Published online Jan 16, 2022. doi: 10.4253/wjge.v14.i1.49
Peer-review started: April 27, 2021
First decision: June 13, 2021
Revised: June 16, 2021
Accepted: December 11, 2021
Article in press: December 11, 2021
Published online: January 16, 2022
Processing time: 260 Days and 21.2 Hours
Endoscopic resection, especially endoscopic submucosal dissection (ESD), is increasingly performed in elderly patients with early gastric cancer, and lesions beyond the expanded indications are also resected endoscopically in some patients. It is essential to assess whether gastric ESD is safe and suitable for elderly patients and investigate what type of lesions carry an increased risk of ESD-related complications.
To assess the efficacy and feasibility of gastric ESD for elderly patients, and define high-risk lesions and prognostic indicators.
Among a total of 1169 sessions of gastric ESD performed in Kanagawa Cancer Center Hospital from 2006 to 2014, 179 sessions (15.3%) were performed in patients aged ≥ 80 years, and 172 of these sessions were done in patients with a final diagnosis of gastric cancer. These patients were studied retrospectively to evaluate short-term outcomes and survival. The short-term outcomes included the rates of en bloc resection and curative resection, complications, and procedure-related mortality. Curability was assessed according to the Japanese Gastric Cancer Treatment Guidelines 2010. Fisher’s exact test was used to statistically analyze risk factors. Clinical characteristics of each group were compared using Fisher’s exact test and Mann-Whitney U test. Survival rates at each time point were based on Kaplan-Meier estimation. Overall survival rates were compared between patients with gastric cancer in each group with use of the log-rank test. To identify prognostic factors that jointly predict the hazard of death while controlling for model overfitting, we used the least absolute shrinkage and selection operator (LASSO) Cox regression model including factors curative/ noncurative, age, gender, body mass index, prognostic nutritional index, Charlson comorbidity index (CCI), Glasgow prognostic score, neutrophil-to-lymphocyte ratio, and antithrombotic agent use. We selected the LASSO Cox regression model that resulted in minimal prediction error in 10-fold cross-validation. P < 0.05 was considered statistically significant.
The en bloc dissection rate was 97.1%, indicating that a high quality of treatment was achieved even in elderly patients. As for complications, the rates of bleeding, perforation and aspiration pneumonitis were 3.4%, 1.1% and 0.6%, respectively. These complication rates indicated that ESD was not associated with a particularly higher risk in elderly patients than in nonelderly patients. A dissection incision > 40 mm, lesions associated with depressions, and lesions with ulcers were risk factors for post-ESD bleeding, and location of the lesion in the upper third of the stomach was a risk factor for perforation in elderly patients (P < 0.05). Location of the lesion in the lower third of the stomach tended to be associated with a higher risk of bleeding. The overall survival (OS) did not differ sig
Gastric ESD is feasible even in patients aged ≥ 80 years. Observation without additional surgery after noncurative ESD is reasonable, especially in elderly patients with CCI ≥ 2.
Core Tip: This was a retrospective study to evaluate the efficacy and feasibility of gastric endoscopic submucosal dissection in elderly patients aged ≥ 80 years. The rates of en bloc dissection, bleeding, perforation and aspiration pneumonitis were 97.1%, 3.4%, 1.1% and 0.6%, respectively. These rates are similar to the rates in nonelderly patients reported previously. Risk factors for bleeding were incision > 40 mm, lesions associated with depressions, and ulcerative lesions. A risk factor for perforation was location in the upper third of the stomach. Charlson comorbidity index ≥ 2 was an indicator of poor prognosis regardless of curability.
- Citation: Inokuchi Y, Ishida A, Hayashi K, Kaneta Y, Watanabe H, Kano K, Furuta M, Takahashi K, Fujikawa H, Yamada T, Yamamoto K, Machida N, Ogata T, Oshima T, Maeda S. Feasibility of gastric endoscopic submucosal dissection in elderly patients aged ≥ 80 years . World J Gastrointest Endosc 2022; 14(1): 49-62
- URL: https://www.wjgnet.com/1948-5190/full/v14/i1/49.htm
- DOI: https://dx.doi.org/10.4253/wjge.v14.i1.49
Early gastric cancer (EGC) is defined as gastric cancer confined to the mucosa and submucosa[1]. Increasing numbers of EGCs are being detected in Japan[2,3], and EGCs currently account for > 60% of all detected cases of gastric cancer[4]. Since the deve
The elderly population is increasing rapidly in Japan. The average life span is 80.50 years for men and 86.83 years for women, according to statistics reported by the Ministry of Health, Labour and Welfare, Japan in 2014. Surgery carries an increased risk in elderly patients because of poor physical status or serious underlying diseases[9,10]. Thus ER, especially ESD, is being increasingly performed in elderly patients[10-14]. Because this trend is expected to continue, it is necessary to assess whether ESD is actually safe and suitable for elderly patients. In addition, more clearly defining high-risk lesions associated is prerequisite to safe treatment.
A total of 1169 sessions of ESD were performed to treat gastric diseases (mainly EGCs and gastric adenomas, as well as some non-neoplastic lesions) in Kanagawa Cancer Center Hospital between January 2006 and December 2014, and 179 (15.3%) of these sessions were performed in a total of 131 patients who were aged ≥ 80 years. Among the resected specimens, gastric cancers were finally diagnosed in 175 lesions treated by 172 sessions of ESD in 124 patients. These cases were studied retrospectively.
Around-the-lesion biopsy was performed beforehand to confirm the margin of the lesions, if necessary. On the day of ESD, the margin was identified again using white light endoscopy, chromoendoscopy with indigo carmine solution, and narrow-band imaging. All-around-the-lesion marking was carried out with the use of small multiple cautery units. Submucosal injection was performed to lift the mucosal layer. Glyceol (10% glycerol and 5% fructose; Chugai Pharmaceutical Co., Tokyo, Japan) or MucoUp (0.4% sodium hyaluronate; Johnson & Johnson, New Brunswick, NJ, United States) with a small amount of indigo carmine was used as the injection solution. A circumferential mucosal incision and submucosal dissection were performed using a needle knife (Olympus Optical Co. Ltd., Tokyo, Japan). The high-frequency generators used were ICC200 or VIO300D (ERBE Elektromedizin GmbH, Tübingen, Germany).
