Published online Aug 16, 2021. doi: 10.4253/wjge.v13.i8.319
Peer-review started: February 18, 2021
First decision: March 14, 2021
Revised: March 21, 2021
Accepted: July 14, 2021
Article in press: July 14, 2021
Published online: August 16, 2021
Processing time: 174 Days and 23.2 Hours
Thoracoscopic esophagectomy is related to an extended lymphadenectomy, and a high number of retrieved lymph nodes, compared to the transhiatal approach; however, its association with an improvement in overall survival (OS) is debatable.
To compare thoracoscopic esophagectomy with transhiatal esophagectomy in patients with adenocarcinoma of the esophagogastric junction (AEGJ) in terms of survival, number of lymph nodes, and complications.
In total, 147 patients with AEGJ were selected retrospectively from 2002 to 2019, and divided into Group A for thoracoscopic esophagectomy, and group B for transhiatal esophagectomy. OS, disease-free survival, postoperative complications, and number of nodes, were similarly evaluated.
One hundred and thirty (88%) were male; the mean age was 64 years. Group A had a mean age of 61.1 years and group B 65.7 years (P = 0.009). Concerning the extent of lymphadenectomy, group A showed a higher number of retrieved lymph nodes (mean of 31.89 ± 8.2 vs 20.73 ± 7; P < 0.001), with more perioperative complications, such as hoarseness, surgical site infections, and respiratory complications. Although both groups had similar OS rates, subgroup analysis showed better survival of transthoracic esophagectomy in patients with earlier diseases.
Both methods are safe, having similar morbidity and mortality rates. Transthoracic thoracoscopic esophagectomy allows a more extensive resection of the lymph nodes and may have better oncological outcomes during earlier stages of the disease. Prospective studies are warranted to better evaluate these findings.
Core Tip: The type of access during esophagectomy to adenocarcinoma of esophagogastric junction tumor is on debate. Thoracoscopic esophagectomy produces higher numbers of retrieved lymph nodes than transhiatal esophagectomy but is associated with more perioperative complications. The relationship between lymphadenectomy’s extension and survival outcomes is debatable. We compared both access and found better survival in early staging of patients treated by thoracoscopic esophagectomy, probably due to the extension of lymphadenectomy and acceptable complication rate. These findings reveal a new place of thoracoscopic esophagectomy for adenocarcinoma of the esophagogastric junction tumor in the multimodal era.
- Citation: Takeda FR, Obregon CA, Navarro YP, Moura DTH, Ribeiro Jr U, Aissar Sallum RA, Cecconello I. Thoracoscopic esophagectomy is related to better outcomes in early adenocarcinoma of esophagogastric junction tumors. World J Gastrointest Endosc 2021; 13(8): 319-328
- URL: https://www.wjgnet.com/1948-5190/full/v13/i8/319.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i8.319
Esophageal cancer is one of the most lethal neoplasms worldwide (with about 17000 new cases per year), and the sixth leading cause of cancer deaths (286000 deaths per year)[1]. The most frequent histologic type of esophageal neoplasm is squamous cell carcinoma, responsible for 76% of cases, followed by adenocarcinoma in Eastern countries[2]. In our institution, adenocarcinoma increased from 15% to 32.5% over the last thirteen years[3]. In the same way, the prevalence of adenocarcinoma of the esophagogastric junction (AEGJ) is rising in Western countries, mostly due to the higher prevalence of risk factors such as obesity[4].
The topographic distribution of metastatic lymph nodes of AEGJ varies according to the Siewert classification. In Siewert type I, the main lymphatic drainages are predominantly in the middle and lower mediastinum; in type II, in the lower mediastinum, thoracoabdominal transition, and abdominal part; and in type III, almost entirely abdominal[5]. Regarding surgical treatment, Siewert type II leads the indication for the transhiatal approach, and Siewert type I leads for the transthoracic approach[6,7]. Despite controversy over access to esophagectomy, transthoracic access is preferred by several Western surgeons[8-10], partly because most advocate an infracarinal lymphadenectomy[11]. However, the addition of minimally invasive techniques, associated with a lower number of postoperative complications and morbidity rates, makes transthoracic esophagectomy by thoracoscopy one of the main options. Yet, extensive radical resection has not shown better survival than transhiatal en bloc esophagectomy with extended lymphadenectomy[12]. Some studies find that the extremely invasive procedure leads to an increase in morbidity and mortality[13,14], which might interfere with overall survival (OS).
