Published online Oct 16, 2022. doi: 10.4253/wjge.v14.i10.628
Peer-review started: March 10, 2022
First decision: June 16, 2022
Revised: July 13, 2022
Accepted: September 13, 2022
Article in press: September 13, 2022
Published online: October 16, 2022
Processing time: 215 Days and 8.3 Hours
Tracheoesophageal fistulas (TEFs) can be described as a pathological comm
We report the cases of 14 patients with different comorbidities such as being over
The incidence of TEFs increased during the coronavirus disease 2019 pandemic (from 0.5% to 1.5%). We believe that endoscopic treatment should be considered as an option for this group of patients, since evidence reported in the literature is still a growing area. Therefore, we propose an algorithm to lead intervention in patients presenting with TEFs due to prolonged intubation.
Core Tip: Due to the significant increase of tracheoesophageal fistulas in the context of severe coronavirus disease 2019 (COVID-19) pneumonia, and the high frequency of risk factors in patients with COVID-19, we recommend early identification and correction of these factors, such as frequent measurement of the cuff pressure and, if possible, periodic evaluation of the tracheal mucosa with bronchoscopy to identify early precursor lesions of tracheoesophageal fistula. Regarding treatment, provide initial endoscopic management until optimal conditions for surgical management are reached. Endoscopic management should be selected according to the size and location of the fistula.
- Citation: Gomez Zuleta MA, Gallego Ospina DM, Ruiz OF. Tracheoesophageal fistulas in coronavirus disease 2019 pandemic: A case report. World J Gastrointest Endosc 2022; 14(10): 628-635
- URL: https://www.wjgnet.com/1948-5190/full/v14/i10/628.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i10.628
Tracheoesophageal fistulas (TEFs) are defined as abnormal communications between the esophagus and the trachea or bronchi, leading to the passage of oral and gastric secretions into the respiratory tract[1]. TEFs can be classified into two main categories: Congenital or acquired. The congenital form is frequently associated with type C esophageal atresia (85%), presenting in an isolated manner in 4% of cases. Characteristically, clinical manifestations of this condition develop early in life[2-4]. On the other hand, acquired TEFs mainly affect adults and are most frequently found in the cervicothoracic junction. TEFs can be malignant or benign. Each type constitutes approximately half of the acquired cases[4].
Malignant TEFs are a catastrophic complication of invasive neoplasms of the esophagus (squamous cell carcinoma), trachea, lung, or mediastinum[4-6]. On the other hand, benign fistulas mainly develop due to prolonged mechanical ventilation (through an endotracheal tube or tracheostomy); blunt trauma to the neck and chest; traumatic or surgical injury of the esophagus; granulomatous mediastinal infections; previous esophageal stents, or ingestion of foreign bodies/corrosives[5]. In patients under
The most common clinical presentation of TEFs includes respiratory distress, dysphagia, cough after swallowing (ONO sign), malnutrition, and recurrent pulmonary infections. The severity of symptoms largely depends on their size and location[8,9]. A diagnosis should be made by combining characteristic findings on thoracic imaging (esophagogram and chest tomography with 3D reconstruction) and those on endoscopic studies such as bronchoscopy and upper endoscopy. These studies are also essential when planning the best treatment option for each patient[1,8,10,11].
The mean survival reported for patients with TEFs is less than 3 mo from the time of diagnosis. As such, adequate treatment should include an immediate multidisciplinary approach, including specialists in critical care, interventional pulmonology, gastroenterology, and thoracic surgery. Currently, there are few case reports regarding TEFs due to prolonged intubation in patients with coronavirus disease 2019 (COVID-19)[12-16]. We herein present a case series on patients with COVID-19 who develop TEFs and discuss diagnostic and therapeutic approaches.
Before creating this case series, we obtained informed consent from each patient or their legal guardians. We included patients who were admitted to a university hospital in the city of Bogotá, Colombia in the period between November 2020 and December 2021. We identified 14 adult patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pneumonia who developed TEFs as a complication of prolonged mechanical ventilation.
We present the sociodemographic variables of the patients and relevant information on their past medical histories in Table 1. The average age was 53.5 years (range 38-72 years). Half of the sample was composed by men. Comorbidities were found in 85.7% of the patients, with the most frequent being obesity/overweight, diabetes mellitus, and systemic hypertension.
