer in Australia. However, the data on the performance of quality metrics, through adequate adenoma detection, bowel pre
To determine the quality of colonoscopy in Australian teaching hospitals and their association with proceduralist specialty, trainee involvement, and location.
We retrospectively evaluated 2443 consecutive colonoscopy procedure reports from 1 January to 1 April, 2018 from five public teaching tertiary hospitals in Australia (median 60 years old, 49% male). Data for bowel preparation quality, procedure completion rates, and detection rates of clinically significant adenomas, conventional adenomas, and serrated lesions was collected and compared to national criteria for quality in colonoscopy. Participating hospital, proceduralist specialty, and trainee involvement indicators were used for stratification. Data was analysed using Chi-squared tests of independence, Mann-Whitney U, One-way ANOVA, and multivariate binary logistic regression.
Fifty-two point two percent (n = 1276) and 43.3% (n = 1057) were performed by medical and surgical proceduralists respectively, whilst 29.8% (n = 728) involved a trainee. Inadequate bowel preparation affected 7.3% of all procedures. The procedure completion rate was 95.1%, which increased to 97.5% after adjustment for bowel preparation quality. The pooled cancer, adenoma, and serrated lesion detection rates for all five hospitals were 3.5%, 40%, and 5.9% respectively. Assessed hospitals varied significantly by patient age (P < 0.001), work-force composition (P < 0.001), adequacy of bowel preparation (P < 0.001), and adenoma detection rate (P < 0.001). Two hospitals (40%) did not meet all national criteria for quality, due to a procedure completion rate of 94.5% or serrated lesion detection rate of 2.6%. Although lower than the other hospitals, the difference was not significant. Compared with surgical specialists, procedures performed by medical specialists involved older patients [65 years (inter-quartile range, IQR 58-73) vs 64 years (IQR 56-71); P = 0.04] and were associated with a higher adenoma detection rate [odds ratio (OR) 1.53; confidence interval: 1.21-1.94; P < 0.001]. Procedures involving trainee proceduralists were not associated with differences in the detection of cancer, adenoma, or serrated lesions, compared with specialists, or according to their medical or surgical background. On multivariate analysis, cancer detection was positively associated with patient age (OR 1.04; P < 0.001) and negatively associated with medical compared to surgical proceduralists (OR 0.54; P = 0.04). Conventional adenoma detection rates were independently associated with increasing patient age (OR 1.04; P < 0.001), positively associated with medical compared to surgical proceduralists (OR 1.41; P = 0.002) and negatively associated with male gender (OR 0.53; P < 0.001).
Significant differences in the quality of colonoscopy in Australia exist, even when national ben
Core Tip: We evaluated the quality of colonoscopy performed at five teaching hospitals in Australia, using bowel preparation quality, procedure completion, and detection of cancer, adenoma, and serrated lesions as main indicators. In our retrospective analysis of 2443 procedures, the collective performance met national benchmarks for quality. However, two hospitals individually failed to meet all national benchmarks and we observed significant differences in key metrics of adenoma detection and adequacy of bowel preparation for colonoscopy across all hospitals. Higher adenoma detection rates were also independently shown amongst medical compared with surgical proceduralists, and amongst female patients.
- Citation: Ow TW, Sukocheva OA, Tran V, Lin R, Lee SZ, Chu M, Angelica B, Rayner CK, Tse E, Iyngkaran G, Bampton PA. Quality of colonoscopy performed by medical or surgical specialists and trainees in five Australian hospitals. World J Gastrointest Endosc 2022; 14(11): 672-683
- URL: https://www.wjgnet.com/1948-5190/full/v14/i11/672.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i11.672
Metrics conventionally used in the assessment of quality in colonoscopy are centred around its role in the prevention and early detection of colorectal cancer (CRC) and other gastrointestinal (GI) complications. These include the adenoma detection rate (ADR), generally considered the gold-standard indicator of quality, the adequacy of bowel cleansing and rate of procedure completion[1,2]. The im
The Gastroenterological Society of Australia has recently implemented a recertification program using self-reported data to assess the performance of colonoscopy. Current nominated benchmarks include an ADR of 25% in eligible procedures, completion rate of at least 95% in patients with intact colons, and serrated lesion detection rate (SLDR) of 4%[5]. This can provide valuable data on adenoma detection, procedure completion, and bowel preparation rates. However, the data submitted for recertification typically relates to work performed for patients with private health insurance. This does not reflect the quality of procedures in government-funded universal healthcare, in which a quarter of all colonoscopies in Australia are performed[6]. Considering that patients of lower socio-economic background are not only at risk of the poorest outcomes of CRC and other GI complications, but are also reliant upon this pathway for access to healthcare, it is important to ensure its quality[6].
However, assessment of performance data from this section is limited to a handful of single-centre studies[7-10]. Furthermore, the quality of procedures performed by proceduralists-in-training in Australia remain unreported. Ensuring the quality of colonoscopy in this sector therefore also supports both current and future screening and surveillance practice. We measured the quality of colonoscopy performed in five public teaching hospitals in Australia. We aimed to assess not only the quality of the performed colonoscopies, but also key areas for further improvement and targeted solutions for po
We performed a retrospective, multicentre, cohort study across five hospitals (identified as Site 1-5) in South Australia and the Northern Territory with electronic records of colonoscopy and pathology data spanned over three months. Together, the catchment population for the five hospitals is estimated to be just over one million people. Ethical approval was granted by the Central Adelaide Local Health Network ethics committee.