This study aimed to analyze the results of AEGJ surgical treatment, comparing transhiatal esophagectomy and transthoracic esophagectomy access by thoracoscopy, including outcomes such as complications and mortality rates, and extension of lymphadenectomy as represented by the number of resected lymph nodes.
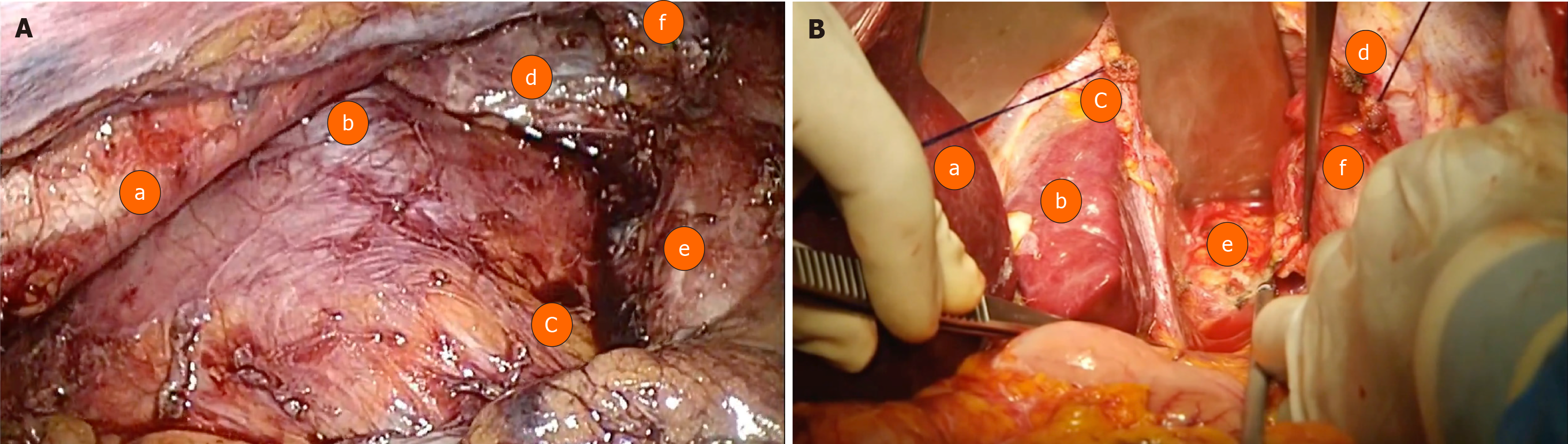
This is a retrospective study following the STROBE Statement Checklist analysing patients with a histological diagnosis of AEGJ, Siewert I and II types, who underwent surgical treatment [transthoracic esophagectomy by thoracoscopy (group A) (Figure 1A) and transhiatal esophagectomy (group B) (Figure 1B)] between 2002 and 2019 at Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo and Instituto do Câncer do Estado de São Paulo. As this is a retrospective analysis, the Ethics committee of both institutions exempted the need for approval.
The following epidemiological data were analyzed and compared between group A and B: age, gender, body mass index, preoperative functional assessment by the Zubrod scale (Eastern Cooperative Oncology Group), and a relevant personal medical history (diabetes, cardiovascular disease, etc.).
Transhiatal esophagectomy: This procedure involves a dissection of the combined cervical and abdominal esophagus without opening the thorax. Improved by Pinotti[15], with transection of the diaphragm, it allowed dissection under direct view of almost the entire mediastinum, thereby avoiding the inconvenience of blunt dissection of the esophagus.
After opening the diaphragm, the infracarinal lymphadenectomy is performed around the bilateral pleural, added to resection of lymph nodes around the hepatic artery, left gastric artery and vein, and the celiac trunk. In the abdominal section, the stomach is released in the great curvature, preserving the arch from the gastroepiploic vessels. The stomach is transposed into the cervical region through the posterior mediastinum, with cervical gastroplasty performed (preparation of the isoperistaltic gastric tube) with linear staplers and oversuturing.
Transthoracic thoracoscopic esophagectomy: After selective intubation of the left bronchus, the patient is placed in a prone position, along with five trocars. The first one at 12 mm is introduced at the inferior limit of the right scapula. The other four trocars are positioned under direct visualization (after positive intrathoracic insufflation of 8 mmHg of CO2).
Three other trocars (two 10 mm and one 5 mm) are arranged with the first in a semicircular line from the medial border of the scapula to the posterior right costal border. Finally, the fifth trocar is positioned at the midpoint of this line, next to the spine.
