Published online Sep 16, 2021. doi: 10.4253/wjge.v13.i9.356
Peer-review started: March 11, 2021
First decision: April 6, 2021
Revised: May 17, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: September 16, 2021
Processing time: 182 Days and 12.5 Hours
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer related death in the world. The early detection and removal of CRC precursor lesions has been shown to reduce the incidence of CRC and cancer-related mortality. Endoscopic resection has become the first-line treatment for the removal of most precursor benign colorectal lesions and selected malignant polyps. Detailed lesion assessment is the first critical step in the evaluation and management of colorectal polyps. Polyp size, location and both macro- and micro- features provide important information regarding histological grade and endoscopic resectability. Benign polyps and even malignant polyps with superficial submucosal invasion and favorable histological features can be adequately removed endoscopically. When compared to surgery, endoscopic resection is associated with lower morbidity, mortality, and higher patient quality of life. Conversely, malignant polyps with deep submucosal invasion and/or high risk for lymph node metastasis will require surgery. From a practical standpoint, the most appropriate strategy for each patient will need to be individualized, based not only on polyp- and patient-related characteristics, but also on local resources and expertise availability. In this review, we provide a broad overview and present a potential decision tree algorithm for the evaluation and management of colorectal polyps that can be widely adopted into clinical practice.
Core Tip: Endoscopic resection is a proven strategy for the management of benign and selected malignant colorectal polyps. When compared to surgery, endoscopic resection is less costly and associated with improved clinical outcomes and patient satisfaction. Detailed lesion assessment, including endoscopic imaging and histopathology, play a critical role in directing subsequent treatment strategies. Ultimately, the most appropriate intervention will depend on various factors, including patient and lesion characteristics, as well as local resources and expertise availability. Establishing the multidisciplinary collaboration between referring physicians, endoscopists, surgeons and pathologists is the basis for ensuring best practices for the management of colorectal polyps.
- Citation: Mathews AA, Draganov PV, Yang D. Endoscopic management of colorectal polyps: From benign to malignant polyps. World J Gastrointest Endosc 2021; 13(9): 356-370
- URL: https://www.wjgnet.com/1948-5190/full/v13/i9/356.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i9.356
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer death in the world[1]. A well-recognized characteristic of CRC carcinogenesis is that most cancers arise from precursor benign polyps[2]. The increasingly widespread adoption of colonoscopy has reduced CRC incidence and mortality via the early detection and removal of these precursor lesions and even early cancers[3,4]. In this review, we provide a broad overview and decision algorithm on the endoscopic evaluation and management of colorectal polyps.
Colorectal polyps are growths or protuberances into the lumen above the adjacent colonic mucosa. The two major histologic types of neoplastic polyps that serve as direct precursors to most CRCs are conventional adenomas and serrated polyps[5].
Adenomas are commonly regarded as the prototypical precursor of CRC, given that nearly 85%-90% of sporadic CRCs derive from adenomas[6]. These lesions are identified histologically by epithelial clusters of dysplastic glands; and are divided into tubular, tubulovillous, or villous types according to the World Health Organization (WHO) classification system[7]. The adenoma-carcinoma sequence is characterized by chromosomal instability and a stepwise progression of gradual genetic and epigenetic mutations that culminate in the transformation of these precancerous lesions to CRC[8-10].
Serrated polyps encompass three main types:
Hyperplastic polyps (HPs): are the most common type of serrated polyp. They are usually small (less than 5 mm), predominantly located in the rectosigmoid colon, and are not associated with a risk for malignant transformation[6].
Sessile serrated lesions (SSLs): The term SSL is often used interchangeably with sessile serrated adenomas (SSAs). These lesions are traditionally larger than HPs, predominantly in the right colon, and according to the WHO criteria, distinguished from HPs based on the presence of crypt distortion on histology[7].
Traditional serrated adenomas (TSAs): TSAs are more commonly located in the distal colon and may have an erythematous “pine cone” gross appearance on endoscopy[11,12]. Histologically, TSAs feature prominent cytoplasmic eosinophilia, elongated nuclei and ectopic crypts[7].
Unlike HPs, both SSL/SSAs and TSAs have malignant potential and account for approximately 15%-30% of all sporadic CRCs[6,11]. The inactivation of tumor suppressor genes via hypermethylation plays a critical role in the progression of serrated polyps to cancer, which is the basis of the CpG island methylator phenotype pathway[11-13]. From a histological standpoint, it is important to note that unlike conventional adenomas, not all SSL/SSAs have dysplasia. As opposed to SSL/SSAs without dysplasia, serrated polyps with dysplasia have advanced molecular changes; although there is some controversy in what constitutes these dysplasia patterns[14]. Irrespectively, SSL/SSAs with dysplasia should be distinguished from those without dysplasia given their significantly higher risk for progression to CRC[15].
CRC is defined as the invasion of neoplastic cells beyond the muscularis mucosa. As opposed to other organs in the gastrointestinal tract, the colonic mucosa is devoid of lymphatics. Therefore, neoplastic lesions confined to the muscularis mucosa have a negligible risk for lymph node metastasis (LNM) and, according to the National Comprehensive Cancer Network, do not meet the clinically accepted definition for CRC[16]. These lesions are defined as benign (non-malignant) polyps.
The term malignant polyp is used to describe a colorectal lesion in which neoplastic cells have invaded into, but not beyond the submucosa[17]. Hence, a malignant polyp represents early CRC and is categorized as pT1 according to the American Joint Committee on Cancer tumor-node metastasis classification system[18]. It has been estimated that at least 0.2% to 8.3% of colorectal polyps are malignant polyps[19-22].
Detailed lesion assessment is the first critical step in the evaluation and management of colorectal polyps. Every polyp should be evaluated according to its size, location, and carefully inspected for macro- and micro- features. These details may provide important information regarding its histological grade and direct subsequent management decisions.
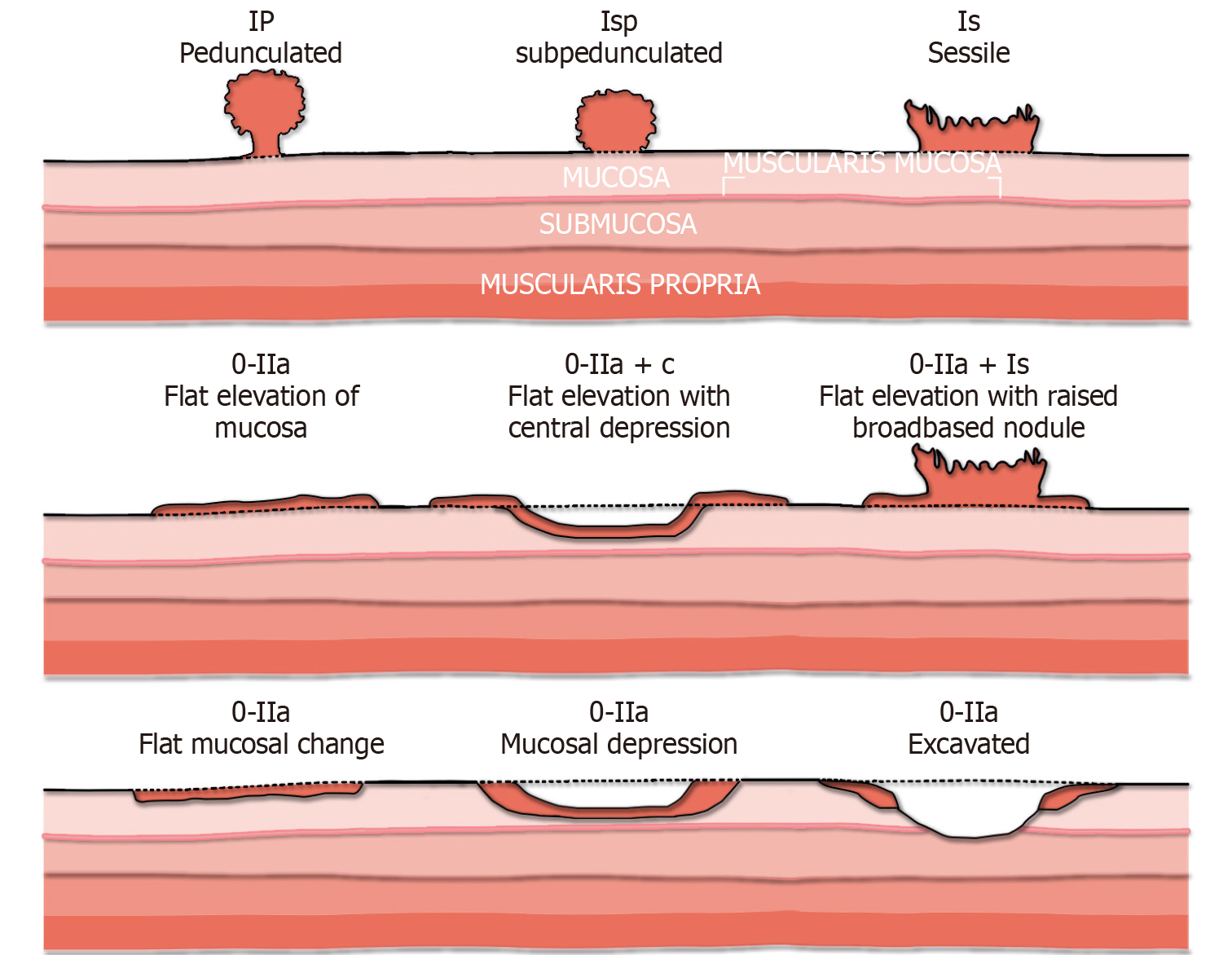
Paris classification: The Paris classification is a consensus system widely used to describe colorectal polyp morphology[23]. Although studies have shown only moderate agreement among experts using the Paris classification, it serves as a validated standardized nomenclature that helps categorize colorectal polyps and stratify according to the risk of CRC. Broadly speaking, lesions are categorized as polypoid (type 0-I) or non-polypoid (type 0-II) (Figure 1). The polypoid type can be either pedunculated (type 0-Ip) or sessile (type 0-Is). Nonpolypoid type 0-II can be further subdivided into those that are superficially elevated (0-IIa), flat (0-IIb), or depressed (0-IIc). Excavated lesions are designated type 0-III. The risk of CRC [i.e. submucosal invasion (SMI)] has been shown to be directly proportional to polyp size and the presence of depression: with the risk being as high as 40% in smaller lesions (6-10 mm) to nearly all lesions measuring more than 20 mm[24-26].