We searched GI endoscopy databases (ProVationMD) for colonoscopy procedures performed between 1 January, 2018 to 31 March, 2018 inclusive at each participating site. We excluded patients undergoing a flexible sigmoidoscopy, where only the left side of the colon was viewed. Patients younger than 18 years were also excluded as conventional quality metrics are not typically applied in the paediatric po
We collected data including patient age, gender, proceduralist speciality, trainee participation, trainee specialty, and site for each procedure. We examined the records of each patient for a history of CRC, prior colonic resection, and inflammatory bowel disease (IBD). We evaluated the quality of bowel pre
Adequacy of bowel preparation was defined by a description of fair, good, or excellent according to the Aronchick scale. Alternatively, a score of 6 or greater, with no individual segment less than 2, was used according to the Boston Bowel Preparation Scale[13]. The rate of inadequate bowel preparation was determined by the proportion of procedures which did not meet the above criteria when rated against either scale. The rate of indeterminate bowel preparation quality otherwise determined according to the proportion of procedures where an alternative or no scoring system was applied.
Procedure completion was defined by documented (either written or photographic) progress to the caecum or terminal ileum, in patients with an intact colon (the absence of a history of CRC or prior colonic resection). The procedure completion rate was defined by the proportion of procedures in which this was achieved. The adjusted procedure completion rate was defined by the proportion of colonoscopies with adequate bowel preparation where procedure completion was achieved.
We adapted conventional criteria for ADR to define the population (or eligible procedures) for which the detection rates for the various lesions (CRC, conventional adenomas, and serrated lesions) were determined. Typically this involves patients, aged 50 and over, who are undergoing their index co
The CRC detection rate was defined as the proportion of eligible procedures in which the cancer was identified and confirmed on histology. These cases were subsequently excluded for the calculation of detection rates for conventional adenomas and serrated lesions due to the possibility that a newly diagnosed CRC may influence proceduralists’ further efforts to find and resect synchronous non-malignant lesions. The ADR and SLDR were thus defined by the proportion of procedures in which at least one conventional adenoma or serrated lesion respectively was identified on histology amongst the remaining procedures[15]. The clinically significant lesion detection rate (CSLDR) was determined according to the proportion of procedures where either a conventional adenoma, serrated lesion or both were identified amongst eligible procedures without a new CRC diagnosis.
Contemporary World Health Organisation histological definitions for conventional adenomas (tubular, tubulovillous, or villous adenoma) and serrated lesions (sessile serrated lesion, traditional serrated lesion or large hyperplastic polyp ≥ 10 mm) were used[16].
We determined the rates of inadequate bowel preparation and procedure completion for all hospitals, and stratified the results according to hospital, proceduralist specialty (medical/surgical), presence or absence of a trainee, and trainee specialty. Amongst eligible procedures, those with a new diagnosis were used to calculate the cancer detection rate. We analysed the remaining procedures to determine the ADR, SLDR, and CSLDRs. Lesions identified on colonoscopy without available histology were not counted when calculating detection rates. The detection rates for cancer, adenoma, serrated lesions, and clinically significant lesions were also stratified according to the same groups as above. We did not compare the outcomes of procedures performed by nurse endoscopists to those of medical or surgical specialists as they were only employed at a single hospital and thus subject to a significant risk of sampling bias.
The primary outcome was ADR. According to a recent meta-analysis showing an expected ADR of 40% with a confidence interval of 95% and a margin of error of 5%, we assessed a minimum sample of 369 patients[17].
Descriptive statistics was adapted to characterise the data. Chi-squared tests of independence were used to analyse nominal data. Mann-Whitney U test and one-way ANOVA tests were used for comparison of non-parametric data. Multivariate binary logistic regression was used to determine contributing factors for detection rates for cancer, adenomas, and serrated lesions. The significance level was set at 0.05. IBM SPSS Statistics version 27 was used.
A total of 2443 consecutive colonoscopies were performed from January to April of 2018. 49% (n = 1198) of the patients were male with a median age of 60 (inter-quartile range 50-70). Prior to exclusions, 69.1% (n = 1688) of procedures were performed on individuals aged 50 or greater; 6.4% (n = 156) of procedures were indicated for a personal history of CRC; 7.9% (n = 192) had undergone prior surgical resection; and 6.5% (n = 159) of procedures were indicated for IBD. Bowel preparation was documented as adequate in 86.9% (n = 2123), indeterminate in 5.8% (n = 142), and inadequate in 7.3% (n = 178) of procedures, respectively. Procedure completion was confirmed in 95.1% (n = 2114) after 9% (n = 220) of procedures were excluded for either a history of CRC or prior surgical resection. After excluding additional procedures for inadequate or indeterminate bowel preparation quality (n = 288), the adjusted procedure completion rate was 97.5%.