Dissection of the esophagus is performed from the lower to upper mediastinum. Extensive lymphadenectomy takes place: periesophageal, periaortic, supradiaphragmatic, and pericardial lymph nodes are dissected. The right and left infracarinal lymph nodes are resected, which exposes the right and left bronchi to their origin in the carina.
In order to facilitate esophageal mobilization and the lymphadenectomy, the azygos vein is ligated and transected (preferentially with a laparoscopic stapler).
After dissection, the right pleural space is drained, and the trocars are withdrawn. The patient is placed supine in order to proceed with the abdominal part (which occurs similarly to that described in the open transhiatal esophagectomy).
The main outcomes of this study include resected lymph nodes, complications and deaths. Once the surgical specimen is removed, the lymph nodes are immediately dissected by the surgeon and separated based on lymph node stations. This material is sent for anatomopathological study (N), together with the surgical specimen, each in formaldehyde. The resected lymph nodes (LDs) for patients in groups A and B were compared. The lymph nodes affected (LA) and the status of the dissected and affected (LD/LA) in each group were evaluated. Postoperative complications analyzed include cervical fistulae, chylothorax, respiratory disorders (pneumonia, atelectasis, pleural effusions, and respiratory failure), hoarseness (paralysis or paresis of vocal cords), and infection (mediastinal collections and abscesses).
Data were reported as number (%) or mean ± SD. Categorical variables were compared using Pearson’s chi-squared test or Fisher’s exact test, and continuous variables were compared using Student’s t-test. Survival outcomes were compared using the Kaplan-Meier method and the log-rank test. The Cox proportional hazards model was used to identify relevant prognostic factors, with significant covariables from the univariate analyses selected for the multivariate model. The results were reported as hazard ratios and 95%CIs. Differences were considered statistically significant at P values of < 0.05, and all analyses were performed using IBM SPSS software (version 20, IBM Corp., Armonk, NY, United States).
Fifty-four patients underwent transthoracic esophagectomy by thoracoscopy (group A) and 93 transhiatal approach (group B). Forty-seven patients from group A (87.0%) and forty-three patients from group B (46.2%) received neoadjuvant treatment (chemotherapy associated with radiotherapy as needed).
Epidemiological data are shown in Table 1. Age was higher in patients undergoing transhiatal esophagectomy (P = 0.009); however, the other parameters analyzed were similar.
| Characteristics | Group | Total | P value | ||
| Thoracoscopygroup A | Transhiatalgroup B | ||||
| n = 54, n (%) | n = 93, n (%) | n = 147, n (%) | |||
| Gender | Female | 6 (11.1) | 11 (11.8) | 17 (11.6) | 0.8961 |
| Male | 48 (88.9) | 82 (88.2) | 130 (88.4) | ||
| Age (yr) | mean ± SD | 61.11 ± 9.03 | 65.72 ± 10.73 | 64.03 ± 10.35 | 0.0092 |
| Mean (vmin-vmax) | 62.50 (37-84) | 65.00 (36-94) | 64.00 (36-94) | ||
| BMI class | BMI < 25 kg/m2 | 46 (85.2) | 78 (83.9) | 124 (84.4) | 0.8331 |
| BMI > 25 kg/m2 | 8 (14.8) | 15 (16.1) | 23 (15.6) | ||
| Pre-surgical ECOG§ | Score 0 | 50 (92.6) | 79 (84.9) | 129 (87.8) | 0.1731 |
| Score 1 | 4 (7.4) | 14 (15.1) | 18 (12.2) | ||
| Diabetes | No | 39 (72.2) | 67 (72.0) | 106 (72.1) | 0.9811 |
| Yes | 15 (27.8) | 26 (28.0) | 41 (27.9) | ||
| Cardiovascular diseases | No | 21 (38.9) | 34 (36.6) | 55 (37.4) | 0.7781 |
| Yes | 33 (61.1) | 59 (63.4) | 92 (62.6) | ||
The absolute number of respiratory complications was higher in patients undergoing thoracoscopy esophagectomy, although no significant difference was observed between groups A and B. The most frequent respiratory complications involved segmental atelectasis. One patient experienced a residual pneumothorax, probably related to low flow of the peripheral air fistula.
Temporary paralysis of vocal cords, translated by hoarseness and surgical site infections, were more frequent in group A (both with P = 0.017).
Most infectious complications were related to atelectasis, complicated by bronchopneumonia (with diagnosis made through radiological findings, laboratory tests, and clinical evaluation).