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total, n (%) |
| Age (years) | 60 | 58 | 72 | 52 | 46 | 63 | 56 | 46 | 41 | 61 | 49 | 39 | 69 | 38 | |
| Sex | M | F | M | F | F | F | F | F | M | M | M | M | M | F | |
| BMI | 25.1 | 34.3 | 23.9 | 28.6 | 32 | 19.1 | 28 | 27 | 26 | 29.5 | 23 | 32 | 27.1 | 23.4 | |
| Past medical history | |||||||||||||||
| Diabetes mellitus | - | X | - | - | X | - | X | X | - | - | - | X | X | - | 6 (42.8) |
| Systemic hypertension | - | - | X | - | - | X | X | X | - | - | - | X | X | - | 6 (42.8) |
| Obesity/Overweight | X | X | - | X | X | - | X | X | X | X | - | X | X | - | 10 (71.4) |
| Other | - | - | PC | - | - | H | - | - | H | AF | - | - | - | - |
The clinical characteristics of the patients are shown in Table 2. The most common symptoms, which lead all patients to attend the emergency room, were cough and dyspnea. All of the subjects were diagnosed with severe pneumonia due to COVID-19. At least 64.2% presented with septic shock, requiring vasoactive support. All patients required invasive mechanical ventilation for more than 14 d. Acute respiratory distress syndrome (ARDS) was documented in 13 patients, and this variable was no available for assessment in one patient. All patients were treated with a steroid (dexamethasone: 6 mg s.c., q.d. for 10 d), and the steroid was prematurely stopped in one patient due to diabetic ketoacidosis during treatment. All patients received enteral nutrition through nasoenteral tubes.
| Case | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total, n (%) |
| Reason for consultation | |||||||||||||||
| Fever | X | - | X | X | X | X | - | X | X | - | X | X | X | - | 10 (71.4) |
| Cough | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 14 (100) |
| Dyspnea | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 14 (100) |
| Clinical findings | |||||||||||||||
| Viral pneumonia SARS CoV2 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 14 (100) |
| SOFA | 2 | 6 | ND | 4 | 8 | ND | ND | 10 | ND | ND | ND | ND | 6 | ND | |
| Clinical course | |||||||||||||||
| Invasive mechanical ventilation | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 14 (100) |