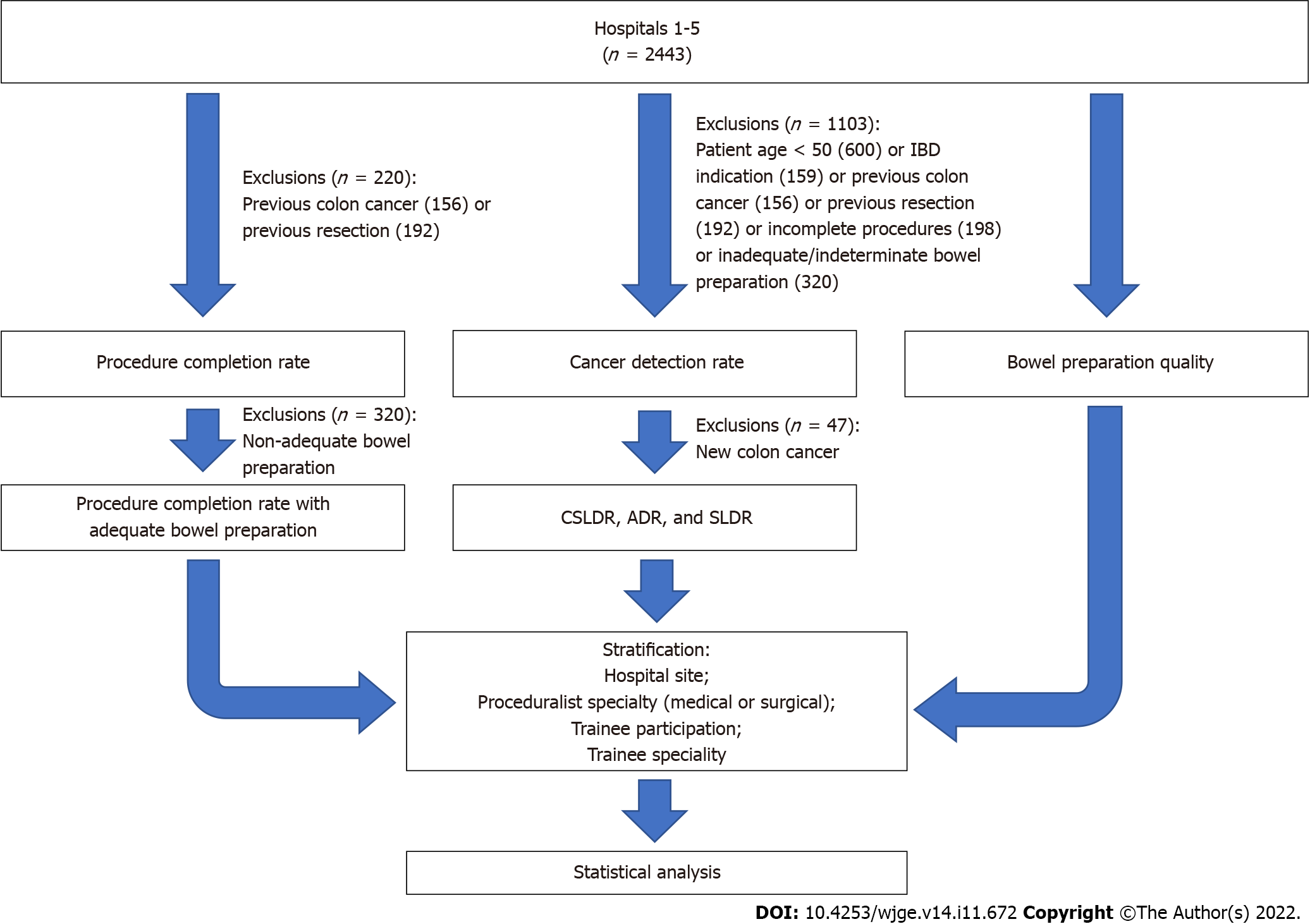
Of the total 2443 procedures, we excluded 600 that were conducted in patients under 50 years old; and a further 74 with IBD; 137 with CRC; 34 with prior bowel surgery; 77 incomplete procedures; and 181 with inadequate or indeterminate bowel preparations (Figure 1). Consequently, 1340 (54.9%) procedures were considered eligible for the determination of detection rates for cancer, conventional adenomas, and serrated lesions. Cancer was detected in 1.9% (n = 47) of patients. Conventional adenomas and serrated lesions were identified in 40% (n = 517) and 5.9% (n = 76) of the remaining procedures, respectively.
Our analysis indicated that 43.3% (n = 1057) and 52.2% (n = 1276) of procedures were performed by surgical and medical specialty groups, respectively. Nurse endoscopists conducted 4.5% (n = 106) of procedures at a single site. The specialty could not be determined in the remaining four cases where a proceduralist was not named on the colonoscopy report. Amongst all procedures, 29.8% (n = 728) of colonoscopies were attended by trainees. Of these, 45.9% (n = 334) of procedures were attended by a medical trainee.
On analysing outcomes according to specialty group, a total of 551 eligible procedures were performed by surgical proceduralists, with cancer detected in 4.7% (n = 26) of cases (Table 1). Of the remaining procedures, conventional adenomas and serrated lesions were identified in 34% (n = 178) and 4.6% (n = 24) respectively. In comparison, 716 eligible procedures were performed by medical proceduralists, with cancer detected in 2.7% (n = 19) of cases. After excluding new diagnoses of cancer, medical proceduralists identified conventional adenomas and serrated lesions in 44% (n = 307) and 6.6% (n = 46).
| Medical, n = 716 | Surgical, n = 551 | P value | OR (95%CI) | |
| Patient age, median (IQR) | 65 (58-73) | 64 (56-71) | 0.04 | - |
| Patient gender (male %) | 49.7 (n = 356) | 48.8 (n = 269) | 0.75 | - |
| Cancer detection rate (%) | 2.7 (n = 19) | 4.7 (n = 26) | 0.052 | 0.55 (0.30-1.01) |
| CSPDR (%) | 46.6 (n = 325) | 35.6 (n = 187) | < 0.001 | 1.58 (1.25-1.99) |
| ADR (%) | 44 (n = 307) | 34 (n = 178) | < 0.001 | 1.53 (1.21-1.94) |
| SLDR (%) | 6.6 (n = 46) | 4.6 (n = 24) | 0.13 | 1.47 (0.89-2.45) |
Further analysis indicated that, compared with medical specialists, surgeons performed their procedures on a significantly younger patient group (P = 0.04). The overall cancer detection rate was lower among medical compared to surgical specialists, although the difference was not found to be significant (P = 0.052). The odds of detecting a clinically significant polyp or adenoma, however, were significantly higher amongst medical than surgical specialists [P < 0.001, odds ratio (OR) 1.58, (95% confidence interval (CI): 1.25-1.99); P < 0.001, OR 1.53, (95%CI: 1.21-1.94)] (Table 1).