Mortality within days was similar between the two groups. In group A, one death was reported due to cervical fistula with drainage to the mediastinum, while another was due to acute myocardial infarction. In group B, two deaths were related to cardiogenic shock. One patient died of massive bronchoaspiration, and one due to a fistula to the mediastinum.
Table 2 shows the main complications and mortality observed for the total number of patients in both groups.
| Group | Total | P value | |||
| Thoracoscopygroup A | Transhiatalgroup B | ||||
| n = 54, n (%) | n = 93, n (%) | n = 147 , n (%) | |||
| Complications | No | 27 (50.0) | 60 (64.5) | 87 (59.2) | 0.0841 |
| Yes | 27 (50.0) | 33 (35.5) | 60 (40.8) | ||
| Fistulae | No | 48 (88.9) | 80 (86.0) | 128 (87.1) | 0.6171 |
| Yes | 6 (11.1) | 13 (14.0) | 19 (12.9) | ||
| Chylothorax | No | 53 (98.1) | 93 (100) | 146 (99.3) | 0.3672 |
| Yes | 1 (1.9) | 0 | 1 (0.7) | ||
| Respiratory disorders | No | 46 (85.2) | 85 (91.4) | 131 (89.1) | 0.2441 |
| Yes | 8 (14.8) | 8 (8.6) | 16 (10.9) | ||
| Hoarseness | No | 50 (92.6) | 93 (100) | 143 (97.3) | 0.0172 |
| Yes | 4 (7.4) | 0 | 4 (2.7) | ||
| Infections | No | 50 (92.6) | 91 (97.9) | 143 (97.3) | 0.0172 |
| Yes | 4 (7.4) | 2 (2.1) | 4 (2.7) | ||
| Mortality | 2 (3.7) | 4 (4.3) | 6 (4.08%) | 0.3422 | |
In group A, 15 to 73 lymph nodes were resected (mean 31.89 + 8.2) and 1 to 25 Lymph nodes were affected (mean 3.96 + 1.7). In Group B, 14 to 48 Lymph nodes were resected (mean 20.73 + 7); 1 to 14 Lymph nodes were affected (mean 4.25 + 1).
The number of resected lymph nodes in group A was higher (P < 0.001). There was no difference in the number of lymph nodes affected (P = 0.721) or the DL/AL ratio in both groups (P = 0.666). The data regarding resected lymph nodes are summarized in Table 3.
| Group | Total | P value | |||
| Thoracoscopygroup A | Transhiatalgroup B | ||||
| n = 54, n (%) | n = 93, n (%) | n = 147, n (%) | |||
| Dissected lymph nodes | mean ± SD | 31.89 ± 17.65 | 20.73 ± 12.70 | 24.83 ± 15.62 | < 0.0011 |
| Metastatic lymph nodes | Median (vmin-vmax) | 30 (3-73) | 19 (2-85) | 22 (2-85) | |
| Median (vmin-vmax) | 2 (0-25) | 1 (0-34) | 1 (0-34) | ||
| AL/DL (%) | mean ± SD | 15.59 (21.44) | 20.56 (28.12) | 18.73 (25.90) | 0.6961 |
| Median (vmin-vmax) | 5.86 (0-92.31) | 5.88 (0-97.14) | 5.88 (0-97.14) | ||
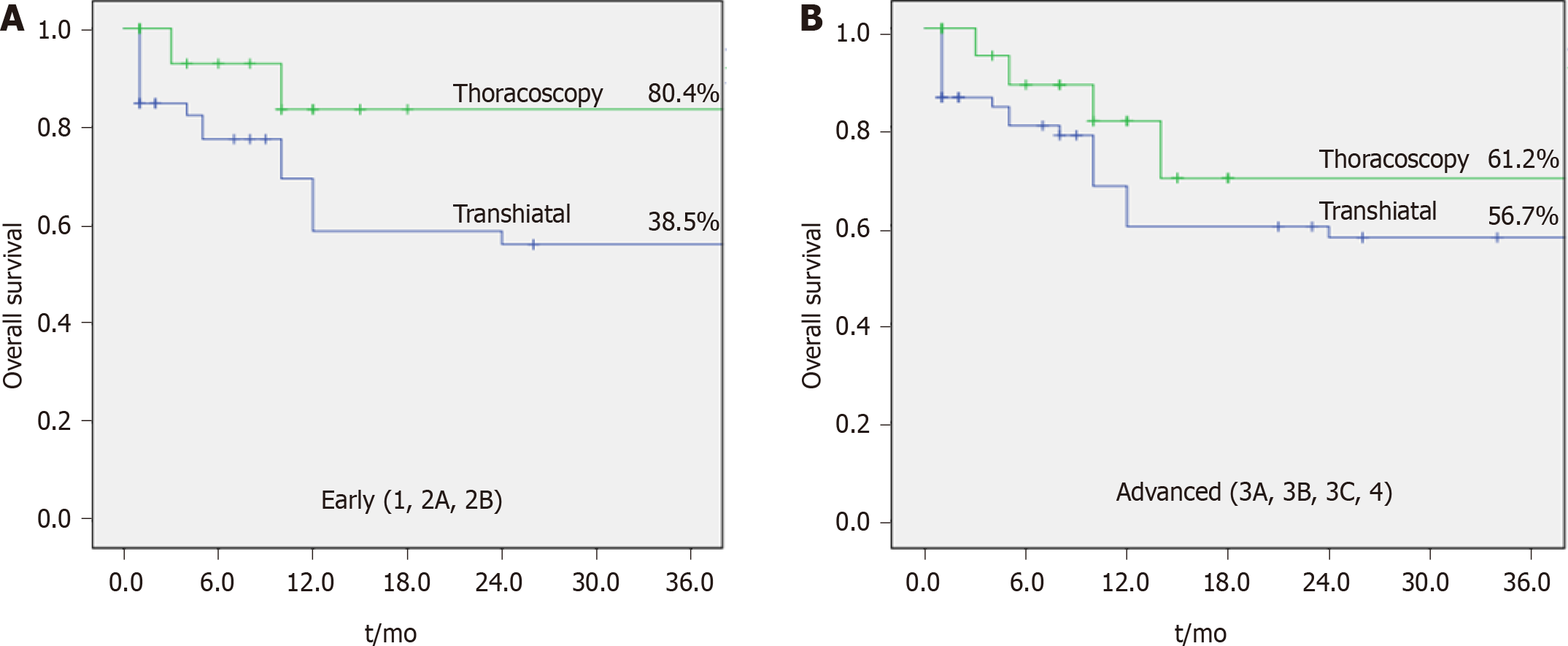
With regard to OS and disease-free survival (DFS), there is no statistically significant difference between groups (Table 4). However, when results are analyzed by clinical stage, longer survival is observed in patients with earlier disease (up to stage 2B), undergoing thoracoscopic esophagectomy (P = 0.001, Figure 2 and Table 4).
| Disease-free survival | Univariate analysis | Multivariate analysis | ||||
| Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Male (vs female) | 0.99 | 0.47–2.08 | 0.975 | |||