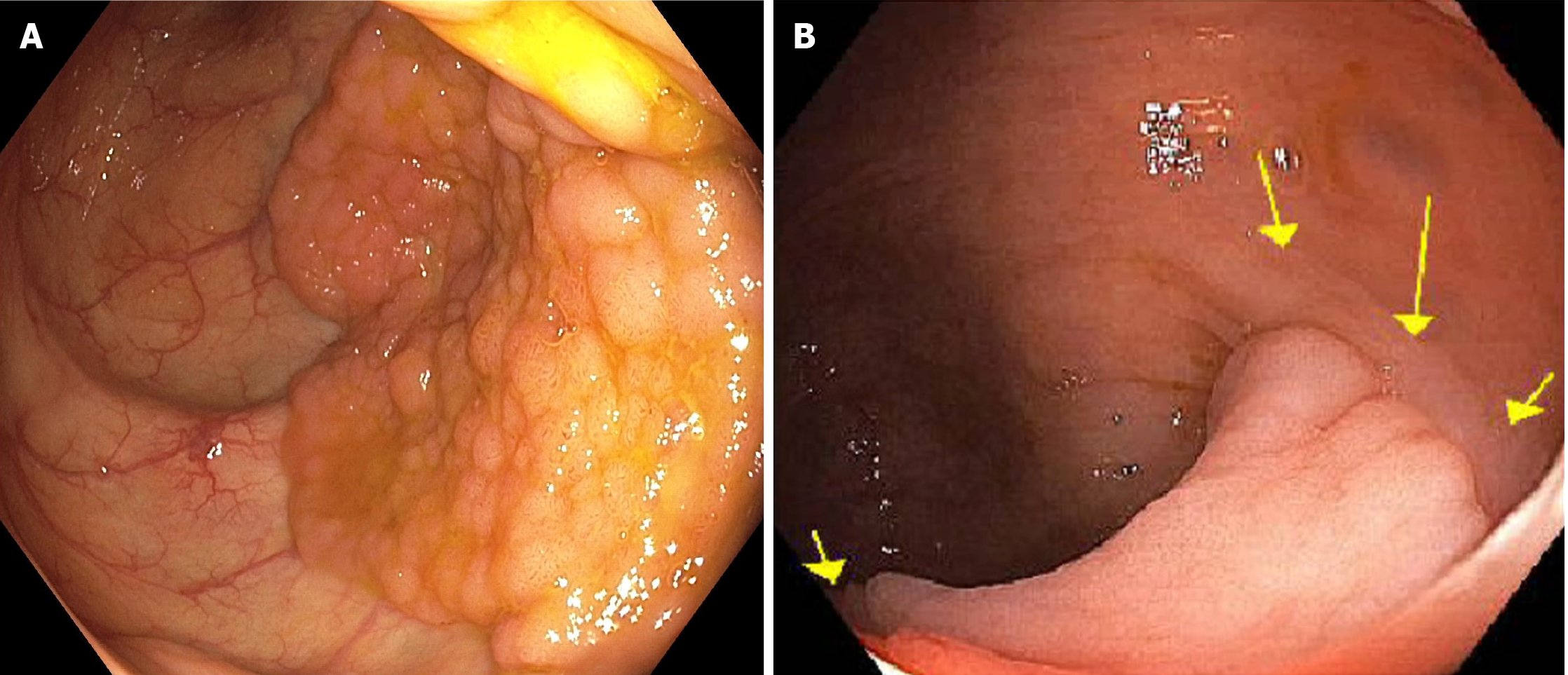
Lateral spreading tumors: Superficial non-polypoid colorectal lesions measuring more than 10 mm in diameter extending laterally rather than vertically are commonly referred as laterally spreading tumors (LSTs). The incidence of LSTs on routine colonoscopy is approximately 9%[25], and these can be broadly subdivided into the granular (LST-G) or non-granular (LST-NG) types (Figure 2). Similar to the Paris classification, LST morphology provides prognostic information regarding the risk for SMI. LST-G with a homogenous nodular pattern have a low risk of local invasion (< 2%) compared to LST-G with mixed-size nodules, in which the risk can be as high as 30% for those measuring more than 30 mm in size[27]. As opposed to the nodularity in LST-Gs, LST-NGs are characterized by a smooth surface and can be either flat or pseudo-depressed. In all, LST-NG with pseudo-depression carries the highest risk of SMI among LSTs (31.6%; 95%CI: 19.8%-43.4%)[28]. In addition to morphology, location is another important factor, with LST-G mixed type or LST-NG lesions in the rectosigmoid colon carrying the highest risk for malignancy[29].
In addition to its gross morphology, the surface vascular and pit pattern of a polyp can provide information about the risk of SMI and thereby assist with management decisions. Multiple classification systems have been developed for polyp characterization and are outside the scope of this review. As part of this overview, we briefly discuss the Narrow Band Imaging International Colorectal Endoscopic (NICE) classification system and Kudo pit pattern nomenclature, which are possibly the most commonly utilized classification systems in the West.