| ARDS | X | X | ND | X | X | X | X | X | X | X | X | X | X | X | |
| Vasoactive | X | X | ND | X | X | X | ND | X | X | X | ND | ND | X | ND | |
| Shock | X | X | ND | X | X | X | ND | X | X | X | ND | ND | X | ND | |
| Steroids | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 14 (100) |
| Dispositivo vía esofagica | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 14 (100) |
| Cuff pressure measurement | - | - | - | X | - | - | - | - | X | - | - | - | - | - | 2 (14.2) |
| Tracheostomy | X | X | - | X | - | - | X | - | X | X | X | X | - | X | 9 (64.2) |
| Gastrostomy | X | - | - | X | - | X | X | - | X | X | X | X | - | X | 9 (64.2) |
| Diagnosis | |||||||||||||||
| Upper gastrointestinal endoscopy | X | X | X | X | X | X | X | X | X | X | X | X | X | X | 14 (100) |
| Axial computed tomography of the neck | X | X | X | X | X | X | N | X | - | N | X | X | N | X | 13 (92.8) |
| Complications | |||||||||||||||
| Tracheitis | X | - | - | - | - | - | - | - | - | - | - | X | X | - | 3 (21.4) |
| Pneumonia | - | - | X | X | X | X | X | X | X | X | - | - | - | X | 9 (64.2) |
| Bacteremia | - | X | - | - | - | - | - | - | - | - | X | - | - | X | 3 (21.4) |
| Clostridioidal infection | - | - | X | - | - | - | - | - | - | - | - | - | - | X | 2 (14.2) |
| Acute kidney injury | - | X | - | - | - | - | - | X | X | X | X | - | X | - | 6 (42.8) |
| Treatment | |||||||||||||||
| OTS clip | X | - | X | X | - | - | X | - | - | - | - | - | - | - | 4 (28.5) |
| TTS endoclip | - | - | - | - | - | - | X | - | - | X | - | - | - | - | 2 (14.2) |
| Self-expanding metallic stent | - | X | - | - | - | - | - | X | - | - | - | X | - | - | 3 (21.4) |
| Surgery | X | - | - | X | - | X | - | - | X | - | X | X | - | X | 7 (50) |
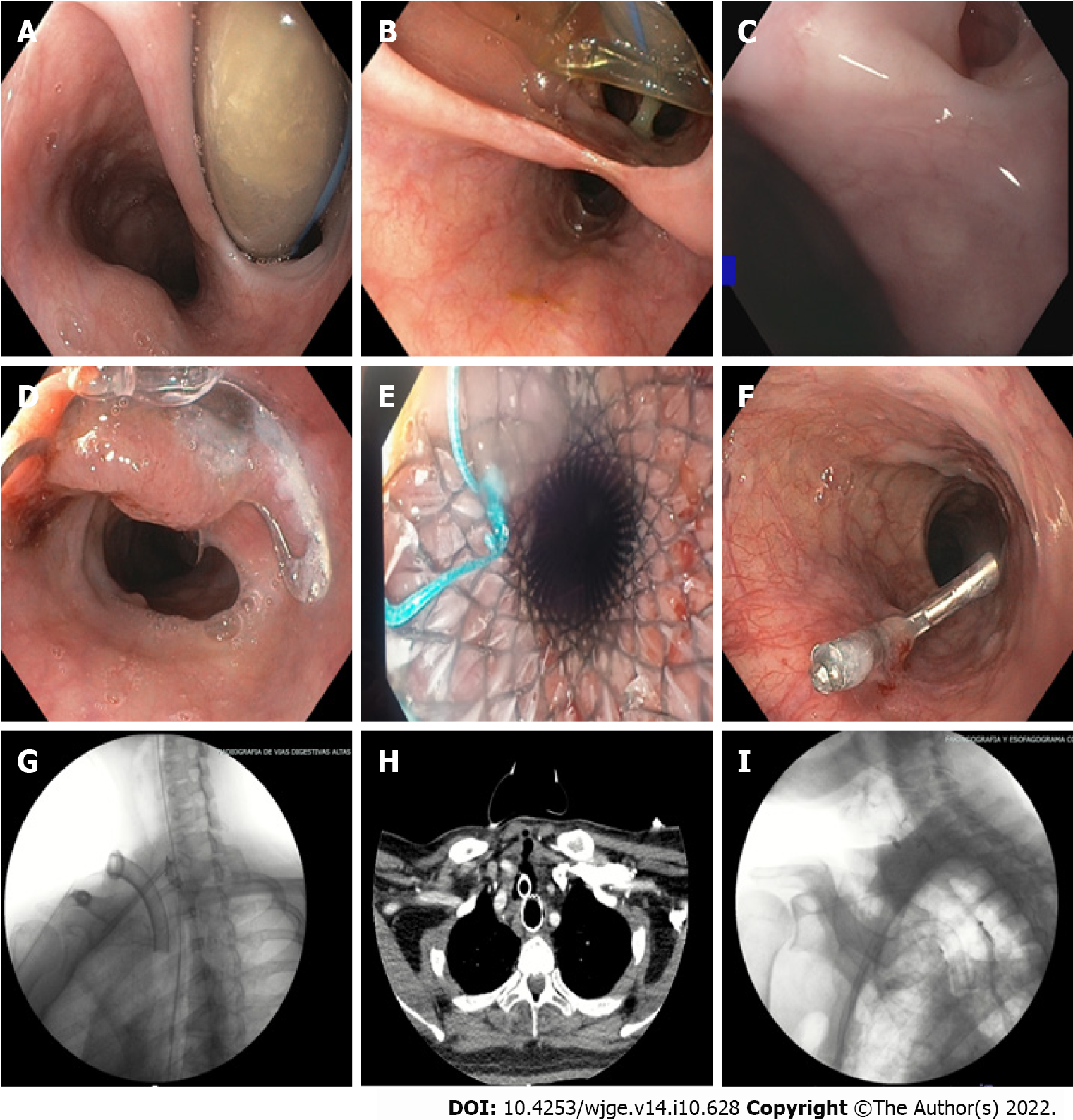
The pressure of the endotracheal cuff was measured in only two patients (14.2%), being greater than 35 cmH2O in both cases. TEFs were documented by endoscopic study of the upper digestive tract (100%) and in some cases with three-dimensional reconstruction of neck computed tomography (71.4%). All TEFs were found in the proximal esophagus, with an average distance of 16.7 cm from the dental arch, and the average diameter was 18.2 mm (range 3 mm-40 mm) (Figure 1).