| Age (< 62 yr vs > 62 yr) | 0.87 | 0.56–3.14 | 0.873 | |||
| Siewert 1 vs 2 | 1.11 | 0.14–8.89 | 0.921 | |||
| TH vs TT (1, 2A) | 1.71 | 1.01–2.90 | 0.046 | 1.73 | 1.00–2.99 | 0.049 |
| Post-operative complications | 1.22 | 0.56-2.06 | 0.961 | |||
| G3 (vs G1/G2) | 1.14 | 0.61–2.13 | 0.690 | |||
| LN+/LN- | 2.61 | 1.71-3.56 | 0.001 | 1.77 | 0.99-3.24 | 0.101 |
| pT3/pT4 status (vs pT0/T1/pT2) | 2.21 | 1.86–7.31 | 0.003 | 1.56 | 0.97-3.89 | 0.102 |
| pN+ (vs pN0) | 2.54 | 1.57-5.78 | 0.05 | 1.43 | 0.88-3.32 | 0.103 |
| Pathological exam | ||||||
| Lymphatic | 0.78 | 0.39-1.29 | 0.783 | |||
| Venous | 1.67 | 0.35-2.72 | 0.246 | |||
| Neural | 0.78 | 0.67-1.89 | 0.183 | |||
| Overall survival | Univariate analysis | Multivariate analysis | ||||
| Variables | HR | 95%CI | P value | HR | 95%CI | P value |
| Age (< 62 yr vs > 62 yr) | 0.98 | 0.89-5.13 | 0.821 | |||
| Siewert 1 vs 2 | 1.31 | 0.16-10.68 | 0.799 | |||
| TH vs TT (1, 2A) | 2.01 | 1.19-3.39 | 0.009 | 1.79 | 1.03-3.09 | 0.038 |
| Post-operative complications | 1.03 | 0.60-1.74 | 0.927 | |||
| G3 (vs G1/G2) | 2.37 | 1.36-4.16 | 0.003 | 2.54 | 1.33-4.82 | 0.005 |
| LN+/LN- | 1.72 | 1.00-3.48 | 0.050 | 1.21 | 0.87-3.46 | 0.732 |
| pT3/pT4 status (vs pT0/T1/pT2) | 5.95 | 1.81-19.61 | 0.003 | 9.96 | 2.43-40.74 | 0.001 |
| pN+ (vs pN0) | 1.68 | 1.38-3.90 | 0.002 | 1.18 | 0.86-4.99 | 0.735 |
| Pathological exam | ||||||
| Lymphatic | 0.47 | 0.23-1.78 | 0.109 | |||
| Venous | 0.49 | 0.20-1.06 | 0.076 | |||
| Neural | 1.80 | 0.96-3.35 | 0.065 | |||
Other factors associated with OS in the univariate analysis include transhiatal approach, grade 3, metastatic lymph node, pT3/4, and lymphatic invasion in the tumor specimen. The multivariable analysis demonstrated better results related to transhiatal access in early staging tumors, hazard ratio 1.73 (95%CI: 1.00-2.99, P = 0.049). Factors associated to DFS were: transhiatal approach, metastatic lymph node, pT3/4, and lymphatic invasion in the tumor specimen (Table 4).
AEGJ is one of the neoplasms with the highest global rate of increased incidence through the last years, associated with risk factors such as obesity and gastroesophageal reflux disease[16].
In Brazil and many Western countries, it is still a disease with a poor prognosis, mainly because about 65% are T3 or T4 at the time of diagnosis. Recently, Tustumi et al[3] published a cross-sectional study performed in our center, in which more than 550 patients with esophageal cancer had an OS rate of 20.2% for AEGJ (types I, II, and III). The percentage of curative-intent surgery in AEGJ was 30.4%, with a mean survival rate of 58% after five years follow-up.