The short-term outcomes included the rates of en bloc resection and curative resection, complications, and procedure-related mortality. Curability was assessed according to the Japanese Gastric Cancer Treatment Guidelines 2010[15]. A curative resection was defined as satisfying all the following conditions: en bloc resection, negative horizontal and vertical margin, no lymphovascular infiltration, and absolute or expanded indication for ER. Differentiated type intramucosal cancer ≤ 20 mm in size without ulceration was categorized as a lesion of absolute indication. A lesion of expanded indications was as follows: Differentiated type intramucosal cancer > 20 mm in size without ulceration; differentiated type intramucosal cancer ≤ 30 mm in size with ulceration; differentiated type submucosal superficial cancer ≤ 30 mm in size; and undifferentiated type intramucosal cancer ≤ 20 mm in size without ulceration.
As for complications, bleeding, perforation and aspiration pneumonitis were assessed. Bleeding was defined as the occurrence of melena or hematemesis; detection of ongoing hemorrhage; or the presence of coagulated blood in the stomach with apparent bleeding spots on endoscopic examination, which was basically performed routinely in all patients on the next day of ESD. Perforation was confirmed by observation of mesenteric fat during ESD or by detection of free air on X-ray films. Aspiration pneumonitis was diagnosed on the basis of clinical findings and X-ray films. Procedure-related mortality was defined as death within 30 d due to complications. In patients who had complications, patient-related factors, such as World Health Organization performance status and underlying disease, as well as lesion-related factors, such as location, size, and macroscopic aspects were investigated.
For evaluation of long-term outcomes, a patient who had experienced noncurative ESD within the last 5 years (n = 1) and patients who underwent additional surgery after ESD (n = 3) were excluded from the target of analysis. Overall survival (OS) was evaluated starting from the date of ESD to the date of death or the last verified date of survival. To determine the prognostic indicators for elderly patients with EGC treated by ESD, we also evaluated the clinical characteristics of the patients who did not undergo additional surgery after ESD (n = 120), using age, gender, body mass index (BMI), prognostic nutritional index (PNI), Charlson comorbidity index (CCI), Glasgow prognostic score (GPS), neutrophil-to-lymphocyte ratio (NLR), and use of antithrombotic agents.
To estimate affecting factors related to complications, relative risks were calculated. Fisher’s exact test was used to statistically analyze risk factors. Clinical characteristics of each group were compared using Fisher’s exact test and Mann-Whitney U test. Survival rates at each time point were based on Kaplan-Meier estimation. OS rates were compared with the log-rank test between patients with gastric cancer in each group. To identify prognostic factors that jointly predict the hazard of death while controlling for model overfitting, the least absolute shrinkage and selection operator (LASSO) Cox regression model including factors curative/noncurative, age, gender, BMI, PNI, CCI, GPS, NLR and antithrombotic agent use was used (R package glmnet)[16]. We selected the LASSO Cox regression model that resulted in minimal prediction error in 10-fold cross-validation. P < 0.05 was considered statistically significant.
All statistical analyses were conducted using the EZR software, version 1.54 (Saitama Medical Center, Jichi Medical University, Saitama, Japan)[17] and R version 4.0.3 (The R Foundation for Statistical Computing, Vienna, Austria). The statistical review of the study was performed by a biomedical statistician.
Short-term outcomes are shown in Table 1. Within 172 sessions of ESD, two different specimens of multiple lesions were resected at the same time in three sessions; only one specimen was resected for each treatment in 168 sessions; and one lesion was unresectable in one session. A total of 174 specimens were thus resected from 175 lesions in 172 sessions of ESD. The en bloc dissection rate and the curative dissection rate were 97.1% and 77.1%, respectively. Six lesions (3.4%) had postoperative bleeding, two (1.1%) had intraoperative perforation, and one patient (0.6%) had aspiration pneumonitis after ESD. Blood transfusion was required in one patient. There were no procedure-related deaths.
| Location of the lesions (n = 175)1 | |
| Upper third | 33 (18.9) |
| Middle third | 57 (32.6) |
| Lower third | 85 (48.6) |
| Size of dissected specimen (n = 174)2 | |
| Range | 9-110 mm |
| Median | 30 mm |
| Average | 33.4 mm |
| ESD quality (n = 175)2 | |
| En bloc dissection | 170 (97.1) |
| Fractional dissection | 4 (2.3) |
| Not dissected endoscopically | 1 (0.6) |
| Curability (n = 175)1 | |
| Curative dissection | 135 (77.1) |
| Non-curative dissection | 40 (22.9) |
| Complications | |
| ESD sessions (n = 172) with any complication | 8 (4.7) |
| Bleeding (n = 175)1 | 6 (3.4) |
| Perforation (n = 175)1 | 2 (1.1) |
| Aspiration pneumonitis (n = 172)3 | 1 (0.6) |
| Procedure-related death (n = 172)3 | 0 |
The characteristics of the treated lesions and patients are shown in Table 2. Mac
| (A) Lesions (n = 175) | |
| Macroscopic type | |
| Protruded type (0-I, 0-I+IIa, 0-I+IIb, 0-I+IIc) | 24 (13.7) |
| Flat type (0-IIa, 0-IIa+IIc, 0-IIb, 0-IIc, 0-IIc+IIa) | 150 (85.7) |
| Advanced (type 1) | 1 (0.6) |
| Ulceration | |
| UL (+) | 22 (12.6) |
| UL () | 153 (87.4) |
| Depth of invasion | |
| M | 152 (86.9) |
| ≥ SM | 23 (13.1) |
| (B) Patients (n = 124) | |
| Underlying disease | |
| Circulatory | 38 (30.6) |
| Respiratory | 9 (7.3) |
| Renal | 0 |
| Antithrombotic agent | |
| Taking | 28 (22.6) |
In the present study of elderly patients, lesions that did not meet the indication criteria were also treated. The details of noncurative lesions and noncurative factors are shown in Table 3. Among 40 noncurative lesions, 32 (80.0%) were differentiated type, and eight (20.0%) were undifferentiated type. The noncurative factors were depth of invasion in 30.0%, oversize in 20.0%, positive ulceration associated with undifferentiated components in 12.5%, and positive or uncertain lymph vascular invasion in 35.0% of the noncurative lesions.