All of the patients had bacterial infectious complications, including tracheitis (21.4%), pneumonia (64.2%), and bacteremia (21.4%). Therefore, they required treatment with broad-spectrum antibiotics leading to Clostridioides difficile infection in 14.2% of the sample. Six patients developed terminal acute kidney injury requiring renal replacement therapy. For the closure of TEFs, eight patients were taken to temporary or definitive endoscopic treatment: Four needed over the scope (OTS) clips, achieving successful endoscopic closure in two. Clip placement failed in one of the patients due to tissue fibrosis; a recurring defect was documented in another patient. Three patients received temporary management with a fully coated metallic stent (SEMS), managing to completely cover the defect. Hemoclips (TTS endoclips) were used in two patients. In one patient, with a 3 mm TEF, adequate closure of the defect was achieved; while in another patient, temporary reduction in diameter was achieved, allowing further management with an OTS clip (Figure 1). In six patients, a surgical approach was indicated given the location and size of the fistula. Surgical management was also provided to the patient with failure to therapy with the OTS clip, achieving successful correction of the defect. On follow-up, recurrence of TEFs was observed in only one patient treated with an OTS clip, and an increase in the size of the fistula was detected, for which surgical therapy was considered, successfully closing the defect. Despite the efforts made, 42.8% (6/14) died due to infectious complications, with two patients dying before receiving surgical management.
Comorbidities were found in 85.7% of the patients, with the most frequent being obesity/overweight (71.4%), diabetes mellitus (42.8%), and systemic hypertension (42.8%).
Half of the sample was composed by women with an average weight of 72.4 kg (body mass index [BMI] 27.4). The men had an average weight of 82 kg (BMI 26.6). The pressure of the endotracheal cuff was measured in only two patients (14.2%), being greater than 35 cmH2O in both cases.
Three dimensional reconstruction of neck computed tomography was performed in 13 patients (92.8%), identifying the presence of a fistula in 71.4%. At the time of diagnosis, all patients were on invasive mechanical ventilation, so esophagogram was not performed in any of them.
TEFs were documented by endoscopic study of the upper digestive tract (100%) and in some cases with three-dimensional reconstruction of neck computed tomography (71.4%). All TEFs were found in the proximal esophagus, with an average distance of 16.7 cm from the dental arch, and the average diameter was 18.2 mm (range 3-40 mm) (Figure 1).
For the closure of TEFs, eight patients were taken to temporary or definitive endoscopic treatment: Four needed OTS clips, achieving successful endoscopic closure in two (video 1). Clip placement failed in one of the patients due to tissue fibrosis; a recurring defect was documented in another patient. Three patients received temporary management with a fully coated metallic stent (SEMS), managing to completely cover the defect. Hemoclips (TTS endoclips) were used in two patients. In one patient, with a 3mm TEF, adequate closure of the defect was achieved, while in another patient, temporary reduction in diameter was achieved, allowing further management with an OTS clip (Figure 1). In six patients, a surgical approach was indicated given the location and size of the fistula. Surgical management was also provided to the patient with failure to therapy with the OTS clip, achieving successful correction of the defect. On follow-up, recurrence of TEFs was observed in only one patient treated with the OTS clip, and an increase in the size of the fistula was detected, for which surgical therapy was considered, successfully closing the defect.
Despite the efforts made, 42.8% (6/14) of the patients died due to infectious complications, with two patients dying before receiving surgical management.
Acquired TEFs are a rare clinical entity, with incidence rates approaching 0.5%. Up to 75% of cases are due to trauma related to endotracheal cuff overinflation or prolonged mechanical ventilation[4,8,17]. The pressure exerted by the endotracheal tube cuff erodes the tracheal mucosa, leading to ischemic destruction of the tracheal cartilage, which creates a communication with the esophageal wall[4,8].