Several factors associated with treatment contributed to improved survival of patients with AEGJ in recent years, among them, neoadjuvant treatment stands out[7,17]. Based on the most recent data, neoadjuvant chemotherapy and radiotherapy (similar to the CROSS trial) were performed for esophageal tumors and for both pre- and postoperative chemotherapy in patients with predominantly gastric tumors.
Regarding surgical approach, transhiatal esophagectomy was initially performed by Akiyama et al[18] in Japan in 1975; Orringer et al[19] in the United States in 1978; and Pinotti[15] in Brazil in 1976, which was the preferred approach for AEGJ. Several studies suggest fewer pulmonary complications than the transthoracic approach, despite a limited surgical view and difficult mediastinal lymph node resection; it became the preferred access route in AEGJ in Siewert types I and II at our institution for over twenty-five years. After the introduction of minimally invasive surgery with thoracoscopic access and standardization of the thoracic lymphadenectomy, and reasonable morbidity results[17], we modified our approach in types I and II AEGJ to transthoracic by thoracoscopy.
It is well-known that post-operative complications after esophagectomy are associated with a worse prognosis[20]. In particular, a higher incidence of respiratory infections (pneumonia and tracheobronchitis) is described in patients undergoing thoracoscopy, due to the fact that there is selective intubation and a longer duration of mechanical ventilation. We also observed this in our series, with respiratory complications occurring in 10.9% of patients in group A.
Another complication with an exclusive incidence in group A was hoarseness, probably secondary to mediastinal lymphadenectomy-with consequent manipulation of recurrent laryngeal nerves. In all, four cases were reported in our series. Of these, none evolved with severe speech dysfunction or bronchoaspiration, or the need for a tracheostomy.
The main surgical complication of both surgeries was anastomotic fistula. In this study, it was observed in 12.9% of cases, with no statistical difference between groups. Its prevalence ranges from 15.8% to 30%; although it is accompanied by low mor
Regarding surgery-related mortality rate, this study reported 3.7% in the thoracoscopy group and 4.3% in the transhiatal group, with 4.0% overall mortality, showing acceptable results compared to rates up to 15.4% as reported in a systematic review[22].
The number of lymph nodes resected by thoracoscopy was higher (31.89 lymph nodes on average) than transhiatal (20.73 lymph nodes on average), with a significant statistical difference (P < 0.001). However, the number of affected nodes were similar.
With regard to long-term results, what was previously known is that both the transhiatal and transthoracic techniques resulted in similar oncological outcomes, with a tendency for greater perioperative morbidity with the transthoracic pathway[22-24], which is similar to our results.