When we compared 370 eligible procedures performed with trainees present against 968 performed by specialists, no significant differences in the cancer, adenoma, and serrated lesion detection rates were found (Table 2). Similarly, no significant differences in the lesion detection rates were found amongst the procedures attended by trainees according to their background specialty (Table 3).
| With trainees, n = 370 | Without trainees, n = 968 | P value | OR (95%CI) | |
| Patient age, median (IQR) | 64 (57-72) | 64 (57-72) | 0.83 | - |
| Patient gender (male %) | 53.5 (n = 198) | 47.4 (n = 463) | 0.06 | - |
| Cancer detection rate (%) | 4.1 (n = 15) | 3.3 (n = 32) | 0.51 | 1.24 (0.66-2.31) |
| CSPDR (%) | 41.4 (n = 147) | 42.7 (n = 400) | 0.67 | 0.95 (0.74-1.21) |
| ADR (%) | 38.9 (n = 138) | 40.4 (n = 378) | 0.62 | 0.94 (0.73-1.21) |
| SLDR (%) | 4.8 (n = 17) | 6.3 (n = 59) | 0.30 | 0.74 (0.43-1.30) |
| Medical trainees, n = 370 | Surgical trainees, n = 968 | P value | OR (95%CI) | |
| Patient age, median (IQR) | 59.5 (47-71) | 59 (48.75-69) | 0.30 | - |
| Patient gender (male %) | 49.7 (n = 166) | 52.3 (n = 206) | 0.49 | - |
| Cancer detection rate (%) | 2.3 (n = 5) | 3.3 (n = 10) | 0.49 | 0.68 (0.23-2.02) |
| CSPDR (%) | 38.2 (n = 81) | 32.5 (n = 94) | 0.19 | 1.28 (0.89-1.86) |
| ADR (%) | 36.3 (n = 77) | 29.1 (n = 84) | 0.09 | 1.39 (0.95-2.03) |
| SLDR (%) | 5.7 (n = 12) | 5.2 (n = 15) | 0.82 | 1.1 (0.5-2.39) |
Following this, sites were compared for the quality of endoscopic procedures. Prior to exclusions (n = 2443), there were significant variations in the age of patients undergoing colonoscopy (P < 0.001); the procedure completion rate (P < 0.001); proportion of procedures performed by surgical or medical proceduralists (P < 0.001); degree of trainee involvement (P < 0.001); and bowel preparation quality (P < 0.001) (Table 4). Following univariate analysis, significant differences were observed in the detection of conventional adenomas (P = 0.01) and clinically significant polyps (P = 0.01), but not for cancer (P = 0.38) or serrated lesions (P = 0.31).
| Site 1 (n = 254) | Site 2 (n = 396) | Site 3 (n = 604) | Site 4 (n = 790) | Site 5 (n = 399) | P value | Overall (n = 2443) | |||
| Patient age, median (IQR) | 56 (46-66) | 61 (50-71) | 59.5 (49-70) | 61 (50-71) | 60 (50-71) | < 0.001 | 60 (50-70) | ||
| Patient gender (male %) | 56.7 (n = 144) | 45.7 (n = 181) | 48.0 (n = 290) | 48.4 (n = 382) | 50.4 (n = 201) | 0.08 | 49.0 (n = 1197) | ||
| Proceduralist | |||||||||
| Surgical (%) | 87.4 (n = 222) | 33.1 (n = 131) | 35.6 (n = 215) | 35.9 (n = 284) | 51.4 (n = 205) | < 0.001 | 43.3 (n = 1057) | ||
| Medical (%) | 12.6 (n = 32) | 66.9 (n = 265) | 64.1 (n = 387) | 50.5 (n = 399) | 48.4 (n = 193) | < 0.001 | 52.2 (n = 1276) | ||
| Trainee (%) | 59.4 (n = 151) | 38.9 (n = 154) | 8.1 (n = 49) | 28.1 (n = 222) | 38.1 (n = 152) | < 0.001 | 29.8 (n = 728) | ||
| Medical (%) | 0 (n = 0) | 61.7 (n = 95) | 91.8 (n = 45) | 39.2 (n = 87) | 70.4 (n = 107) | - | 45.9 (n = 334) | ||
| Surgical (%) | 100 (n = 151) | 38.3 (n = 59) | 8.2 (n = 4) | 60.8 (n = 135) | 29.6 (n = 45) | - | 54.1 (n = 394) | ||
| Inadequate bowel preparation (%) | 13.4 (n = 34) | 8.1 (n = 32) | 2.6 (n = 16) | 7.2 (n = 57) | 9.8 (n = 39) | < 0.001 | 7.3 (n = 178) | ||
| Indeterminate bowel preparation (%) | 0.0 (n = 0) | 2.8 (n = 11) | 1.5 (n = 9) | 4.3 (n = 34) | 22.1 (n = 88) | < 0.001 | 5.8 (n = 142) | ||
| Procedure completion (%) | 94.3 (n = 215) | 92.2 (n = 319) | 98.2 (n = 556) | 95.1 (n = 686) | 93.4 (n = 338) | < 0.001 | 95.1 (n = 2114) | ||
| Procedure completion (%) with adequate preparation | 98.0 (n = 195) | 94.5 (n = 294) | 99.2 (n = 537) | 98.0 (n = 627) | 96.3 (n = 233) | 0.99 | 97.5 (n = 1886) | ||
| Eligible procedures | 121 | 216 | 381 | 462 | 160 | 1340 | |||
| Cancer detection (%) | 5.0 (n = 6) | 2.3 (n = 5) | 2.9 (n = 11) | 3.5 (n = 16) | 5.6 (n = 9) | 0.38 | 3.5 (n = 47) | ||
| CSPDR (%) | 30.4 (n = 35) | 40.8 (n = 86) | 48.6 (n = 180) | 42.6 (n = 190) | 41.1 (n = 62) | 0.01 | 42.8 (n = 553) | ||
| ADR (%) | 27.8 (n = 32) | 39.3 (n = 83) | 45.7 (n = 169) | 39.5 (n = 176) | 37.7 (n = 57) | 0.01 | 40.0 (n = 517) | ||