NICE classification system: Narrow-band imaging (NBI) is a form of digital chromoendoscopy that enables detailed assessment of the capillary mucosal pattern of polyps by filtering white light into specific wavelengths to enhance the superficial microvascular structures. Using NBI, the NICE classification system provides a validated criterion for the optical diagnosis of colorectal polyps[30,31]. In this classification scheme, polyps can be divided into three categories (type 1, 2 or 3) based on their appearance (Table 1). NICE type 1 and 2 polyps are benign and can be resected endoscopically. Conversely, type 3 Lesions, characterized by disrupted/missing vessel pattern and amorphous or absent surface pattern on NBI, are highly suggestive of deep SMI, and thereby not amenable to endoscopic resection.
| Color | Vessels | Pits | Association | |
| Type 1 | Same or lighter than background | No or lacy vessels | Dark or white spots of uniform size | Hyperplastic or serrated polyps |
| Type 2 | Browner than background | Brown vessels | Oval or tubular white pits | Adenomatous polyps |
| Type 3 | Dark brown | Disrupted or missing vessels | Amorphous or absent pits | Deep submucosal invasion |
Japan NBI Expert Team classification system: The Japan NBI Expert Team (JNET) introduced an NBI magnifying endoscopic classification system for colorectal polyps in 2014[32]. The JNET system is mainly used in Asian countries and less frequently in the Western Hemisphere. By focusing on vessel and surface pattern, the JNET system classifies colorectal polyps into four types (Types 1, 2A, 2B, and 3); each type representing the histological feature of the polyps (Table 2). Similar to NICE, irregular /amorphous vessel and surface patterns on the JNET classification system are indicative of a higher likelihood of submucosal invasive cancer.
| Type 1 | Type 2A | Type 2B | Type 3 | |
| Vessel pattern | Invisible | Regular caliber and distribution (meshed/spiral) | Variable caliber, irregular distribution | Loose vessel areas, interruption of thick vessels |
| Surface pattern | Uniform dark or white spots similar to surrounding mucosa | Regular (tubular/branched/papillary) | Irregular or obscure | Amorphous areas |
| Most likelyhistology | Hyperplastic or sessile serrated polyps | Low grade dysplasia | High grade dysplasia/shallow submucosal invasive cancer | Deep submucosal invasive cancer |
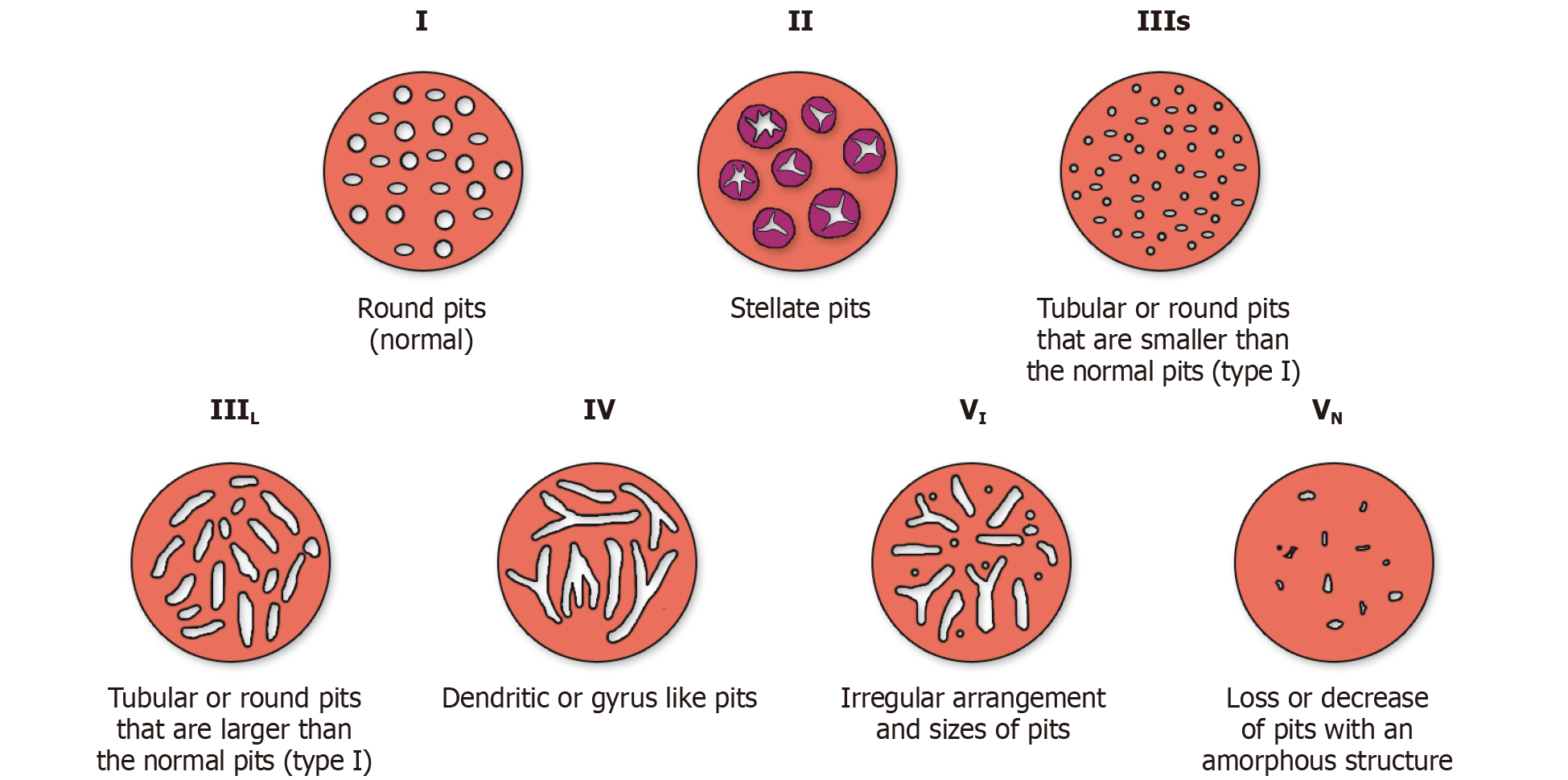
Kudo pit pattern: Kudo and colleagues first highlighted the feasibility of examining and classifying pit patterns to distinguish type of polyps by using magnifying endoscopy[33]. This scheme broadly categorizes pit patterns into 7 types based on the pit appearance and structure (Figure 3). Most colorectal polyps (Kudo pit pattern types I through IV) fall within the spectrum of benign polyps that can be managed endoscopically. On the other hand, lesions with Kudo pit pattern V (amorphous, non-structured pit pattern) are often indicative of deep SMI, CRC and therefore the need for surgery[26,34].
Accurate histopathological assessment is critical in determining adequacy of endoscopic resection. In this section, we briefly discuss some of the specific histopathological criteria associated with risk of recurrence and LNM in the context of malignant polyps.
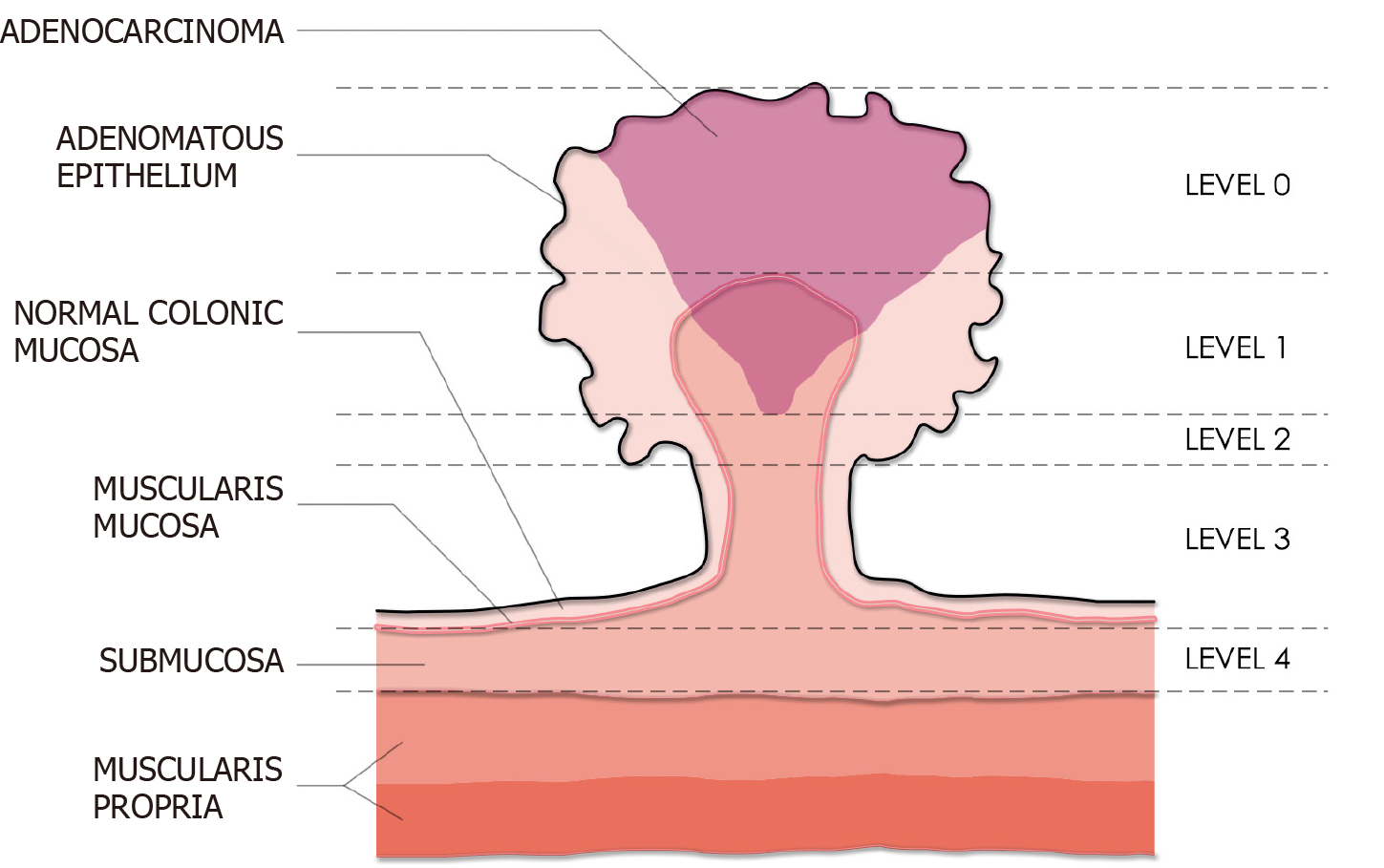
Haggitt classification of pedunculated polyps: Haggitt et al[35] developed a classification system to describe the level of invasion in pedunculated polyps. This system categorizes polyps into five classes: level 0 to 4 (Figure 4). Level 0 corresponds to neoplastic cells limited to the mucosa without breaching the muscularis mucosa, thereby not meeting the clinical definition of CRC. Level 1 corresponds to those pedunculated polyps in which cancer cells have invaded the submucosa of the polyp head. Level 2 and 3 indicate cancer cells invading into the submucosa of the neck (junction between head and stalk) and any region of the stalk, respectively. Lastly, level 4 denotes invasion of cancer cells into the submucosa of the colorectal wall below the stalk of the polyp, but not into the muscularis propria.