However, when we analyzed OS and DFS for each clinical stage in isolation, we observed a trend of encouraging results in group A in the earlier stages (up to 2B).
Despite the close follow-up, this study has limitations such as the retrospective design and thus, patients were not randomly selected. There were some disparities in the neoadjuvant treatment between groups (87% in thoracoscopic vs 46% in transhiatal) which may be considered a limitation. However, the study aimed to assess overall survival on AEGJ tumors considering a cohort of patients in a “real-world” setting. The neoadjuvant therapy was indicated just in patients > 3A staged. Therefore, neoadjuvant treatment did not interfere in the early stage subgroup analysis. Regarding advanced stages, we believe that the possible limitation related to the difference between groups receiving neoadjuvant chemotherapy was minimized by the multivariate analysis.
Both esophagectomy approaches have low morbidity and mortality, given the magnitude of the procedures. Hoarseness and infectious complications were more significant in transthoracic esophagectomy by thoracoscopy. However, it allowed the resection of a more significant number of lymph nodes. In addition, this method is apparently associated with higher OS and DFS at earlier stages and may be a better approach. Further studies are required to confirm our findings.
Extension of lymphadenectomy during esophagectomy is on debate for adenocarcinoma of the esophagogastric junction. Thoracoscopic transthoracic access is consider superior regarding retrieved lymphonodes comparing to transhiatal esophagectomy, but overall survival is questionable.
To understand the relationship between extension of lymphadenectomy and survival according to type of surgical approach.
To compare outcomes after thoracoscopic esophagectomy and transhiatal approach for adenocarcinoma of the esophagogastric junction.
Retrospective review of medical records of patients were assessed. A total of 147 patients with adenocarcinoma of the esophagogastric junction were selected from 2002 to 2019, and divided into group A (thoracoscopic esophagectomy), and group B (transhiatal esophagectomy). Overall survival (OS), disease-free survival, post
Concerning the extent of lymphadenectomy, group A showed a higher number of retrieved lymph nodes (mean of 31.89 ± 8.2 vs 20.73 ± 7; P < 0.001), with more perioperative complications, such as hoarseness, surgical site infections, and respiratory complications. Although both groups had similar OS rates, subgroup analysis showed better survival of transthoracic esophagectomy in patients with earlier diseases.
Both methods are safe, having similar morbidity and mortality rates. Transthoracic thoracoscopic esophagectomy allows a more extensive resection of the lymph nodes and may have better oncological outcomes during earlier stages of the disease.
Prospective randomized trials addressing topics as long-term survival, the role of neoadjuvant therapies and costs.
| 1. | Parkin DM, Pisani P, Ferlay J. Estimates of the worldwide incidence of 25 major cancers in 1990. Int J Cancer. 1999;80:827-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 2. | Sobin LH, Wittekind C. International Union Against Cancer (UICC): TNM Classification of Malignant Tumors. 5th ed. New York: John Wiley and Sons, 1997. |
| 3. | Tustumi F, Takeda FR, Kimura CM, Sallum RA, Ribeiro U Junior, Cecconello I. ESOPHAGEAL CARCINOMA: IS SQUAMOUS CELL CARCINOMA DIFFERENT DISEASE COMPARED TO ADENOCARCINOMA? Arq Gastroenterol. 2016;53:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Matsuno K, Ishihara R, Ohmori M, Iwagami H, Shichijyo S, Maekawa A, Kanesaka T, Yamamoto S, Takeuchi Y, Higashino K, Uedo N, Matsunaga T, Morishima T, Miyashiro I. Time trends in the incidence of esophageal adenocarcinoma, gastric adenocarcinoma, and superficial esophagogastric junction adenocarcinoma. J Gastroenterol. 2019;54:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Kurokawa Y, Takeuchi H, Doki Y, Mine S, Terashima M, Yasuda T, Yoshida K, Daiko H, Sakuramoto S, Yoshikawa T, Kunisaki C, Seto Y, Tamura S, Shimokawa T, Sano T, Kitagawa Y. Mapping of Lymph Node Metastasis From Esophagogastric Junction Tumors: A Prospective Nationwide Multicenter Study. Ann Surg. 2021;274:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 179] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 6. | Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392-400; discussion 400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 407] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 7. | Noordman BJ, van Klaveren D, van Berge Henegouwen MI, Wijnhoven BPL, Gisbertz SS, Lagarde SM, van der Gaast A, Hulshof MCCM, Biermann K, Steyerberg EW, van Lanschot JJB; also on behalf of the CROSS-study group. Impact of