| SLDR (%) | 2.6 (n = 3) | 5.2 (n = 11) | 5.1 (n = 19) | 7.4 (n = 33) | 6.6 (n = 10) | 0.31 | 5.9 (n = 76) | ||
However, some differences were found to be no longer significant when multivariate analysis was performed (Tables 4 and 5). Our analysis indicates that two factors were associated with cancer detection: increasing patient age, and procedures performed by surgical specialists (Tables 4 and 5). Adenoma detection was increased with increasing patient age, female gender, and procedures performed by medical proceduralists. We also observed a trend towards the increased detection of serrated lesions amongst male patients, but this did not reach the significance level (P = 0.054).
| Coefficient | OR (95%CI) | P value | |
| Cancer | |||
| Site | 0.11 | 1.11 (0.87-1.43) | 0.40 |
| Patient age | 0.04 | 1.04 (1.02-1.07) | < 0.001 |
| Patient gender (male) | -0.45 | 0.64 (0.36-1.14) | 0.13 |
| Trainee (present) | -0.12 | 0.89 (0.46-1.73) | 0.73 |
| Proceduralist (medical)1 | -0.61 | 0.54 (0.30-0.97) | 0.04 |
| Adenomas | |||
| Site | -0.01 | 0.99 (0.90-1.09) | 0.84 |
| Patient age | 0.04 | 1.04 (1.03-1.05) | < 0.001 |
| Patient gender (male) | -0.65 | 0.53 (0.42-0.65) | < 0.001 |
| Trainee (present) | 0.22 | 1.24 (0.96-1.61) | 0.10 |
| Proceduralist (medical)1 | 0.34 | 1.41 (1.13-1.76) | 0.002 |
| Serrated lesions | |||
| Site | 0.08 | 1.08 (0.90-1.3) | 0.42 |
| Patient age | 0.00 | 1.00 (0.99-1.02) | 0.57 |
| Patient gender (male) | 0.41 | 1.51 (0.99-2.29) | 0.05 |
| Trainee (present) | 0.26 | 1.29 (0.78-2.14) | 0.33 |
| Proceduralist (medical)1 | 0.28 | 1.32 (0.87-2.02) | 0.19 |
Although heterogeneity of colonoscopy practice in Australia has been previously described, there are limited reports about its quality, or its association with proceduralist specialty or the involvement of trainees[6]. To our knowledge, this is the first paper to assess quality outcome measures in colonoscopy for surgical and medical specialists, and their trainees across multiple Australian hospitals.
While the collective rates for lesion detection, procedure completion, and adequacy of bowel pre
One area where hospital sites differed significantly was in the quality of bowel preparation. The importance of this metric is attributable to its association with ADR and procedure completion[2,18]. The rates of inadequate preparation within our analysis were comparable with the 9%-13% previously observed in two Australian studies[19,20]. A validated scale for bowel preparation quality (Boston Bowel Preparation or Aronchick), however, was only adopted in one of these[19]. Although either scale was used in 94.2% of colonoscopy procedures assessed in our study, unvalidated approaches were used in up to 22.1% of procedures at individual sites. The exclusion of these procedures from the calculation of completion and detection rates may have been a significant source of bias, potentially limiting our analysis. Considering that suboptimal bowel preparations also justify the re-booking of procedures, ensuring the standardised adoption of validated scales in participating centres should be a priority for quality assurance.
The ADR across all sites in our study comfortably surpassed national benchmarks for quality. Although this was similar to rates reported in a recent meta-analysis of the international literature, direct comparisons should be interpreted with caution due to differences in the definitions used in our study[17]. Whilst ADR has traditionally been determined amongst patients over 50 undergoing an index colonoscopy for the indication of a positive bowel cancer screening test, we included all indications except IBD or prior colorectal surgery as per our national recertification program. However, we additionally excluded non-adequate bowel preparation and incomplete procedures so that the ADR might be a more accurate indicator of technical proficiency. Consequently, this would allow for quality improvement initiatives to be better targeted. Although ADR differed between sites, this was no longer significant on multivariate analysis.