| (A) Details of noncurative lesions (n = 40) | |||||
| Depth of invasion | |||||
| M | SM1 | SM2 | ≥ MP | ||
| Histological type | |||||
| Differentiated (tub1, tub2, pap) | 19 | 4 | 8 | 1 | |
| Undifferentiated (por, sig, muc) | 4 | 2 | 2 | 0 | |
| (B) Estimated non-curative factors of 40 non-curative lesions, n (%) | |||||
| Depth of invasion | |||||
| ≥ SM2, differentiated | 8 (20) | ||||
| ≥ SM, undifferentiated | 4 (10) | ||||
| Lesion size | |||||
| ≥ 30 mm, differentiated, UL (+) | 2 (5) | ||||
| ≥ 30 mm, differentiated, SM1 | 1 (2.5) | ||||
| ≥ 20 mm, undifferentiated | 5 (12.5) | ||||
| Ulceration | |||||
| UL (+) with undifferentiated components | 5 (12.5) | ||||
| Lymphovascular invasion | |||||
| Ly +/uncertain | 7 (17.5) | ||||
| V +/uncertain | 7 (17.5) | ||||
| Surgical margin | |||||
| Positive | 7 (17.5) | ||||
| Uncertain | 21 (52.5) | ||||
| Not dissected endoscopically | 1 (2.5) | ||||
The patients with complications are summarized in Table 4. One patient had both postoperative bleeding and aspiration pneumonitis, and the others had one complication each. None of patients with postoperative bleeding was receiving any antithrombotic agents.
| Age (yr) | Gender | Ps | Underlying disease | Past history | Location1 | Size (mm) | Macroscopic type | Final pathology | Curability | Specimen (mm) | Complications |
| 83 | F | 1 | Post-BHA | L, Ant | 40 | 0-IIc, UL (+) | Tub2 > por2, M, ly0, v0, HM0, VM0 | Noncurative | 60 | Bleeding G2 | |
| 83 | M | 0 | L, Ant | 10 | 0-IIc, UL (+) | Tub1 > tub2, M, ly0, v0, HM0, VM0 | Curative | 20 | Bleeding G2 | ||
| 92 | M | 0 | Laryngeal cancer | U, Post | 50 | Type1 | Surgical resection: pap > tub, SS, ly0, v1, NX, HMX | Noncurative | 522 | Perforation G3 | |
| 89 | M | 3 | Brain cancer | M, Les | 33 | 0-IIc, UL (+) | Sig/por2, M, ly0, v0, HM0, VM0 | Noncurative | 68 | Bleeding G3, pneumonitis G2 | |
| 83 | F | 2 | AD, Depression | U, Les | 15 | 0-IIa | Tub1, M, ly0, v0, HM0, VM0 | Curative | 30 | Perforation G2 | |
| 82 | F | 0 | (1) L, Ant | (1) 20 | (1) 0-IIc | (1) Tub2 > tub1 > por, M, ly0, v0, HM0, VM0 | (1) Curative | 54 | Bleeding G2 | ||
| (2) L, Ant | (2)10 | (2)0-IIc | (2) Tub1-tub2, M, ly0, v0, HM0, VM0 | (2) Curative | |||||||
| 84 | M | 2 | AP, COPD | L, Les | 15 | 0-IIc | Por1, M, ly0, v0, HMX, VMX | Noncurative | 40 | Bleeding G2 | |
| 80 | M | 0 | Colon cancer, EGC | L, Les | 16 | 0-IIa+IIc, UL (+) | Tub1 > tub2 > por, M, ly0, v0, HM0, VM0 | Curative | 47 | Bleeding G2 |
The relation of complications to lesion location and size of resected specimen is summarized in Table 5. Lesion location in the lower third of the stomach and a resected specimen size > 40 mm tended to have higher bleeding rates. Lesion location in the upper third of the stomach and a resected specimen size > 40 mm tended to be associated with higher perforation rates.
| Bleeding (+) | Bleeding (-) | Perforation (+) | Perforation (-) | Total | |
| n = 6 | n = 169 | n = 2 | n = 173 | n = 175 | |
| Location | |||||
| Upper third | 0 | 33 (100) | 2 (6.1) | 31 (93.9) | 33 |
| Middle third | 1 (1.6) | 56 (98.4) | 0 | 57 (100) | 57 |
| Lower third | 5 (5.9) | 80 (94.1) | 0 | 85 (100) | 85 |
| Size of specimen | |||||
| ≤ 20 mm | 1 (3.3) | 29 (96.7) | 0 | 30 (100) | 30 |
| 21-40 mm | 1 (1.0) | 102 (99.0) | 1 (1.0) | 102 (99.0) | 103 |
| 41-60 mm | 3 (8.1) | 34 (91.9) | 1 (2.7)1 | 36 (97.3) | 37 |
| ≥ 61 mm | 1 (20.0) | 4 (80.0) | 0 | 5 (100) | 5 |
The relative risks of lesion location and resected specimen size are shown in Table 6. Resected specimens > 40 mm, macroscopic shape with depressive component, and presence of ulceration were determined to be risk factors for bleeding (P < 0.05). Location of the lesion in the upper third of the stomach was determined to be a risk factor for perforation (P < 0.05).