Surgical Approach on Long-term Survival in Esophageal Adenocarcinoma Patients With or Without Neoadjuvant Chemoradiotherapy. Ann Surg. 2018;267:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Lerut T, Nafteux P, Moons J, Coosemans W, Decker G, De Leyn P, Van Raemdonck D, Ectors N. Three-field lymphadenectomy for carcinoma of the esophagus and gastroesophageal junction in 174 R0 resections: impact on staging, disease-free survival, and outcome: a plea for adaptation of TNM classification in upper-half esophageal carcinoma. Ann Surg. 2004;240:962-72; discussion 972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 290] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 9. | Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg. 2002;236:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 308] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 10. | Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234:360-7; discussion 368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 380] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Lerut T, De Leyn P, Coosemans W, Van Raemdonck D, Scheys I, LeSaffre E. Surgical strategies in esophageal carcinoma with emphasis on radical lymphadenectomy. Ann Surg. 1992;216:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 221] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 12. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H, Tilanus HW, van Lanschot JJ. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1151] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 13. | Rice TW, Rusch VW, Apperson-Hansen C, Allen MS, Chen LQ, Hunter JG, Kesler KA, Law S, Lerut TE, Reed CE, Salo JA, Scott WJ, Swisher SG, Watson TJ, Blackstone EH. Worldwide esophageal cancer collaboration. Dis Esophagus. 2009;22:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 272] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Jung MK, Schmidt T, Chon SH, Chevallay M, Berlth F, Akiyama J, Gutschow CA, Mönig SP. Current surgical treatment standards for esophageal and esophagogastric junction cancer. Ann N Y Acad Sci. 2020;1482:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Pinotti HW. [Extrapleural approach to the esophagus through frenolaparatomy]. AMB Rev Assoc Med Bras. 1976;22:57-60. [PubMed] |
| 16. | Lagergren J, Bergström R, Lindgren A, Nyrén O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N Engl J Med. 1999;340:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2115] [Cited by in RCA: 2041] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 17. | Harada G, Bonadio RRDCC, de Araújo FCC, Victor CR, Sallum RAA, Junior UR, Cecconello I, Takeda FR, de Castria TB. Induction Chemotherapy for Locally Advanced Esophageal Cancer. J Gastrointest Cancer. 2020;51:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Akiyama H, Hiyama M, Miyazono H. Total esophageal reconstruction after extraction of the esophagus. Ann Surg. 1975;182:547-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 56] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg. 1978;76:643-654. [PubMed] |
| 20. | Kataoka K, Takeuchi H, Mizusawa J, Igaki H, Ozawa S, Abe T, Nakamura K, Kato K, Ando N, Kitagawa Y. Prognostic Impact of Postoperative Morbidity After Esophagectomy for Esophageal Cancer: Exploratory Analysis of JCOG9907. Ann Surg. 2017;265:1152-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 21. | Bhasin DK, Sharma BC, Gupta NM, Sinha SK, Singh K. Endoscopic dilation for treatment of anastomotic leaks following transhiatal esophagectomy. Endoscopy. 2000;32:469-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Blencowe NS, Strong S, McNair AG, Brookes ST, Crosby T, Griffin SM, Blazeby JM. Reporting of short-term clinical outcomes after esophagectomy: a systematic review. Ann Surg. 2012;255:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 234] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 23. | Fujiwara H, Shiozaki A, Konishi H, Komatsu S, Kubota T, Ichikawa D, Okamoto K, Morimura R, Murayama Y, Kuriu Y, Ikoma H, Nakanishi M, Sakakura C, Otsuji E. Hand-assisted laparoscopic transhiatal esophagectomy with a systematic procedure for en bloc infracarinal lymph node dissection. Dis Esophagus. 2016;29:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Mori K, Yamagata Y, Aikou S, Nishida M, Kiyokawa T, Yagi K, Yamashita H, Nomura S, Seto Y. Short-term outcomes of robotic radical esophagectomy for esophageal cancer by a nontransthoracic approach compared with conventional transthoracic surgery. Dis Esophagus. 2016;29:429-434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Gastrointestinal Endoscopy; Sociedade Brasileira de Endoscopia Digestiva; Colégio Brasileiro de Cirurgiões.
Specialty type: Surgery
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Laracca GG S-Editor: Zhang H L-Editor: A P-Editor: Wang LYT