Both patient and proceduralist factors can affect adenoma and lesion detection rates[21]. The medical proceduralists in our study demonstrated significantly higher ADRs compared to their surgical counterparts on both univariate (P < 0.001) and multivariate analyses (P = 0.002). The area is controversial with two other Australian studies reporting conflicting results. Lee et al[10] found no difference in ADR amongst 300 procedures completed by medical or surgical specialists in a single centre, whilst Zorron Cheng Tao Pu et al[8] showed a significantly higher ADR, of 36.8% and 30.4% (P < 0.001), amongst medical proceduralists. Our findings are, however, consistent with a recent meta-analysis of 36 international studies which reported results which were similar to ours[22]. This raises important questions about whether the patients of surgical specialists are disadvantaged. However, the possibility of selection bias due to additional factors which influence ADR, such as procedure indication, should be considered[23]. Additional studies to understand the difference between medical and surgical specialists in Australia are thus required.
A higher cancer detection rate amongst surgical specialists was also observed in our multivariate analysis. Although such a finding would appear to contradict the lower ADR, it would most likely reflect a selection bias in the process of referral for colonoscopy. We assumed that patients with more conspicuous CRC diagnoses would more likely be referred to a surgical specialist. However, data on referral indication was not available in this dataset.
Another key finding of the multivariate analysis was the association between gender and ADR. Higher adenoma detection and CRC risk are usually seen in men and thus the finding of increased adenoma detection amongst female patients was unexpected[24,25]. Metabolic risk factors which increase the risk of adenoma development, including smoking, alcohol use, and low physical activity, have however been observed more frequently in women[26,27]. However, data on these lifestyle factors was not available. On the other hand, our findings may alternatively suggest better engagement of females in individuals with increased risk of adenoma and CRC development. Further studies to validate these results and understand the mechanism of increased ADR amongst women in Australia are therefore also required.
No significant differences were found in the primary outcomes between trainee and specialist proceduralists, the detection of serrated lesions, or procedure completion after adjustment for bowel preparation. Further analysis of trainees according to background speciality similarly showed no significant differences. Together, these findings suggest that the quality of procedures involving training proceduralists are comparable to those of specialists. These findings encouraging for patients who may have reservations about the quality of their procedures on teaching lists within the public sector in Australia. As the next generation of proceduralists in Australia, it is vital that good quality colonoscopy is a foundation of their clinical practice.
The sample size at each individual site may be considered as a limitation of the current study which incorporated five study sites (hospitals). Although the included sites represent both regional and metropolitan practice across two states and territories, it may not be reflective of the broader picture of public practice. To our knowledge, however, it is the first and largest multicentre dataset analysis providing an insight into the quality of colonoscopy in training hospitals in Australia.
One of the major limitations of this study is its retrospective design. Indeterminate outcomes resulting from shortfalls in the quality of the documentation were censored from the analyses but could have affected the results. Non-validated bowel preparation quality scoring systems could not be interpreted although it would have been expected that inadequate preparations would have been reported as such. Limited documentation of withdrawal times also meant that this could not be measured within this study, despite its accepted place as a marker of procedure quality. A prospective study design could account for these limitations and may provide more data reliable quality of documentation, however, would be susceptible to bias from the Hawthorne effect[28].
The exclusions for calculating key metrics in this study also differs from those used in prior studies or the National Recertification program[5,29]. Although this may limit the ability to compare the outcomes against national and internationally reported metrics, we would argue that the adjustments allow the metrics to reflect the aspects of practical interest more accurately. Our definitions separated the outcomes of procedure completion, quality of bowel preparation, and lesion detection which can inform targeted quality improvement efforts. This could include split preparations and shorter runway times to improve quality of bowel preparation, technical re-training for issues associated with procedure completion, or monitoring of withdrawal times for lesion detection. Caution should be taken in the assessment of lesion detection rates however due to the incorporation of multiple indications (screening; surveillance; symptomatic presentations) in the definition of the eligible population.
The definition for serrated lesions adopted within this study were in line with the most recent World Health Organization publication[16]. Repeated updates to these definitions have resulted in the reclassification of lesions in prior studies and remain dependent on the expertise of the reporting pathologist. The absence of a centralised expert pathologist for the assessment of resected lesions of the bowel may have resulted in the misclassification of some lesions, particularly serrated ones. Although we detected no differences in the detection of serrated lesions in our study, it is possible that this may have been masked by misclassification. Careful consideration of the definitions employed in colonoscopy is required for the interpretation of quality outcomes.
Despite potential limitations, our study offers novel clinical insights into the quality of procedures currently being performed in Australian public hospitals. These results highlight the need for quality procedural reporting and bowel preparation, as well as further research into factors which may result in lower ADRs amongst surgeons and men.
Our study indicates that the quality of colonoscopy collectively in the Australian public sector meets national benchmarks. Even when national benchmarks targets were achieved, significant differences in the quality of bowel preparation, and ADRs according to proceduralist specialty and patient gender were found. Two sites of the five assessed did not individually meet all the requirements. Improving bowel preparation should therefore be a key target for quality improvement initiatives. Our analysis suggested that sampling bias was a significant contributing factor which requires attention and control in future investigations. Additional studies to understand why surgical proceduralists detect fewer adenomas than their medical counterparts, and why women in Australia have higher rates of adenoma are required.
There is increasing attention on the quality of colonoscopy performed in Australia due to its vital role in the prevention of colorectal cancer, and its relative under-utilisation among rural and lower socioeconomic communities. However, quality of colonoscopy in Australia has seldom been reported outside of single-centre studies. The largest database, the National Re-certification Program, attempts to address this but largely reflects the quality of work being performed in private hospital settings. Government funded procedures are not well represented in this data, yet accounts for 25% of colonoscopy work, and remains the main pathway for patients without private insurance and within the lowest socioeconomic strata to access this care. We sought to characterise the quality of colonoscopy in this sector, with the aim of informing quality improvement initiatives.