| (A) Relative risk of location lower third, size > 40 mm, macroscopic shape, presence or absence of ulceration, and depth of invasion for bleeding | ||||
| Bleeding (+) | Relative risk | P value | ||
| Location | ||||
| Lower third | 5.9% (5/85) | 5.3 | 0.11 | |
| Upper third, middle third | 1.1% (1/90) | |||
| Dissected size | ||||
| ≥ 41 mm | 9.5% (4/42) | 6.3 | 0.030 | |
| ≤ 40 mm | 1.5% (2/133) | |||
| Macroscopic shape | ||||
| Depressive component (+) | 8.2% (6/73) | 0.005 | ||
| Depressive component () | 0% (0/102) | |||
| Ulceration | ||||
| UL (+) | 18.2% (4/22) | 13.9 | 0.003 | |
| UL () | 1.3% (2/153) | |||
| Depth of invasion | ||||
| ≥ SM | 3.9% (6/152) | 1 | ||
| M | 0% (0/23) | |||
| (B) Relative risk of location upper third, size > 40 mm, macroscopic shape, presence or absence of ulceration, and depth of invasion for perforation | ||||
| Perforation (+) | Relative risk | P value | ||
| Location | ||||
| Upper third | 6.3% (2/32) | 0.033 | ||
| Middle third, lower third | 0% (1/143) | |||
| Dissected size | ||||
| ≥ 41 mm | 2.4% (1/42) | 3.2 | 0.423 | |
| ≤ 40 mm | 0.8% (1/133) | |||
| Macroscopic shape | ||||
| Depressive component (+) | 0% (0/73) | - | 0.511 | |
| Depressive component () | 2.0% (2/102) | |||
| Ulceration | ||||
| UL (+) | 0% (0/22) | - | 1 | |
| UL () | 1.3% (2/153) | |||
| Depth of invasion | ||||
| ≥ SM | 0.7% (1/152) | 6.6 | 0.246 | |
| M | 4.3% (1/23) | |||
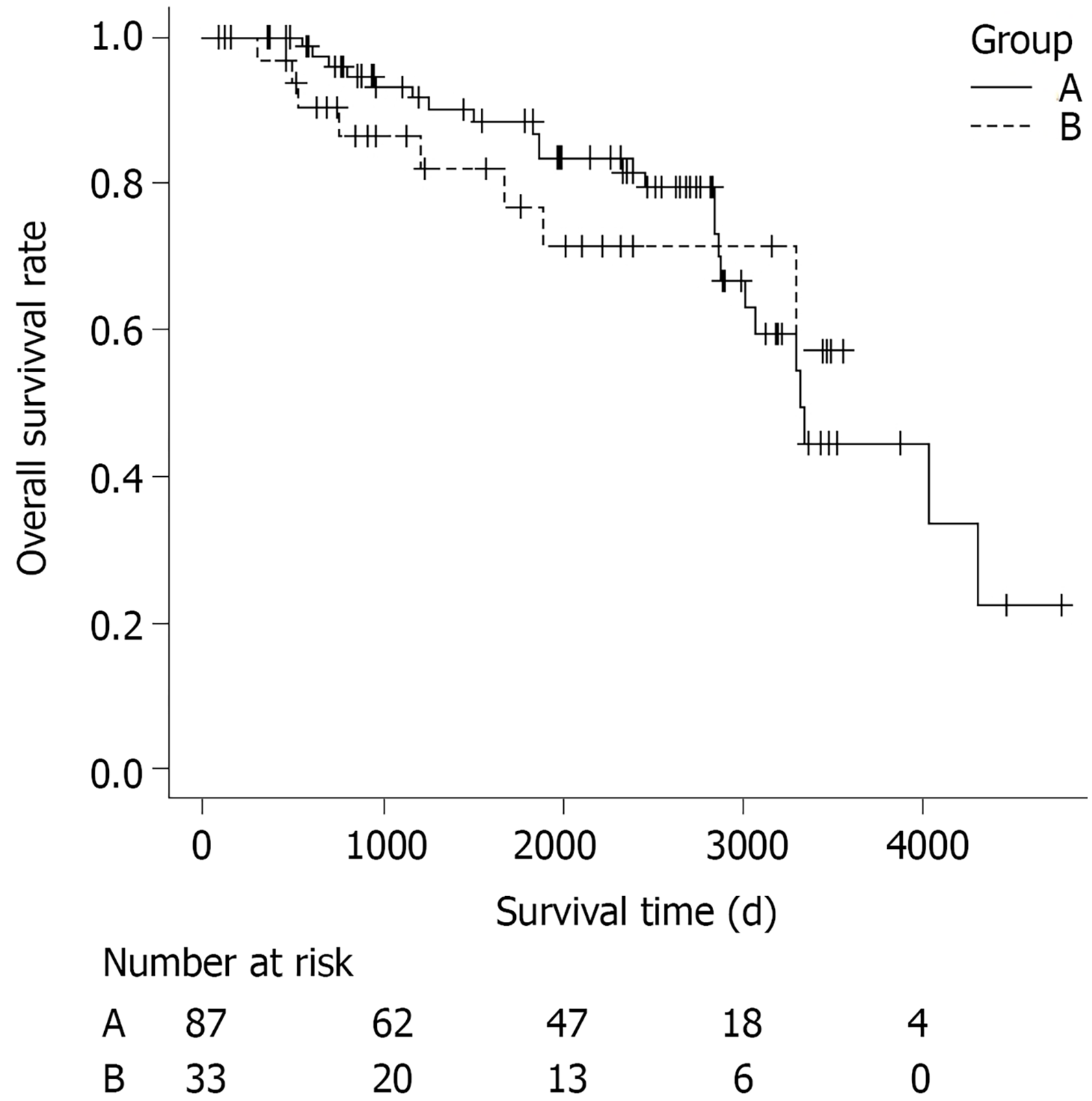
Survival curves according to the curability are shown in Figure 1. The patients were divided into two groups: Those who underwent only curative ESD (curative ESD group, n = 87), and those who underwent noncurative ESD without additional surgery (noncurative ESD group, n = 33). Patients who had undergone dissection more than once were classified as noncurative when ESD was noncurative at least once. A total of 32 patients (26.7%) died during a median follow-up period of 2005 d (range, 83-4774 d). Twenty-four of the patients who died were in the curative ESD group and eight were in the noncurative ESD group. The cause of death was gastric cancer in none of them. The OS rate did not differ significantly between the curative and the non-curative ESD groups (P = 0.69).