The current health situation, due to the SARS-CoV-2 pandemic, which significantly increased cases of severe pneumonia and ARDS, led to a parallel increase in TEFs associated with prolonged endotracheal intubation. We found that 14 out of 894 patients undergoing mechanical ventilation for severe COVID-19 pneumonia, developed TEFs (incidence 1.56%). In most patients, several risk factors were simultaneously found; these included prolonged mechanical ventilation, hypotension, steroid use, diabetes mellitus, obesity, and excessive movement of the endotracheal tube due to frequent position changes (supine-prone)[18]. We hypothesize that monitoring of the endotracheal cuff pressure was insufficient, possibly due to overcrowding in critical care units, as well as the exhaustion, anxiety, and depression developed by healthcare workers during the pandemic[19,20,21,22].
Spontaneous closure of TEFs is rare, and therefore requires the use of different treatment approaches, including endoscopic and surgical options[4,7,23]. Among the endoscopic options is the use of fully coated metallic stents (SEMS), OTS clips, TTS endoclips, and suture systems among others[24-27]. These procedures have allowed for high success rates (73%-83%) regarding closure of perforations, leaks, and gastrointestinal fistulas[28]. However, due to a low incidence of TEFs, no consensus guidelines on the management of this entity currently exist, particularly concerning patients with SARS-CoV-2 infection. It has been reported that mechanical ventilation increases the risk for suture dehiscence. Furthermore, comorbidities and the critical condition of patients with severe COVID-19 pneumonia usually lead to deferral of surgical procedures until after mechanical ventilation withdrawal. This is why considering endoscopic interventions as initial management in critically ill patients with tracheoesophageal fistula associated with mechanical ventilation due to COVID-19 should be sought.
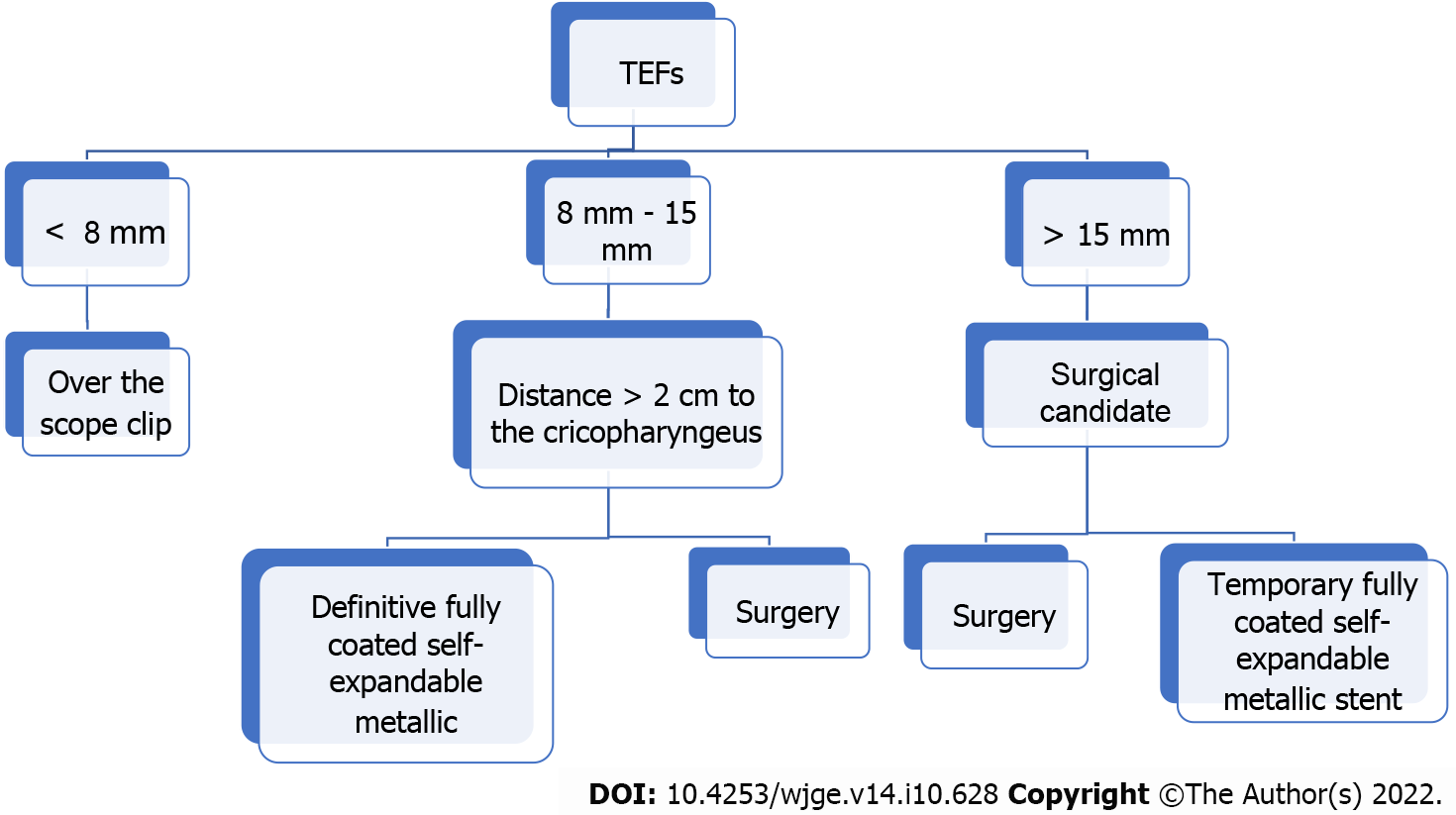
We present a treatment algorithm for this group of patients in Figure 2. Our approach is determined by the size and location of the fistula, using OTS clips for defects below the size of 8 mm. For lesions between 8 and 15 mm, we suggest to use SEMS as long as the fistula is more than 2 cm distal to the cricopharyngeus where the stent can be properly fixed. In lesions larger than 15 mm, we propose upfront surgical treatment, as well as when the fistulas are less than 2 cm from the cricopharyngeus (because at this distance the stent may lead to foreign body sensation). When the patient is not a good surgical candidate and has lesions larger than 15 mm located more than 2 cm away from the cricopharyngeus, a fully SEMS can be placed as bridging therapy until the patient becomes stable and in better condition for surgical treatment. Although we have a small sample size, to the best of our knowledge, this is the first study to illustrate the management of this type of patients in the context of the coronavirus pandemic.