The key quality metrics for colonoscopy are bowel preparation quality, procedure completion rate, and lesion detection rates (cancer, adenomas, and clinically significant serrated lesions). Serrated lesions have also received increasing attention recently, resulting in their incorporation within current national re-certification guidelines. We hope to determine if there are deficiencies in these metrics according to national guidelines and by comparison between participating hospital sites. We also sought to determine if there are significant differences in the detection rates of lesions according to consultant specialty (medical vs surgical), training level (specialist vs trainee), hospital site, and trainee background (medical vs surgical). The outcomes of this research can drive further inquiry into understanding the reasons for these differences and potential solutions.
We aimed to determine the lesion (cancer, adenoma, clinically significant serrated lesion) detection rates, quality of bowel preparation, procedure completion rates among teaching hospitals in Australia. Additionally, we wished to compare the outcomes according to proceduralist specialty, hospital, involvement of trainees, and trainee specialty. We were able to realize all these outcomes, however the analysis of outcomes according to sites was limited by the small sample sizes at some of the participating hospitals. Further studies to explore the link between proceduralist specialty, gender, and adenoma detection rates in Australia are warranted. Additional research regarding methods to improve these outcomes is also indicated.
This was a retrospective cohort study involving consecutive colonoscopies performed over five publicly-funded teaching hospitals in Australia. Currently available colonoscopy quality metrics in Australia are either self-reported and reflect privately funded procedural work or pertain to fewer procedures at single centres. To our knowledge, this is the first study to describe colonoscopy quality across multiple large teaching endoscopy units in the public sector of Australia.
The overall quality of colonoscopy performed in participating hospitals met all specified national benchmarks (adenoma detection rate/procedure completion rate/serrated lesion detection rate). Two hospitals did not meet all benchmarks, due to either a low procedure completion or serrated lesion detection rate, when assessed individually. However, these results were not significantly different when compared with their peers. Significant differences between hospitals were identified on the remaining outcomes of bowel preparation, and detection of cancers and adenomas. Medical specialists detected adenomas in significantly more procedures than their surgical counterparts. In procedures attended by trainees, the detection rate of clinically significant lesions (cancer, adenoma, serrated lesions) was no different to those only involving specialists. Trainee specialty similarly did not affect lesion detection rates. The difference in adenoma detection rate between medical and surgical specialists was confirmed on multivariate analysis. An additional unexpected finding on the multivariate analysis was an association between female gender and adenoma detection. The findings highlight the need for further research to understand the differences between the colonoscopy procedures performed by medical and surgical specialists, and the reasons why female gender in this cohort of patients was an independent risk factor for adenoma detection. Furthermore, it suggests the need for additional sampling in lower-volume endoscopy units for the assessment of quality in colonoscopy.
Our study suggests that although the overall quality of colonoscopy in publicly funded Australian hospitals reach national standards, significant variations exist between hospitals, according to pro
Further research is required to explain the disparity in adenoma detection rates between medical and surgical specialists performing colonoscopy, and to determine why female, rather than male gender, is an independent predictor for adenoma in Australia.
We thank Schoeman M, Lidums I, Cock C, Fraser R, Campbell K.
| 1. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1668] [Article Influence: 139.0] [Reference Citation Analysis (1)] |
| 2. | Clark BT, Rustagi T, Laine L. What level of bowel prep quality requires early repeat colonoscopy: systematic review and meta-analysis of the impact of preparation quality on adenoma detection rate. Am J Gastroenterol. 2014;109:1714-23; quiz 1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 3. | Maida M, Morreale G, Sinagra E, Ianiro G, Margherita V, Cirrone Cipolla A, Camilleri S. Quality measures improving endoscopic screening of colorectal cancer: a review of the literature. Expert Rev Anticancer Ther. 2019;19:223-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 4. | Jensen CD, Doubeni CA, Quinn VP, Levin TR, Zauber AG, Schottinger JE, Marks AR, Zhao WK, Lee JK, Ghai NR, Schneider JL, Fireman BH, Quesenberry CP, Corley DA. Adjusting for patient demographics has minimal effects on rates of adenoma detection in a large, community-based setting. Clin Gastroenterol Hepatol. 2015;13:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Australian Commission on Safety and Quality in Health Care. Colonoscopy Clinical Care Standard. Sydney: ACSQHC; 2020 First released 2018. Updated (minor revisions) January 2020. [cited 26 July 2022]. In: Australian Commission on Safety and Quality in Health Care [Internet]. Available from: https://www.safetyandquality.gov.au/sites/default/files/2020-04/colonoscopy_clinical_care_standard_updated_2020.pdf. |