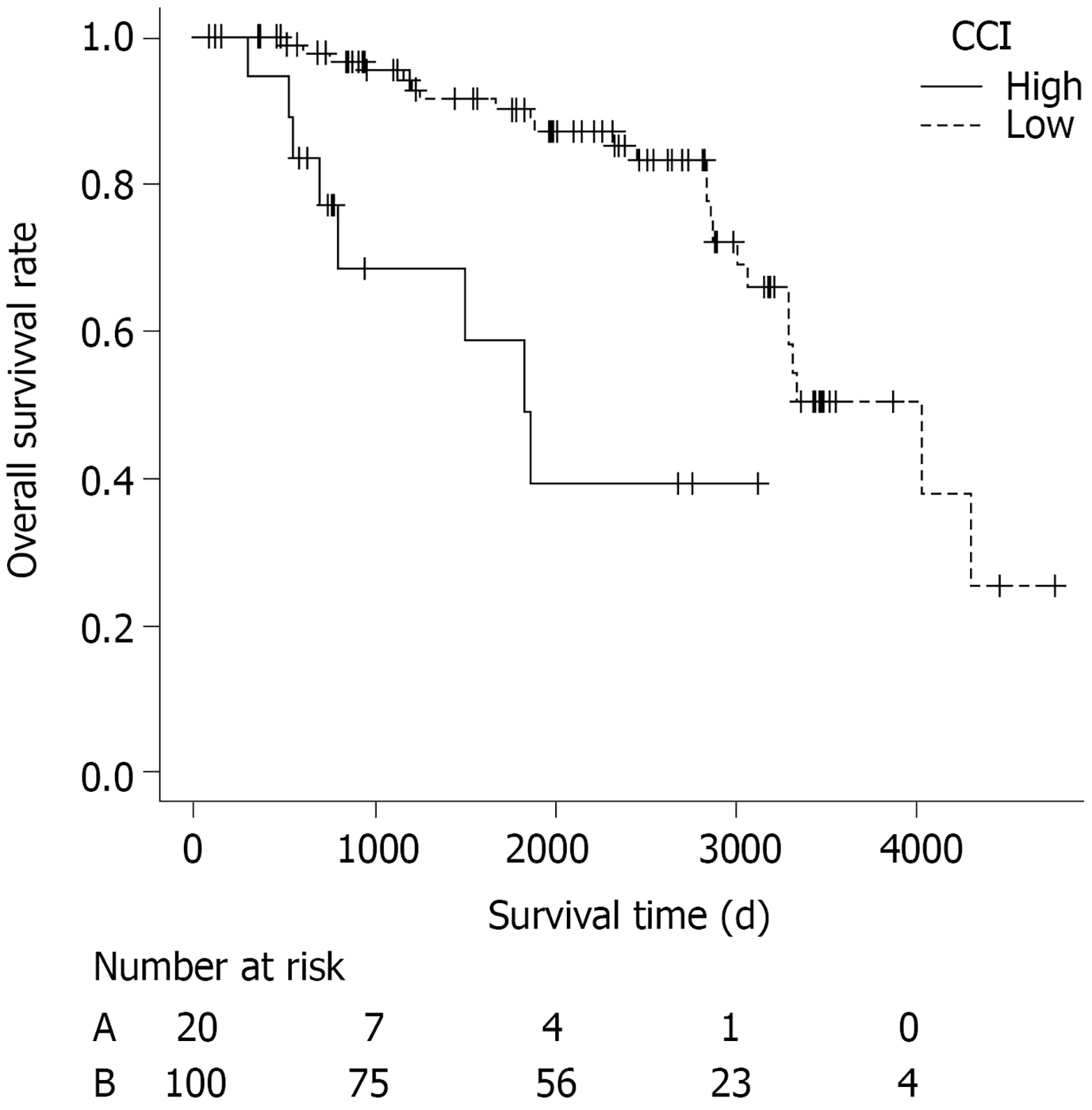
Prognostic factors for OS using LASSO in the patients who did not undergo additional surgery (n = 120) are shown in Table 7. Among these clinical characteristics, gender and CCI, one of most widely used and validated comorbidity scoring system to measure comorbidity status, were significantly associated with OS. As median CCI in each group was 1, patients were divided in two groups according to CCI ≤ 1 or > 1. The survival curve of patients with low CCI ≤ 1 (n = 100) and those with high CCI ≥ 2 (n = 20) are shown in Figure 2. The OS rate was significantly different between the two groups (P < 0.001).
| Cox LASSO | |
| Curability | |
| Noncurative | – |
| Patient | – |
| Age | – |
| Gender: Male | 0.416 |
| BMI | – |
| PNI | – |
| CCI > 1 | 0.477 |
| GPS | – |
| NLR | – |
| Antithrombotic agent (+) | – |
In Japan, the morbidity rate of gastric cancer has been rapidly decreasing according to the Center for Cancer Control and Information Services, National Cancer Center, Japan. Nonetheless, the number of EGCs treated endoscopically has dramatically increased. The increased use of ER seems to be attributed to three reasons. The first reason is the expansion of the indications for ER. Because ER is a local resection procedure without lymphadenectomy, the indications for ER are limited to conditions expected to have no lymph node metastasis[15]. Previous studies of patients who underwent surgery for gastric cancer have evaluated conditions associated with no lymph node metastasis. The second reason is progress in endoscopic techniques[6-8]. The final reason is the minimal invasiveness of ESD. ESD is far less invasive than open surgery, and can prevent symptoms associated with a small capacity of stomach after surgery.