Due to the significant increase in diagnosis of TEFs in patients with severe pneumonia due to COVID-19, and the high frequency of risk factors for TEFs in these patients, we recommend early identification and prevention of these conditions, in addition to frequent measurement of the endotracheal cuff pressure. If possible, we recommend periodic evaluation of the tracheal mucosa by bronchoscopy to identify early lesions that could lead to the development of TEFs. Regarding treatment, we suggest providing initial endoscopic management in small fistulas (below 15 mm) or until optimal conditions for surgical management are met (if larger than 15 mm). Definitive endoscopic treatment may be offered according to the size and location of the fistula.
| 1. | Zhou C, Hu Y, Xiao Y, Yin W. Current treatment of tracheoesophageal fistula. Ther Adv Respir Dis. 2017;11:173-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | Slater BJ, Rothenberg SS. Tracheoesophageal fistula. Semin Pediatr Surg. 2016;25:176-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Hasan L, Sharma B, Goldenberg SA. Acquired Tracheoesophageal Fistulas: A Case Report and Review of Diagnostic and Management Challenges. Cureus. 2022;14:e23324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am. 2003;13:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 5. | Davydov M, Stilidi I, Bokhyan V, Arzykulov G. Surgical treatment of esophageal carcinoma complicated by fistulas. Eur J Cardiothorac Surg. 2001;20:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Rodriguez AN, Diaz-Jimenez JP. Malignant respiratory-digestive fistulas. Curr Opin Pulm Med. 2010;16:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Macchiarini P, Verhoye JP, Chapelier A, Fadel E, Dartevelle P. Evaluation and outcome of different surgical techniques for postintubation tracheoesophageal fistulas. J Thorac Cardiovasc Surg. 2000;119:268-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Santosham R. Management of Acquired Benign Tracheoesophageal Fistulae. Thorac Surg Clin. 2018;28:385-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Kaur D, Anand S, Sharma P, Kumar A. Early presentation of postintubation tracheoesophageal fistula: Perioperative anesthetic management. J Anaesthesiol Clin Pharmacol. 2012;28:114-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Raman D, Shaw IH. Acquired tracheo-oesophageal fistula in adults. Continuing Education in Anaesthesia Critical Care & Pain (3):105-108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Kim HS, Khemasuwan D, Diaz-Mendoza J, Mehta AC. Management of tracheo-oesophageal fistula in adults. Eur Respir Rev. 2020;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | García-Herreros LG, Jiménez A, Cabrera LF, Vinck EE, Pedraza M. Early presentation of post-intubation tracheoesophageal fistula with severe tracheal stenosis in COVID-19 patient. Ann R Coll Surg Engl. 2021;103:e144-e147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | oomi, S, Talib, U, Farooq S, Chohan A, & Kumar R (2020). Tracheoesophageal fistula: a rare complication of prolonged intubation in covid-19. Chest. . [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Cuaño PMGM, Pilapil JCA, Larrazabal RJB, Villalobos RE. Acquired tracheoesophageal fistula in a pregnant patient with COVID-19 pneumonia on prolonged invasive ventilation. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Rosati R, De Nardi P, Dell'Acqua A, Calvi MR, Elmore U, Scarparo E, Beretta L. Tracheoesophageal Fistula in a COVID-19 Ventilated Patient: A Challenging Therapeutic Decision. Case Rep Surg. 2021;2021:6645518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Pereira C, Silva R, Campello GC, Moura F. Tracheoesophageal fistula in a COVID-19 patient. Saudi J Anaesth. 