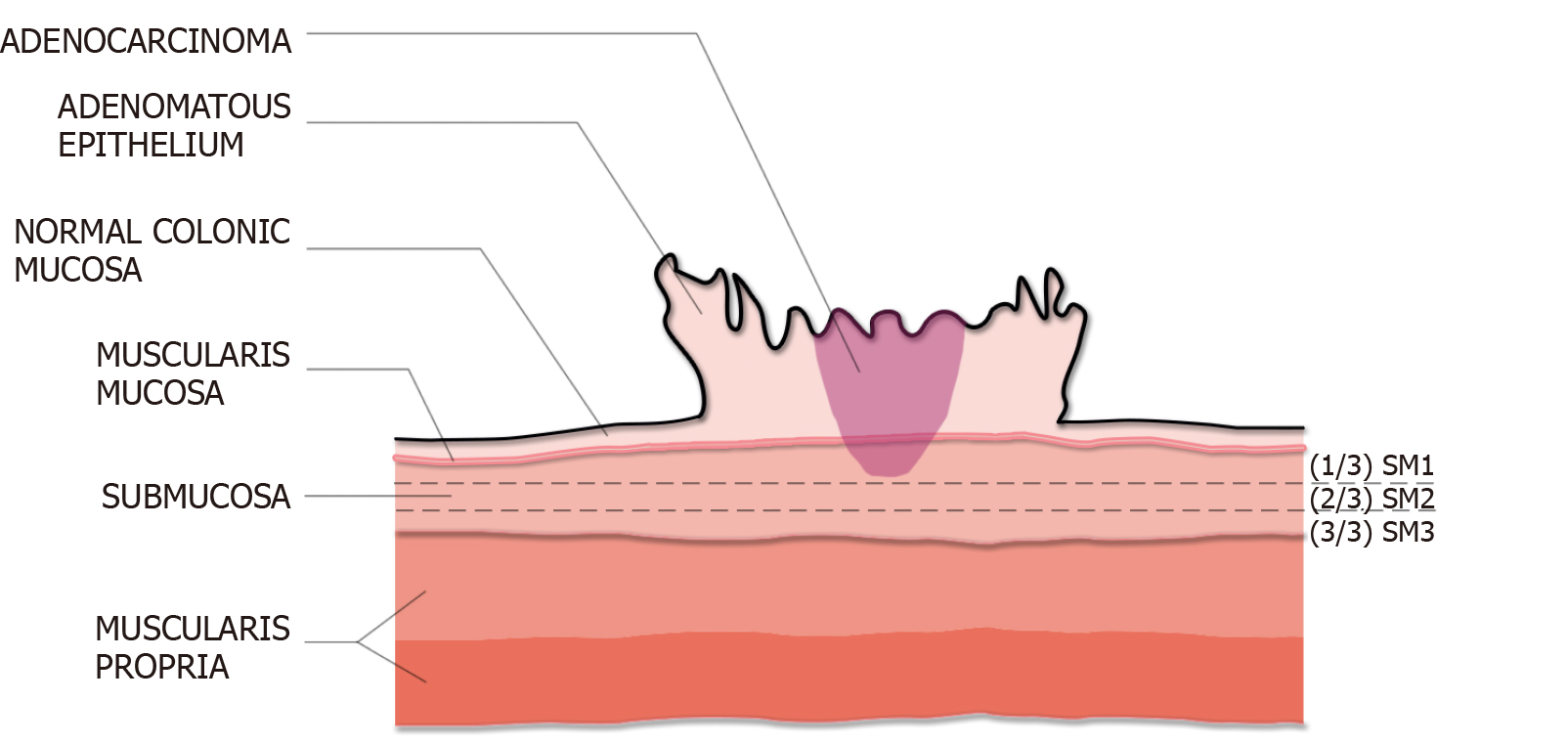
Kudo and Kikuchi classification of sessile polyps: Both Kudo et al[36] and Kikuchi et al[37] introduced the concept of classifying sessile polyps into three levels based on the degree of SMI: Sm1–invasion into the upper third of the submucosa; Sm2–invasion into the middle third; and Sm3–invasion into the lower third (Figure 5). The main challenge of implementing this classification system in routine clinical practice is the need for a significant portion of the submucosa within the resected specimen to define the deepest border of the submucosa. Hence, for practical purposes, this scheme has been largely modified to measure the depth of SMI from the muscularis mucosa. A SMI depth of 1000 µm is used to differentiate those lesions with superficial (< 1000 µm) vs deep (≥ 1000 µm) invasion. Deep SMI has been shown to be highly associated with risk for lymph node spread (10%-18%), independent of other histological features[38-40].
In addition to depth of invasion, several histological features have been identified as predictors for LNM.
Tumor differentiation: Three tumor grades have been used to described CRC based on the degree of glandular differentiation: grade 1 (well-differentiated), grade 2 (moderately differentiated), and grade 3 (poorly differentiated). When compared to grade 1 or 2, poorly differentiated adenocarcinomas have been shown to be associated with a significantly higher incidence of lymphatic spread [odds ratio (OR): 5.60; 95%CI: 2.90-10.82; P < 0.00001] and cancer-related mortality[39].
Lymphovascular invasion: Lymphovascular invasion (LVI) is recognized as a poor prognostic indicator and predictor of patient outcome. The presence of LVI in malignant polyps has been associated with an increased risk of regional LNM (OR: 4.81; 95%CI: 3.14-7.37; P < 0.0001)[39].
Tumor budding: Tumor budding is defined as a single or cluster of up to 5 tumor cells at the advancing front of the tumor[5,40]. This phenomenon has been recognized as a potential indicator of aggressive tumor biology with substantial evidence identifying it as a significant risk factor for LNM (OR: 7.74; 95%CI: 4.47-13.39, P < 0.001)[39].