Although minimal invasiveness is undoubtedly attractive for elderly patients because they have higher incidences of underlying diseases than younger patients have and are sometimes in poor general condition[9,10], the feasibility of ESD remains to be fully evaluated. In our study, complications occurred only in 4.7% of patients, without any procedure-related deaths. In previous studies of elderly patients, the rate of bleeding ranged from 2.5% to 9.6%[10-14], except for the study by Hirasaki et al[10], which reported a bleeding rate of 43.4%[3], and the rates of perforation and of pneumonia ranged from 1.5% to 5.0% and 0.5% to 2.2%, respectively. In most of these studies, ESD was not associated with particularly higher risk in elderly than in nonelderly patients. Indeed, the rates of bleeding and perforation among patients of all ages were reported to range from 3.7% to 15.6% and 1.2% to 6.7%, respectively[18-22]. In nonelderly patients, Lin et al[23] reported that the rates of bleeding, perforation and procedure-related pneumonia were 2.9%, 1.1% and 0.4%, respectively, in their meta-analysis of nine previous studies of gastric ESD. These previous reports and present study suggest that the rates of complications of ESD in elderly patients are not particularly higher than the rates in nonelderly or patients of all ages. Accordingly, we argue that gastric ESD is feasible even in elderly patients aged ≥ 80 years.
However, some studies have reported that ESD carries a higher risk in elderly patients than in younger patients[13,21]. Toyokawa et al[13] reported that the bleeding rate was significantly higher in the elderly group (age ≥ 75 years) than in the nonelderly group (age < 75 years). However, in multivariate analysis, high age was not in itself an independent predictor of bleeding, and the reason why the bleeding rate was higher in the elderly group was unclear. It was also reported by Toyokawa et al[21] in another report that age ≥ 80 years was associated with a significantly higher risk of delayed bleeding after ESD, and they concluded that the use of antiplatelet agents or anticoagulants was not the reason for delayed bleeding in elderly patients. Also in that study, they could not specify the reason why delayed bleeding was pre
Even if gastric ESD is feasible in elderly patients, complications can have severe consequences. To acknowledge the characteristics of lesions associated with higher risks in elderly patients is essential to a safe procedure. Kim et al[22] reported that the risk of perforation associated with ESD is higher for lesions located in the gastric body than those located in the antrum. Toyokawa et al[21] reported that ESD carried a high risk of perforation when EGCs located in the upper third of the stomach were dissected. Our results that lesion location in the upper third of the stomach was a significant risk factor, and lesion size > 40 mm tended toward a higher risk of perforation in elderly patients seem to be consistent with previous studies performed in patients of all ages.
As for bleeding, Chung et al[18] reported that the risk of delayed bleeding after ESD was significantly higher for lesions located in the upper portion of the stomach. In contrast, in our study focusing on elderly patients, lesions located in the lower portion of the stomach tended to have a higher risk of bleeding. As for macroscopic shape, lesions with depressive components such as 0-IIc, 0-IIa + IIc, 0-IIc + IIa, and 0-I + IIc and lesions with ulceration were associated with bleeding after ESD. In contrast, treatment with antithrombotic agents was not associated with bleeding. We speculate that strong peristaltic contractions of the gastric antrum increased the risk of bleeding in the lower portion of the stomach. In addition, a resected lesion size > 40 mm in diameter was determined to be a risk factor for bleeding. Moreover, the median lesion size in patients with bleeding was 50.5 mm (range, 20-68 mm), which was about 70% larger than median lesion size of 30 mm (range, 9-110 mm) in the study group as a whole. We therefore recommend meticulous preventive endoscopic hemostasis after resecting lesions > 40 mm, especially those located in the lower third of the stomach, and lesions with depressive aspects or ulceration, when treating elderly patients.
To prevent aspiration during ESD, an overtube was inserted in all patients. Accordingly, the rate of aspiration pneumonitis was as low as 0.6%. In contrast, Isomoto et al[12] reported that aspiration pneumonitis occurred in 2.2% of patients aged ≥ 75 years, which was more frequent than in younger patients. In contrast, Lee et al[24] reported that the risk of aspiration might be increased by endoscopic procedures with a longer duration.
In the present study of elderly patients, lesions that did not meet the indication criteria were also treated. Accordingly, the curative dissection rate of ESD was only 77.1%. Abe et al[14] reported that the curative rate of ESD was 77.9% in their multicenter study of ESD in patients aged ≥ 80 years, consistent with our results. The question arises whether dissecting lesions beyond expanded indications was meaningless? Kang et al[25] recently reported that even if the lesions are beyond expanded indications, ESD reduces the risk of death from gastric cancer, although it does not completely cure the disease in some patients. In our study, the disease-specific 5-year survival rate and 5-year OS rate in the noncurative ESD group were as high as 100% and 76.9%, respectively. These rates were higher than 5-year survival rate of patients with EGC who did not undergo resection (62.8%) as reported by Tsukuma et al[26]. Furthermore, the OS of the noncurative ESD group was equivalent to that of the curative ESD group. Although the number of patients in our study was small, and our results may have been influenced by selection bias, our findings suggest that ESD might be effective for EGC beyond expanded indications. Indeed, although 32 of 120 recruited patients died during the follow-up period, none of them died of gastric cancer. The causes of death in the other patients were malignancy in other organs in seven patients, respiratory diseases in five patients, and uncertain in 20 patients.