2021;15:447-449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Harley HR. Ulcerative tracheo-oesophageal fistula during treatment by tracheostomy and intermittent positive pressure ventilation. Thorax. 1972;27:338-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Godoy AC, Vieira RJ, Capitani EM. Endotracheal tube cuff pressure alteration after changes in position in patients under mechanical ventilation. J Bras Pneumol. 2008;34:294-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Restauri N, Sheridan AD. Burnout and Posttraumatic Stress Disorder in the Coronavirus Disease 2019 (COVID-19) Pandemic: Intersection, Impact, and Interventions. J Am Coll Radiol. 2020;17:921-926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 20. | Alanazi TNM, McKenna L, Buck M, Alharbi RJ. Reported effects of the COVID-19 pandemic on the psychological status of emergency healthcare workers: A scoping review. Australas Emerg Care. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Kunz M, Strasser M, Hasan A. Impact of the coronavirus disease 2019 pandemic on healthcare workers: systematic comparison between nurses and medical doctors. Curr Opin Psychiatry. 2021;34:413-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Shreffler J, Petrey J, Huecker M. The Impact of COVID-19 on Healthcare Worker Wellness: A Scoping Review. West J Emerg Med. 2020;21:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 396] [Cited by in RCA: 346] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 23. | Shen KR, Allen MS, Cassivi SD, Nichols FC 3rd, Wigle DA, Harmsen WS, Deschamps C. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg. 2010;90:914-8; discussion 919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Cereatti F, Grassia R, Drago A, Conti CB, Donatelli G. Endoscopic management of gastrointestinal leaks and fistulae: What option do we have? World J Gastroenterol. 2020;26:4198-4217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 60] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (2)] |
| 25. | Rogalski P, Daniluk J, Baniukiewicz A, Wroblewski E, Dabrowski A. Endoscopic management of gastrointestinal perforations, leaks and fistulas. World J Gastroenterol. 2015;21:10542-10552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 26. | Chan SM, Auyeung KKY, Lam SF, Chiu PWY, Teoh AYB. Current status in endoscopic management of upper gastrointestinal perforations, leaks and fistulas. Dig Endosc. 2022;34:43-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Ramai D, Bivona A, Latson W, Ofosu A, Ofori E, Reddy M, Adler DG. Endoscopic management of tracheoesophageal fistulas. Ann Gastroenterol. 2019;32:24-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Ross, WA, Lee JH. (2008) Endoscopic Approach to Tracheoesophageal Fistulas in Adults. Tech Gastrointest Endosc. 2008;10:155-163. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Asociación Colombiana De Gastroenterologia; American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Colombia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lui RN, China; Singh I, United States S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