Endoscopic resection should be the first-line preferred approach for the management of non-malignant polyps. Multiple studies have shown that endoscopic resection is more cost-effective, associated with less adverse events and higher patient quality of life when compared to surgery[41-45]. Nonetheless, despite the data favoring endoscopic resection, surgery remains a common practice and increasing trend in the United States over the past two decades[46]. In a recent study on referral patterns for the management of colorectal polyps, we demonstrated that polyps with a baseline histopathology diagnosis of “intramucosal adenocarcinoma” or “carcinoma in-situ” were associated with a significant higher likelihood of being scheduled for surgery as compared to endoscopic resection (OR: 5.72; 95%CI: 1.16-28.19, P = 0.03)[7]. The terms intramucosal adenocarcinoma, intraepithelial carcinoma, carcinoma in-situ or high-grade dysplasia are commonly used interchangeably by pathologists to define lesions in which neoplasia has invaded into the lamina propria but without extension through the muscularis mucosa. In all, these lesions can be adequately treated endoscopically given the absence of lymphatics within the colon mucosa and the aforementioned negligible risk for LNM. However, the inclusion of the word “carcinoma” on the diagnosis can be easily misinterpreted by providers as equivalent to CRC, which in turn can lead to inappropriate management decisions[7,17]. More recently, the terminology for these precursor lesions has been somewhat standardized in the recent 2019 WHO classification of tumors of the digestive system (5th edition)[7,47]. Indeed, the term “dysplasia” is preferred for these precursor lesions in the colon, with the two-tiered system (low- vs high-grade) considered the standard grading system. Conversely, the use of “carcinoma in-situ” and “intramucosal adenocarcinoma” is strongly discouraged so as to reduce the clinical ambiguity associated with these terms[5,7,47].
This standardization of pathological diagnostic reporting unifies these diagnoses under the term high-grade dysplasia, potentially reducing the likelihood of misinterpreting these non-malignant polyps as CRC, and thereby the surgical referrals for otherwise endoscopically resectable lesions.
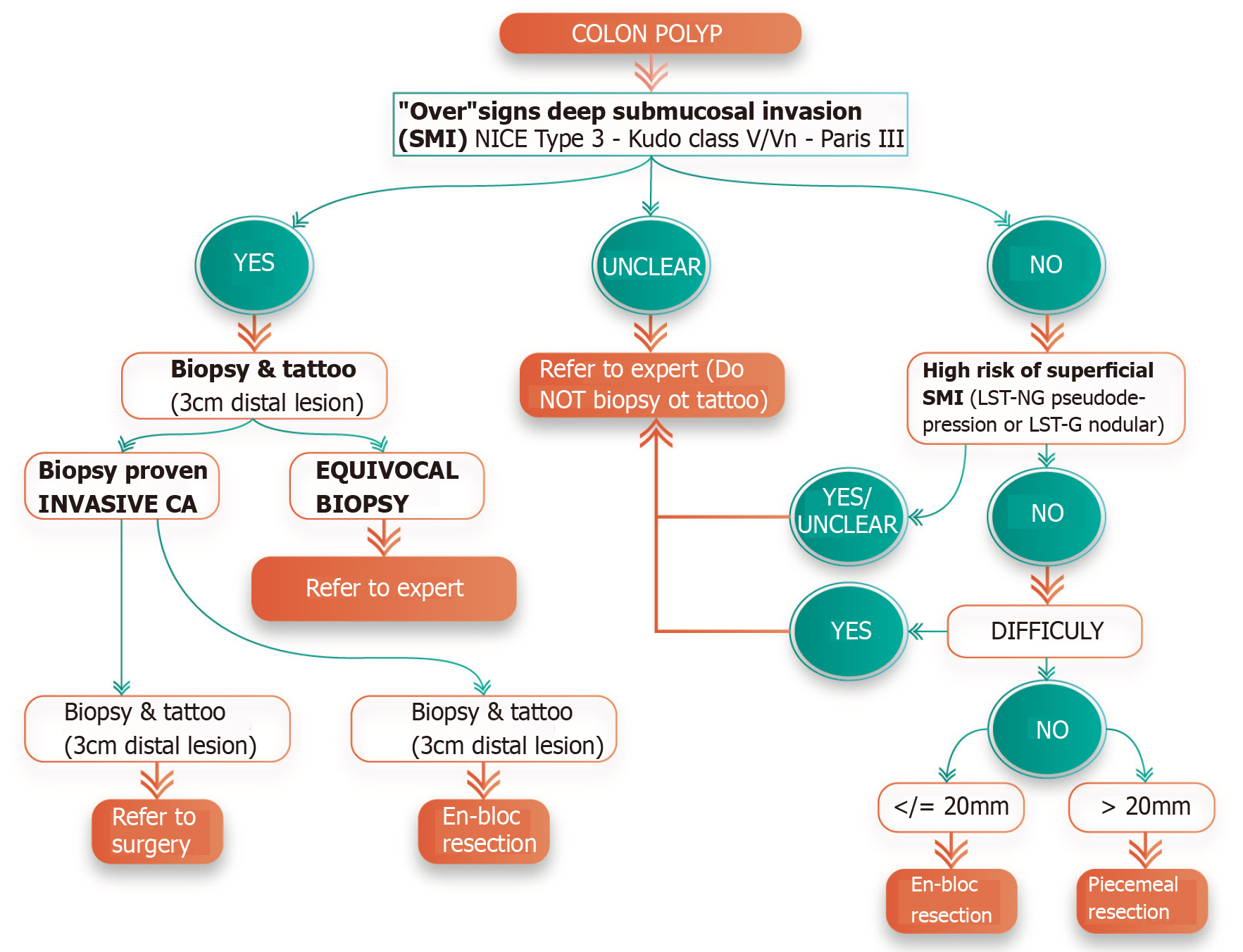
The optimal management of colorectal polyps can be complex and dependent on various factors, including patient and lesion characteristics, as well as local resources and expertise availability. In this section, we propose a potential strategy for the evaluation and management of colorectal polyps that can be adapted in clinical practice. The decision tree is depicted in Figure 6.
Lesions should be carefully evaluated endoscopically for “overt” signs of deep SMI including NICE type 3, Kudo class V, surface ulceration without prior manipulation (i.e. biopsies or resection attempts), or stiffness of the lesion and colon wall[17]. According to the recent recommendations by the United States Multi-Society Task Force (USMSTF) on CRC, non-pedunculated lesions with features of deep SMI should be biopsied (in the area with surface feature disruption), tattooed near the base of the polyp and on the opposite lumen wall, and referred to surgery[48]. These recommendations by the USMSTF stem from data showing that both NICE type 3 and Kudo type V patterns are highly specific predictors of deep SMI, which are associated with LNM and need for surgery[49,50]. However, it should be highlighted that these outcomes on real-time optical diagnosis are derived from endoscopists highly trained in advanced imaging and may not reflect performance in routine clinical practice. In fact, optical diagnosis alone is notoriously endoscopist-dependent and its performance outside of specialized academic centers has been disappointing[51].
Hence, reliance on optical diagnosis alone, as proposed by the USMSTF, may have some potential drawbacks. For one, misclassification of endoscopically resectable polyps as having deep SMI can lead to premature surgical referral and a slew of potentially unnecessary diagnostic staging tests (i.e. EUS, CT, MRI, PET-scan, etc), directly impacting the patient’s mental health and resource utilization[52]. Secondly, tattooing a lesion at or near its base is associated with significant submucosal fibrosis, which in turn can render subsequent endoscopic resection attempts significantly more difficult if not impossible[53-55]. Therefore, if a tattoo is deemed necessary, we recommend strictly tattooing 3 cm distal to the polyp, with appropriate photo documentation of its location with respect to the lesion[56]. Based on the aforementioned issues, we suggest that surgical referral be initiated only for those lesions with biopsy-proven invasive adenocarcinoma (Figure 6). When biopsies are performed, they should be directed to the area exhibiting features of deep SMI. This targeted biopsy strategy increases the yield for histological diagnosis and minimizes the risk of inducing submucosal fibrosis for those lesions that may be amenable for endoscopic intervention. For lesions with the following indeterminate characteristics, we recommend considering referral to a high-volume center with expertise in both endoscopic imaging and resection of complex polyps: Lesions with endoscopic appearance suggestive of deep SMI yet negative for invasive cancer on biopsies[55,57]; Lesions with equivocal endoscopic appearance for deep SMI; Lesions with equivocal biopsy results (i.e. histopathology showing “at least” high-grade dysplasia yet deeper invasion cannot be excluded based on the limited sample).