Tsukuma et al[26] reported that the median interval required for EGC to progress to an advanced stage was 44 mo. Moreover, older patients tended to have shorter intervals to the development of advanced disease, and it was 36 mo in patients aged > 75 years[27]. We thus consider it reasonable to endoscopically resect lesions beyond expanded indications if surgery is unacceptable, with the goal of preventing symptoms that may develop in the future, in patients who are expected to survival for longer than 36 mo.
In this study, local recurrence developed in only one (3.0%) of 33 patients in the noncurative ESD group. Similarly, Abe et al[14] reported that local recurrence developed in 3.3% and distant metastasis developed in 5.5% of patients who did not undergo additional surgery after noncurative ESD. Kusano et al[28] reported that survival was improved by additional surgery following noncurative ER in elderly patients. In contrast, Ahn et al[29] reported that the mortality rate was significantly higher in the presence of lymphovascular invasion than in the absence of such invasion in patients with differentiated EGC who underwent nonsurgical follow-up after noncurative ER. Thus, if possible, additional surgery is advisable after noncurative ESD, even in elderly patients, especially when lymphovascular invasion is confirmed histologically.
CCI was developed to assess the risk of death from comorbidities and has been widely used to evaluate clinical outcomes, such as prognosis or complications. CCI was calculated as the sum of the scores assigned to several comorbidities (myocardial infarction, congestive heart failure, cerebrovascular disease, uncomplicated diabetes, moderate-to-severe chronic kidney disease, moderate-to-severe liver disease, solid tumor, leukemia etc.) based on the original definition[30]. In our study, curability of ESD was not associated with OS rate. CCI was indicated to be the only factor associated with prognosis, among various clinical characteristics such as BMI, PNI, GPS and NLR. However, Iwai et al[31] reported that CCI ≥ 3 and PNI < 47.7 were both significantly associated with lower OS rate. Whether nutritional status is truly a predictor of long-term prognosis is controversial. According to our results, we suggest that observation without additional surgery after noncurative ESD may be considered, especially in elderly patients with CCI > 1.
The limitation of our study was that it was retrospective. Although complications are expected to differ depending on concomitant diseases, we cannot confirm the patients’ characteristics in detail. Moreover, we had only a few cases of bleeding and perforation, as this was a single-center study with a limited number of recruited patients, and our results may have been influenced by selection bias. Therefore, a multicenter prospective trial needs to be performed to confirm the risk factors of ESD related to underlying disease.
Gastric ESD is feasible and permissible in elderly patients aged ≥ 80 years. To ensure a safe procedure, meticulous preventive endoscopic hemostasis is recommended after resecting specimens > 40 mm or lesions with depressive aspects or ulceration, especially those located in the lower third of the stomach, when treating aged patients. Concerning their long-term prognosis, male gender and CCI > 1 are negative predictors.
Endoscopic submucosal dissection (ESD) is increasingly performed in elderly patients with early gastric cancer (EGC).
Whether gastric ESD is safe and suitable for elderly patients, type of lesions which carry an increased risk of procedure-related complications, indicators of prognosis for elderly patients after ESD are unclear.
To investigate short-term and long-term outcomes of gastric ESD for elderly patients, and to determine the risk factors of procedure-related complications and the indicators of prognosis.
This study included patients aged ≥ 80 years who underwent ESD for EGC in Kanagawa Cancer Center Hospital. These patients were studied retrospectively to evaluate short-term outcomes and survival of gastric ESD.
The en bloc dissection rate was as high as 97.1%, and the complication rates of bleeding, perforation and aspiration pneumonitis were as low as 3.4%, 1.1% and 0.6%, respectively, which were similar to the rates of ESD for nonelderly patients. A dissection incision > 40 mm, lesions associated with depressions, and lesions with ulcers were risk factors for bleeding, and location of the lesion in the upper third of the stomach was a risk factor for perforation (P < 0.05). The overall survival (OS) did not differ significantly between curative and noncurative ESD groups (P = 0.69). In patients without additional surgery, OS rate was significantly lower in patients with a high Charlson comorbidity index (CCI) ≥ 2 than in patients with a low CCI ≤ 1 (P < 0.001).
Gastric ESD is feasible even in elderly patients aged ≥ 80 years. Meticulous preventive endoscopic hemostasis after resecting specimens > 40 mm, or lesions associated with depressions or ulcers is recommended. CCI is a prognostic indicator. Observation without additional surgery after noncurative ESD is reasonable, especially in elderly patients with CCI ≥ 2.
As our institution is a hub hospital specializing in cancer treatment, relatively healthy patients without severe underlying diseases tend to visit the hospital. Therefore, a selection bias of target patients may have existed in our study. A multicenter prospective trial with a large number of patients is desirable to confirm the feasibility of gastric ESD in patients with various health problems, and the risk factors and the prognostic indicators related to each underlying disease.