| 6. | Duggan A, Skinner IJ, Bhasale AL. All colonoscopies are not created equal: why Australia now has a clinical care standard for colonoscopy. Med J Aust. 2018;209:427-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Bettington M, Walker N, Rahman T, Vandeleur A, Whitehall V, Leggett B, Croese J. High prevalence of sessile serrated adenomas in contemporary outpatient colonoscopy practice. Intern Med J. 2017;47:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Zorron Cheng Tao Pu L, Lu K, Ovenden A, Rana K, Singh G, Krishnamurthi S, Edwards S, Wilson B, Nakamura M, Yamamura T, Ruszkiewicz A, Hirooka Y, Burt AD, Singh R. Effect of time of day and specialty on polyp detection rates in Australia. J Gastroenterol Hepatol. 2019;34:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Wong WJ, Arafat Y, Wang S, Hawes S, Hung K. Colonoscopy withdrawal time and polyp/adenoma detection rate: a single-site retrospective study in regional Queensland. ANZ J Surg. 2020;90:314-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Lee AHH, Lojanapiwat N, Balakrishnan V, Chandra R. Is there a difference in adenoma detection rates between gastroenterologists and surgeons? World J Gastrointest Endosc. 2018;10:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38562] [Cited by in RCA: 40146] [Article Influence: 2361.5] [Reference Citation Analysis (0)] |
| 12. | Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN; REDCap Consortium. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 17058] [Article Influence: 2436.9] [Reference Citation Analysis (0)] |
| 13. | Kastenberg D, Bertiger G, Brogadir S. Bowel preparation quality scales for colonoscopy. World J Gastroenterol. 2018;24:2833-2843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 104] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (10)] |
| 14. | Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, Kobiela J, Franczyk R, Rupinska M, Kocot B, Chaber-Ciopinska A, Pachlewski J, Polkowski M, Regula J. Increased Rate of Adenoma Detection Associates With Reduced Risk of Colorectal Cancer and Death. Gastroenterology. 2017;153:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 400] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 15. | Desai M, Anderson JC, Kaminski M, Thoguluva Chandrasekar V, Fathallah J, Hassan C, Lieberman D, Sharma P. Sessile serrated lesion detection rates during average risk screening colonoscopy: A systematic review and meta-analysis of the published literature. Endosc Int Open. 2021;9:E610-E620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2748] [Article Influence: 458.0] [Reference Citation Analysis (3)] |
| 17. | Tziatzios G, Gkolfakis P, Lazaridis LD, Facciorusso A, Antonelli G, Hassan C, Repici A, Sharma P, Rex DK, Triantafyllou K. High-definition colonoscopy for improving adenoma detection: a systematic review and meta-analysis of randomized controlled studies. Gastrointest Endosc. 2020;91:1027-1036.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Hsu CM, Lin WP, Su MY, Chiu CT, Ho YP, Chen PC. Factors that influence cecal intubation rate during colonoscopy in deeply sedated patients. J Gastroenterol Hepatol. 2012;27:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Kutyla MJ, Gray MA, von Hippel C, Hourigan LF, Kendall BJ, Whaley AJ, O'Connor S, Holtmann GJ. Improving the Quality of Bowel Preparation: Rewarding Patients for Success or Intensive Patient Education? Dig Dis. 2021;39:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Bryant RV, Schoeman SN, Schoeman MN. Shorter preparation to procedure interval for colonoscopy improves quality of bowel cleansing. Intern Med J. 2013;43:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Cavicchi M, Tharsis G, Burtin P, Cattan P, Venezia F, Tordjman G, Gillet A, Samama J, Nahon-Uzan K, Karsenti D. Difference in Physician- and Patient-Dependent Factors Contributing to Adenoma Detection Rate and Serrated Polyp Detection Rate. Dig Dis Sci. 2019;64:3579-3588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 22. | Mazurek M, Murray A, Heitman SJ, Ruan Y, Antoniou SA, Boyne D, Murthy S, Baxter NN, Datta I, Shorr R, Ma C, Swain MG, Hilsden RJ, Brenner DR, Forbes N. Association Between Endoscopist Specialty and Colonoscopy Quality: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:1931-1946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Yang PF, Wong SW. Adenoma Detection Rate in Colonoscopy: Is Indication a Predictor? Surg Laparosc Endosc Percutan Tech. 2016;26:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Lee JY, Park HW, Kim MJ, Lee JS, Lee HS, Chang HS, Choe J, Hwang SW, Yang DH, Myung SJ, Yang SK, Byeon JS. Prediction of the Risk of a Metachronous Advanced Colorectal Neoplasm Using a Novel Scoring System. Dig Dis Sci. 2016;61:3016-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Coe SG, Wallace MB. Assessment of adenoma detection rate benchmarks in women vs men. Gastrointest Endosc. 2013;77:631-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Aleksandrova K, Pischon T, Jenab M, Bueno-de-Mesquita HB, Fedirko V, Norat T, Romaguera D, Knüppel S, Boutron-Ruault MC, Dossus L, Dartois L, Kaaks R, Li K, Tjønneland A, Overvad K, Quirós JR, Buckland G, Sánchez MJ, Dorronsoro M, Chirlaque MD, Barricarte A, Khaw KT, Wareham NJ, Bradbury KE, Trichopoulou A, Lagiou P, Trichopoulos D, Palli D, Krogh V, Tumino R, Naccarati A, Panico S, Siersema PD, Peeters PH, Ljuslinder I, Johansson I, Ericson U, Ohlsson B, Weiderpass E, Skeie G, Borch KB, Rinaldi S, Romieu I, Kong J, Gunter MJ, Ward HA, Riboli E, Boeing H. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. 2014;12:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 27. | Beigh SH, Jain S. Prevalence of metabolic syndrome and gender differences. Bioinformation. 2012;8:613-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Demetriou C, Hu L, Smith TO, Hing CB. Hawthorne effect on surgical studies. ANZ J Surg. 2019;89:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Pullens HJ, Siersema PD. Quality indicators for colonoscopy: Current insights and caveats. World J Gastrointest Endosc. 2014;6:571-583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Manojlovic N, Serbia; Messias LHD, Brazil S-Editor: Gao CC L-Editor: A P-Editor: Gao CC