While we recognize that this biopsy-driven algorithm is not without its limitations, including false negative histopathology for invasive disease due to sampling error, it may potentially curtail the current trend of surgical referrals for endoscopically resectable colorectal polyps. Of note, the exception to this approach includes pedunculated polyps with either biopsy-proven and/or signs of deep SMI limited to the head of the polyp (Haggitt level 0-2). In these cases, even when invasive CRC is present, en-bloc resection at the level of the stalk is associated with favorable prognosis and is often curative[48,58]. Most of these pedunculated polyps can be adequately transected at the stalk with endoscopic polypectomy. In select cases, maneuvering a snare around the large head of a pedunculated polyp with a long, wide stalk can be technically challenging and endoscopic submucosal dissection (ESD) has been reported as an alternate approach to ensure en-bloc resection[59,60].
In the absence of endoscopic features of overt deep SMI, the next step is to evaluate for morphological features associated with an increased risk for superficial SMI, as this may influence the endoscopic resection strategy. Predictors associated with a relative high risk of superficial SMI include the following; polyps with depressed morphology (Paris IIc), LST-NG with depression or bulky sessile appearance (Paris Is component), and LST-G with dominant nodules[26]. While neither lesion size nor location by itself can reliably predict superficial SMI, multiple studies have shown that the risk increases with lesions ≥ 20 mm and LSTs located in the right colon, rectosigmoid, and rectum[26,48].
As outlined by the recent recommendations by the USMSTF on CRC, lesions with suspected superficial SMI should ideally be approached with en-bloc endoscopic resection[48]. En-bloc removal of these lesions is necessary for accurate histological assessment, as piecemeal resection results in fragmented tissue specimens that compromise specimen orientation and interpretability of the resection margins. Inasmuch, the National Comprehensive Cancer Network practice guidelines specify that patients with otherwise endoscopically curable malignant polyps (i.e. those with superficial SMI and favorable histopathological features) who undergo piecemeal endoscopic resection will inevitably still require surgery due to the high risk of understaging the lesion because of compromised pathological interpretation[61]. Hence, the approach to a lesion with suspected superficial SMI is largely dependent on polyp size.
Lesions ≤ 20 mm in size: En-bloc resection may be achievable with endoscopic mucosal resection (EMR) for lesions ≤ 20 mm. Although a recent systematic review and meta-analysis suggested that underwater EMR may be associated with superior en-bloc resection rate when compared to conventional EMR (OR: 1.49; 95%CI: 1.02-2.16; P = 0.04), high-quality comparative studies are scarce. Therefore, the most appropriate strategy remains to be determined[62]. When performing EMR for these lesions, it is important to ensure that the snare encloses an additional margin of normal tissue around the polyp. By including a wider margin, risk of inadvertent incomplete en-bloc resection is decreased, which would otherwise require piecemeal removal.
Lesions > 20 mm in size: These polyps usually require ESD to achieve en-bloc resection. Attempt to en-bloc resect polyps > 20 mm with EMR is associated with a higher risk of potential complications and failure. A recent meta-analysis showed that the pooled proportion of successful en-bloc resection for polyps > 20 mm with either conventional or underwater EMR was unacceptably low (49.7%-58.7%)[62]. Hence, the European Society of Gastrointestinal Endoscopy, the Japan Gastroenterological Endoscopy Society and a recent American Gastroenterological Association clinical practice update recommend ESD as the preferred strategy for the resection of select colorectal lesions with suspected superficial SMI[63-65]. When compared to EMR, ESD is associated with a higher en-bloc and curative resection rate, and lower risk of recurrence[66]. However, ESD is a technically more complex procedure, associated with a steep learning curve and higher rate of serious adverse events[66,67]. Due to these and other factors, the adoption of colonic ESD in the Western Hemisphere has been slower; albeit recent studies from North America have shown comparable outcomes to those reported in Asia. In a recent North American multicenter study, rectal ESD (n = 171) was associated with an en-bloc and complete (R0) resection rate of 82.5% and 74.9%, respectively[54]. Importantly, this study demonstrated that ESD was curative for 82% of these rectal malignant polyps[54]. It is worth noting that compared to surgery in the proximal colon, rectal operations for malignant polyps have an exceedingly high morbidity (40%-45%)[68,69]. Based on the above, referral for ESD to a center with expertise should be the preferred approach for the management of rectal lesions with suspected superficial SMI.
ESD in the proximal colon is more challenging than in the rectum, given issues with bowel peristalsis, scope positioning, and the relatively thinner colon wall[70]. As such, we recommend referring these lesions to a dedicated center with appropriate endoscopic and surgical expertise for multi-disciplinary discussion regarding the most optimal approach on a case-by-case basis.
All colorectal polyps without signs of superficial or deep SMI are benign and have no risk for LNM. Endoscopic resection should be the preferred management strategy over surgery, given the well-established advantages as previously mentioned in this review.
EMR remains the treatment of choice for the removal of benign colorectal polyps[71]. For lesions ≤ 20 mm in size, en-bloc resection should be attempted as this is associated with a lower risk of recurrence and need for re-intervention when compared to piecemeal removal[66,70]. Piecemeal EMR will invariably be necessary for the removal of larger non-pedunculated polyps, which increases the risk of recurrence, reportedly as high as 40%[70]. Recent strategies, including endoscopic ablation of the resection margins appear to decrease recurrence rate following piecemeal EMR[72], albeit future studies are needed to corroborate its efficacy in routine clinical practice.
Irrespective of the EMR approach, complete endoscopic resection (no visible residual tissue) should be the procedural benchmark. Partial resection or endoscopic ablation of residual visible tissue is associated with a prohibitively high risk for recurrence and even more concerning, significantly jeopardizes the ability to endoscopically remove the lesion on subsequent attempts. Notably, colorectal EMR can be technically challenging for complex polyps. Thereby, the USMSTF recommends that lesions ≥ 20 mm should be removed by endoscopists with experience in advanced polypectomy[48].
Several features have been commonly used to define a “difficult polyp”, including variables such as size (usually ≥ 40 mm) and challenging location (i.e. involving the ileocecal valve, appendiceal orifice, dentate line, behind folds)[73]. More broadly, a “difficult polyp” should be defined as any lesion that the endoscopist feels he/she may not be able to completely resect endoscopically with high confidence; therefore, needing to be referred to a center with the appropriate expertise. When referring these lesions, we recommend against routine biopsy. Pretreatment biopsies do not necessarily change the management strategy in the absence of signs of SMI and can induce submucosal fibrosis, leading to prolonged procedure times and higher incomplete resection rates during succeeding endoscopic resection[74,75]. Furthermore, tattooing is not necessary if the lesion is in the cecum or rectum. If the lesion cannot be easily identified on colonoscopy, tattoo for lesion localization should be placed approximately 3 cm distal to the polyp and documented in the endoscopy report.
Endoscopic resection is a proven strategy for the management of benign and select malignant colorectal polyps. When compared to surgery, endoscopic resection is less costly and associated with improved clinical outcomes and patient satisfaction. Detailed lesion assessment, including endoscopic imaging and histopathology, play a critical role in directing subsequent treatment strategies. Ultimately, the most appropriate intervention will depend on various factors, including patient and lesion characteristics, as well as local resources and expertise availability. Establishing the multidisciplinary collaboration between referring physicians, endoscopists, surgeons and pathologists is the basis for ensuring best practices for the management of colorectal polyps.