| 1. | Sano T, Kobori O, Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br J Surg. 1992;79:241-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 257] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 2. | Shimizu S, Tada M, Kawai K. Early gastric cancer: its surveillance and natural course. Endoscopy. 1995;27:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 107] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Kitano S, Shiraishi N. Current status of laparoscopic gastrectomy for cancer in Japan. Surg Endosc. 2004;18:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Foundation for Promotion of Cancer Research. CANCER STATISTICS IN JAPAN-2014 2014; 24. |
| 5. | Gotoda T, Kondo H, Ono H, Saito Y, Yamaguchi H, Saito D, Yokota T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest Endosc. 1999;50:560-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 338] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 6. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1163] [Article Influence: 46.5] [Reference Citation Analysis (5)] |
| 7. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 515] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 8. | Abe N, Yamaguchi Y, Takeuchi H, Izumisato Y, Yanagida O, Masaki T, Mori T, Sugiyama M, Atomi Y. Key factors for successful en bloc endoscopic submucosal dissection of early stage gastric cancer using an insulation-tipped diathermic knife. Hepatogastroenterology. 2006;53:639-642. [PubMed] |
| 9. | Mohri Y, Omori Y, Hiro J, Yokoe T, Konishi N, Tanaka K, Tonouchi H, Kusunoki M. Clinicopathological features and prognosis of gastric cancer patients > or =75 years of age after laparotomy. Int Surg. 2009;94:38-42. [PubMed] |
| 10. | Hirasaki S, Tanimizu M, Nasu J, Shinji T, Koide N. Treatment of elderly patients with early gastric cancer by endoscopic submucosal dissection using an insulated-tip diathermic knife. Intern Med. 2005;44:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Yahagi N, Omata M. Technical feasibility of endoscopic submucosal dissection for gastric neoplasms in the elderly Japanese population. J Gastroenterol Hepatol. 2007;22:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Isomoto H, Ohnita K, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, Ozawa E, Nakao K, Kohno S, Shikuwa S. Clinical outcomes of endoscopic submucosal dissection in elderly patients with early gastric cancer. Eur J Gastroenterol Hepatol. 2010;22:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Toyokawa T, Fujita I, Morikawa T, Okamoto A, Miyasaka R, Watanabe K, Horii J, Gobaru M, Terao M, Murakami T, Tomoda J. Clinical outcomes of ESD for early gastric neoplasms in elderly patients. Eur J Clin Invest. 2011;41:474-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Abe N, Gotoda T, Hirasawa T, Hoteya S, Ishido K, Ida Y, Imaeda H, Ishii E, Kokawa A, Kusano C, Maehata T, Ono S, Takeuchi H, Sugiyama M, Takahashi S. Multicenter study of the long-term outcomes of endoscopic submucosal dissection for early gastric cancer in patients 80 years of age or older. Gastric Cancer. 2012;15:70-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1911] [Article Influence: 127.4] [Reference Citation Analysis (0)] |
| 16. | Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16:385-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 69] [Reference Citation Analysis (2)] |
| 17. | Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9275] [Cited by in RCA: 14517] [Article Influence: 1116.7] [Reference Citation Analysis (0)] |
| 18. | Chung IK, Lee JH, Lee SH, Kim SJ, Cho JY, Cho WY, Hwangbo Y, Keum BR, Park JJ, Chun HJ, Kim HJ, Kim JJ, Ji SR, Seol SY. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 484] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 19. | Kato M, Nishida T, Tsutsui S, Komori M, Michida T, Yamamoto K, Kawai N, Kitamura S, Zushi S, Nishihara A, Nakanishi F, Kinoshita K, Yamada T, Iijima H, Tsujii M, Hayashi N. Endoscopic submucosal dissection as a treatment for gastric noninvasive neoplasia: a multicenter study by Osaka University ESD Study Group. J Gastroenterol. 2011;46:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Sugimoto T, Okamoto M, Mitsuno Y, Kondo S, Ogura K, Ohmae T, Mizuno H, Yoshida S, Isomura Y, Yamaji Y, Kawabe T, Omata M, Koike K. Endoscopic submucosal dissection is an effective and safe therapy for early gastric neoplasms: a multicenter feasible study. J Clin Gastroenterol. 2012;46:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, Morikawa T, Murakami T, Tomoda J. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 22. | Kim M, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, Seo HE, Chung YJ, Kwon JG, Jung JT, Kim EY, Jang BI, Lee SH, Kim KO, Yang CH; Daegu-Kyungpook Gastrointestinal Study Group (DGSG). Predictive risk factors of perforation in gastric endoscopic submucosal dissection for early gastric cancer: a large, multicenter study. Surg Endosc. 2013;27:1372-1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Lin JP, Zhang YP, Xue M, Chen SJ, Si JM. Endoscopic submucosal dissection for early gastric cancer in elderly patients: a meta-analysis. World J Surg Oncol. 2015;13:293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Lee IL, Wu CS, Tung SY, Lin PY, Shen CH, Wei KL, Chang TS. Endoscopic submucosal dissection for early gastric cancers: experience from a new endoscopic center in Taiwan. J Clin Gastroenterol. 2008;42:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Kang MS, Hong SJ, Kim DY, Han JP, Choi MH, Kim HK, Ko BM, Lee MS. Long-term outcome after endoscopic submucosal dissection for early gastric cancer: focusing on a group beyond the expanded indication. J Dig Dis. 2015;16:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Tsukuma H, Oshima A, Narahara H, Morii T. Natural history of early gastric cancer: a non-concurrent, long term, follow up study. Gut. 2000;47:618-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 157] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Tsukuma H, Ioka A, Iishi H, Yamazaki H. Prospective study of early gastric cancer, with considerations concerning clinical practice for gastric cancer. Stomach Intestine. 2008;43:1777-1783. |
| 28. | Kusano C, Iwasaki M, Kaltenbach T, Conlin A, Oda I, Gotoda T. Should elderly patients undergo additional surgery after non-curative endoscopic resection for early gastric cancer? Am J Gastroenterol. 2011;106:1064-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 29. | Ahn JY, Jung HY, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Choi KD, Song HJ, Lee GH, Kim JH, Park YS. Natural course of noncurative endoscopic resection of differentiated early gastric cancer. Endoscopy. 2012;44:1114-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 39699] [Article Influence: 1017.9] [Reference Citation Analysis (0)] |
| 31. | Iwai N, Dohi O, Naito Y, Inada Y, Fukui A, Takayama S, Ogita K, Terasaki K, Nakano T, Ueda T, Okayama T, Yoshida N, Katada K, Kamada K, Uchiyama K, Ishikawa T, Handa O, Takagi T, Konishi H, Yagi N, Itoh Y. Impact of the Charlson comorbidity index and prognostic nutritional index on prognosis in patients with early gastric cancer after endoscopic submucosal dissection. Dig Endosc. 2018;30:616-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garbarino GM, Hu B, Santos-Antunes J S-Editor: Wu YXJ L-Editor: Kerr C P-Editor: Wu YXJ