Graphic illustrations of figures created by Vinay Mathews, RA, NCARB.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56648] [Article Influence: 7081.0] [Reference Citation Analysis (134)] |
| 2. | Conteduca V, Sansonno D, Russi S, Dammacco F. Precancerous colorectal lesions (Review). Int J Oncol. 2013;43:973-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 3. | Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3107] [Cited by in RCA: 3172] [Article Influence: 96.1] [Reference Citation Analysis (1)] |
| 4. | Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF, Stewart ET, Waye JD. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2382] [Article Influence: 170.1] [Reference Citation Analysis (2)] |
| 5. | Kuo E, Wang K, Liu X. A Focused Review on Advances in Risk Stratification of Malignant Polyps. Gastroenterology Res. 2020;13:163-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 6. | Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 1765] [Article Influence: 252.1] [Reference Citation Analysis (2)] |
| 7. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2736] [Article Influence: 456.0] [Reference Citation Analysis (3)] |
| 8. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8093] [Article Influence: 224.8] [Reference Citation Analysis (1)] |
| 9. | Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1422] [Article Influence: 83.6] [Reference Citation Analysis (12)] |
| 10. | Thompson SL, Bakhoum SF, Compton DA. Mechanisms of chromosomal instability. Curr Biol. 2010;20:R285-R295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 459] [Article Influence: 28.7] [Reference Citation Analysis (2)] |
| 11. | Crockett SD, Nagtegaal ID. Terminology, Molecular Features, Epidemiology, and Management of Serrated Colorectal Neoplasia. Gastroenterology. 2019;157:949-966.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 264] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 12. | Rosty C, Hewett DG, Brown IS, Leggett BA, Whitehall VL. Serrated polyps of the large intestine: current understanding of diagnosis, pathogenesis, and clinical management. J Gastroenterol. 2013;48:287-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 13. | O'Brien MJ, Yang S, Clebanoff JL, Mulcahy E, Farraye FA, Amorosino M, Swan N. Hyperplastic (serrated) polyps of the colorectum: relationship of CpG island methylator phenotype and K-ras mutation to location and histologic subtype. Am J Surg Pathol. 2004;28:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Vennelaganti S, Cuatrecasas M, Vennalaganti P, Kennedy KF, Srinivasan S, Patil DT, Plesec T, Lanas A, Hörndler C, Andraws N, Cherian R, Mathur S, Hassan C, Repici A, Klotz D, Musulen E, Risio M, Castells A, Gupta N, Sharma P. Interobserver Agreement Among Pathologists in the Differentiation of Sessile Serrated From Hyperplastic Polyps. Gastroenterology. 2021;160:452-454.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Bettington M, Walker N, Rosty C, Brown I, Clouston A, McKeone D, Pearson SA, Leggett B, Whitehall V. Clinicopathological and molecular features of sessile serrated adenomas with dysplasia or carcinoma. Gut. 2017;66:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 170] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 16. | National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology, Colon Cancer (v3.2018). Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. |
| 17. | Rex DK, Shaukat A, Wallace MB. Optimal Management of Malignant Polyps, From Endoscopic Assessment and Resection to Decisions About Surgery. Clin Gastroenterol Hepatol. 2019;17:1428-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 18. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC cancer staging manual. 8th ed. New York, NY: Springer, 2017: 252-254. |
| 19. | Hackelsberger A, Frühmorgen P, Weiler H, Heller T, Seeliger H, Junghanns K. Endoscopic polypectomy and management of colorectal adenomas with invasive carcinoma. Endoscopy. 1995;27:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 20. | Netzer P, Forster C, Biral R, Ruchti C, Neuweiler J, Stauffer E, Schönegg R, Maurer C, Hüsler J, Halter F, Schmassmann A. Risk factor assessment of endoscopically removed malignant colorectal polyps. Gut. 1998;43:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Coverlizza S, Risio M, Ferrari A, Fenoglio-Preiser CM, Rossini FP. Colorectal adenomas containing invasive carcinoma. Pathologic assessment of lymph node metastatic potential. Cancer. 1989;64:1937-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010;23:1068-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 23. | Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1380] [Article Influence: 60.0] [Reference Citation Analysis (13)] |
| 24. | Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, Axon AT. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 411] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, Matsui S, Friedland S. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 440] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 26. | Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (1)] |
| 27. | Lopez A, Bouvier AM, Jooste V, Cottet V, Romain G, Faivre J, Manfredi S, Lepage C. Outcomes following polypectomy for malignant colorectal polyps are similar to those following surgery in the general population. Gut. 2019;68:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 28. | Bogie RMM, Veldman MHJ, Snijders LARS, Winkens B, Kaltenbach T, Masclee AAM, Matsuda T, Rondagh EJA, Soetikno R, Tanaka S, Chiu HM, Sanduleanu-Dascalescu S. Endoscopic subtypes of colorectal laterally spreading tumors (LSTs) and the risk of submucosal invasion: a meta-analysis. Endoscopy. 2018;50:263-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (2)] |
| 29. | Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, Mahajan H, McLeod D, Bourke MJ. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology. 2017;153:732-742.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 30. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology. 2012;143:599-607.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 428] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 31. | Puig I, López-Cerón M, Arnau A, Rosiñol Ò, Cuatrecasas M, Herreros-de-Tejada A, Ferrández Á, Serra-Burriel M, Nogales Ó, Vida F, de Castro L, López-Vicente J, Vega P, Álvarez-González MA, González-Santiago J, Hernández-Conde M, Díez-Redondo P, Rivero-Sánchez L, Gimeno-García AZ, Burgos A, García-Alonso FJ, Bustamante-Balén M, Martínez-Bauer E, Peñas B, Pellise M; EndoCAR group, Spanish Gastroenterological Association and the Spanish Digestive Endoscopy Society. Accuracy of the Narrow-Band Imaging International Colorectal Endoscopic Classification System in Identification of Deep Invasion in Colorectal Polyps. Gastroenterology. 2019;156:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 32. | Iwatate M, Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Ishikawa H, Murakami Y, Yoshida S, Saito Y; Japan NBI Expert Team (JNET). Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc. 2018;30:642-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (1)] |
| 33. | Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 477] [Article Influence: 14.9] [Reference Citation Analysis (4)] |
| 34. | Togashi K, Konishi F, Ishizuka T, Sato T, Senba S, Kanazawa K. Efficacy of magnifying endoscopy in the differential diagnosis of neoplastic and non-neoplastic polyps of the large bowel. Dis Colon Rectum. 1999;42:1602-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Haggitt RC, Glotzbach RE, Soffer EE, Wruble LD. Prognostic factors in colorectal carcinomas arising in adenomas: implications for lesions removed by endoscopic polypectomy. Gastroenterology. 1985;89:328-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 446] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 36. | Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 562] [Article Influence: 17.0] [Reference Citation Analysis (11)] |
| 37. | Kikuchi R, Takano M, Takagi K, Fujimoto N, Nozaki R, Fujiyoshi T, Uchida Y. Management of early invasive colorectal cancer. Risk of recurrence and clinical guidelines. Dis Colon Rectum. 1995;38:1286-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 451] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 38. | Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, Iwashita A, Ajioka Y, Watanabe H, Watanabe T, Muto T, Nagasako K. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 495] [Article Influence: 22.5] [Reference Citation Analysis (1)] |
| 39. | Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis. 2013;15:788-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 196] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 40. | Choi JY, Jung SA, Shim KN, Cho WY, Keum B, Byeon JS, Huh KC, Jang BI, Chang DK, Jung HY, Kong KA; Korean ESD Study Group. Meta-analysis of predictive clinicopathologic factors for lymph node metastasis in patients with early colorectal carcinoma. J Korean Med Sci. 2015;30:398-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Jayanna M, Burgess NG, Singh R, Hourigan LF, Brown GJ, Zanati SA, Moss A, Lim J, Sonson R, Williams SJ, Bourke MJ. Cost Analysis of Endoscopic Mucosal Resection vs Surgery for Large Laterally Spreading Colorectal Lesions. Clin Gastroenterol Hepatol. 2016;14:271-8.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (2)] |
| 42. | Ma C, Teriaky A, Sheh S, Forbes N, Heitman SJ, Jue TL, Munroe CA, Jairath V, Corley DA, Lee JK. Morbidity and Mortality After Surgery for Nonmalignant Colorectal Polyps: A 10-Year Nationwide Analysis. Am J Gastroenterol. 2019;114:1802-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 43. | Dang H, de Vos Tot Nederveen Cappel WH, van der Zwaan SMS, van den Akker-van Marle ME, van Westreenen HL, Backes Y, Moons LMG, Holman FA, Peeters KCMJ, van der Kraan J, Langers AMJ, Lijfering WM, Hardwick JCH, Boonstra JJ. Quality of life and fear of cancer recurrence in T1 colorectal cancer patients treated with endoscopic or surgical tumor resection. Gastrointest Endosc. 2019;89:533-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 44. | Zogg CK, Najjar P, Diaz AJ, Zogg DL, Tsai TC, Rose JA Jr, Scott JW, Gani F, Alshaikh H, Canner JK, Schneider EB, Goldberg JE, Haider AH. Rethinking Priorities: Cost of Complications After Elective Colectomy. Ann Surg. 2016;264:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | de Neree Tot Babberich MPM, Bronzwaer MES, Andriessen JO, Bastiaansen BAJ, Mostafavi N, Bemelman WA, Fockens P, Tanis PJ, Dekker E. Outcomes of surgical resections for benign colon polyps: a systematic review. Endoscopy. 2019;51:961-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 46. | Peery AF, Cools KS, Strassle PD, McGill SK, Crockett SD, Barker A, Koruda M, Grimm IS. Increasing Rates of Surgery for Patients With Nonmalignant Colorectal Polyps in the United States. Gastroenterology. 2018;154:1352-1360.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 47. | Moon N, Aryan M, Khan W, Jiang P, Madhok I, Wilson J, Ruiz N, Ponniah SA, Westerveld DR, Gupte A, Pooran N, Qumseya B, Forsmark CE, Draganov PV, Yang D. Effect of referral pattern and histopathology grade on surgery for nonmalignant colorectal polyps. Gastrointest Endosc. 2020;92:702-711.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 48. | Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman D, Syngal S, Rex DK. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;159:1916-1934.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (2)] |
| 49. | Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, Rex DK, Soetikno RM. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 50. | Rastogi A, Keighley J, Singh V, Callahan P, Bansal A, Wani S, Sharma P. High accuracy of narrow band imaging without magnification for the real-time characterization of polyp histology and its comparison with high-definition white light colonoscopy: a prospective study. Am J Gastroenterol. 2009;104:2422-2430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 51. | Kuiper T, Marsman WA, Jansen JM, van Soest EJ, Haan YC, Bakker GJ, Fockens P, Dekker E. Accuracy for optical diagnosis of small colorectal polyps in nonacademic settings. Clin Gastroenterol Hepatol. 2012;10:1016-20; quiz e79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 52. | Yang D, Draganov PV. Surgery Referral of Colorectal Polyps Based on Real-Time Optical Diagnosis Alone: There is More to this Than Meets the Eye. Gastroenterology. 2021;160:2215-2216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Ono S, Fujishiro M, Goto O, Kodashima S, Omata M. Endoscopic submucosal dissection for colonic laterally spreading tumors is difficult after target tattooing. Gastrointest Endosc. 2009;69:763-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Yang D, Aihara H, Perbtani YB, Wang AY, Aadam AA, Tomizawa Y, Hwang JH, Zou B, Natov NS, Siegel A, Khoshknab MP, Khashab MA, Ngamruengphong S, Khara HS, Diehl DL, Maniere T, Andrawes S, Benias P, Kumta NA, Ramay F, Kim RE, Samarasena J, Chang K, Hashimoto R, Tharian B, Inamdar S, Lan G, Sethi A, Nosler MJ, Tabash A, Othman MO, Draganov PV. Safety and efficacy of endoscopic submucosal dissection for rectal neoplasia: a multicenter North American experience. Endosc Int Open. 2019;7:E1714-E1722. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Vosko S, Bourke MJ. Gross morphology predicts the presence and pattern of invasive cancer in laterally spreading tumors: Don't overlook the overview! Gastrointest Endosc. 2020;92:1095-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 56. | Rex DK. The Appropriate Use and Techniques of Tattooing in the Colon. Gastroenterol Hepatol (N Y). 2018;14:314-317. [PubMed] |
| 57. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 797] [Article Influence: 88.6] [Reference Citation Analysis (1)] |
| 58. | Matsuda T, Fukuzawa M, Uraoka T, Nishi M, Yamaguchi Y, Kobayashi N, Ikematsu H, Saito Y, Nakajima T, Fujii T, Murakami Y, Shimoda T, Kushima R, Fujimori T. Risk of lymph node metastasis in patients with pedunculated type early invasive colorectal cancer: a retrospective multicenter study. Cancer Sci. 2011;102:1693-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Chiba H, Tachikawa J, Arimoto J, Ashikari K, Kuwabara H, Nakaoka M, Goto T, Higurashi T, Muramoto T, Ohata K, Nakajima A. Endoscopic submucosal dissection of large pedunculated polyps with wide stalks: a retrospective multicenter study. Endoscopy. 2021;53:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 60. | Jawaid S, Draganov PV, Yang D. Endoscopic resection of large pedunculated colon polyps using only a scissor-type knife: a case series. VideoGIE. 2020;5:264-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Benson AB 3rd, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 576] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 62. | Chandan S, Khan SR, Kumar A, Mohan BP, Ramai D, Kassab LL, Draganov PV, Othman MO, Kochhar GS. Efficacy and histologic accuracy of underwater versus conventional endoscopic mucosal resection for large (>20 mm) colorectal polyps: a comparative review and meta-analysis. Gastrointest Endosc. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 63. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 957] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 64. | Tanaka S, Kashida H, Saito Y, Yahagi N, Yamano H, Saito S, Hisabe T, Yao T, Watanabe M, Yoshida M, Kudo SE, Tsuruta O, Sugihara KI, Watanabe T, Saitoh Y, Igarashi M, Toyonaga T, Ajioka Y, Ichinose M, Matsui T, Sugita A, Sugano K, Fujimoto K, Tajiri H. JGES guidelines for colorectal endoscopic submucosal dissection/endoscopic mucosal resection. Dig Endosc. 2015;27:417-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 456] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 65. | Draganov PV, Wang AY, Othman MO, Fukami N. AGA Institute Clinical Practice Update: Endoscopic Submucosal Dissection in the United States. Clin Gastroenterol Hepatol. 2019;17:16-25.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 352] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 66. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 67. | Yang D, Othman M, Draganov PV. Endoscopic Mucosal Resection vs Endoscopic Submucosal Dissection For Barrett's Esophagus and Colorectal Neoplasia. Clin Gastroenterol Hepatol. 2019;17:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 68. | Bokey EL, Chapuis PH, Fung C, Hughes WJ, Koorey SG, Brewer D, Newland RC. Postoperative morbidity and mortality following resection of the colon and rectum for cancer. Dis Colon Rectum. 1995;38:480-6; discussion 486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 216] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 69. | Alves A, Panis Y, Mathieu P, Kwiatkowski F, Slim K, Mantion G; Association Française de Chirurgie (AFC). Mortality and morbidity after surgery of mid and low rectal cancer. Results of a French prospective multicentric study. Gastroenterol Clin Biol. 2005;29:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 70. | Hassan C, Repici A, Sharma P, Correale L, Zullo A, Bretthauer M, Senore C, Spada C, Bellisario C, Bhandari P, Rex DK. Efficacy and safety of endoscopic resection of large colorectal polyps: a systematic review and meta-analysis. Gut. 2016;65:806-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 296] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 71. | Kaltenbach T, Anderson JC, Burke CA, Dominitz JA, Gupta S, Lieberman D, Robertson DJ, Shaukat A, Syngal S, Rex DK. Endoscopic Removal of Colorectal Lesions-Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;158:1095-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 224] [Article Influence: 37.3] [Reference Citation Analysis (3)] |
| 72. | Klein A, Tate DJ, Jayasekeran V, Hourigan L, Singh R, Brown G, Bahin FF, Burgess N, Williams SJ, Lee E, Sidhu M, Byth K, Bourke MJ. Thermal Ablation of Mucosal Defect Margins Reduces Adenoma Recurrence After Colonic Endoscopic Mucosal Resection. Gastroenterology. 2019;156:604-613.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 197] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 73. | Herszényi L. The "Difficult" Colorectal Polyps and Adenomas: Practical Aspects. Dig Dis. 2019;37:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 74. | Kuroha M, Shiga H, Kanazawa Y, Nagai H, Handa T, Ichikawa R, Onodera M, Naito T, Moroi R, Kimura T, Endo K, Kakuta Y, Kinouchi Y, Shimosegawa T, Masamune A. Factors Associated with Fibrosis during Colorectal Endoscopic Submucosal Dissection: Does Pretreatment Biopsy Potentially Elicit Submucosal Fibrosis and Affect Endoscopic Submucosal Dissection Outcomes? Digestion. 2021;102:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 75. | Fukunaga S, Nagami Y, Shiba M, Sakai T, Maruyama H, Ominami M, Otani K, Hosomi S, Tanaka F, Taira K, Tanigawa T, Yamagami H, Watanabe T, Fujiwara Y. Impact of preoperative biopsy sampling on severe submucosal fibrosis on endoscopic submucosal dissection for colorectal laterally spreading tumors: a propensity score analysis. Gastrointest Endosc. 2019;89:470-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nishida T, Xie HP, Yoshida A S-Editor: Zhang H L-Editor: A P-Editor: Wang LYT