Published online May 16, 2021. doi: 10.4253/wjge.v13.i5.137
Peer-review started: December 11, 2020
First decision: January 29, 2021
Revised: February 9, 2021
Accepted: April 11, 2021
Article in press: April 11, 2021
Published online: May 16, 2021
Processing time: 147 Days and 5.5 Hours
In an effort to further reduce the morbidity and mortality profile of laparoscopic cholecystectomy, the outcomes of such procedure under regional anesthesia (RA) have been evaluated. In the context of cholecystectomy, combining a minimally invasive surgical procedure with a minimally invasive anesthetic technique can potentially be associated with less postoperative pain and earlier ambulation.
To evaluate comparative outcomes of RA and general anesthesia (GA) in patients undergoing laparoscopic cholecystectomy.
A comprehensive systematic review of randomized controlled trials with subsequent meta-analysis and trial sequential analysis of outcomes were conducted in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement standards.
Thirteen randomized controlled trials enrolling 1111 patients were included. The study populations in the RA and GA groups were of comparable age (P = 0.41), gender (P = 0.98) and body mass index (P = 0.24). The conversion rate from RA to GA was 2.3%. RA was associated with significantly less postoperative pain at 4 h [mean difference (MD): - 2.22, P < 0.00001], 8 h (MD: -1.53, P = 0.0006), 12 h (MD: -2.08, P < 0.00001), and 24 h (MD: -0.90, P < 0.00001) compared to GA. Moreover, it was associated with significantly lower rate of nausea and vomiting [risk ratio (RR): 0.40, P < 0.0001]. However, RA significantly increased postoperative headaches (RR: 4.69, P = 0.03), and urinary retention (RR: 2.73, P = 0.03). The trial sequential analysis demonstrated that the meta-analysis was conclusive for most outcomes, with the exception of a risk of type 1 error for headache and urinary retention and a risk of type 2 error for total procedure time.
Our findings indicate that RA may be an attractive anesthetic modality for day-case laparoscopic cholecystectomy considering its associated lower postoperative pain and nausea and vomiting compared to GA. However, its associated risk of urinary retention and headache and lack of knowledge on its impact on procedure-related outcomes do not justify using RA as the first line anesthetic choice for laparoscopic cholecystectomy.
Core Tip: Despite the existence of solid level 1 evidence from multiple randomized controlled trials on comparative outcomes of general anesthesia and regional anesthesia (RA) in laparoscopic cholecystectomy and demonstration of feasibility of laparoscopic cholecystectomy under RA, lack of knowledge on the impact of RA on specific procedure related outcomes may discourage surgeons from selecting RA as the first choice of anesthesia for laparoscopic cholecystectomy. Considering our findings, we encourage use of RA in patients who are not fit for general anesthesia but do not hesitate to highlight that available evidence does not justify using RA as the first line anesthetic choice for laparoscopic cholecystectomy.
- Citation: Asaad P, O’Connor A, Hajibandeh S, Hajibandeh S. Meta-analysis and trial sequential analysis of randomized evidence comparing general anesthesia vs regional anesthesia for laparoscopic cholecystectomy. World J Gastrointest Endosc 2021; 13(5): 137-154
- URL: https://www.wjgnet.com/1948-5190/full/v13/i5/137.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i5.137
Gallstone disease is thought to occur in approximately 15% of the population of whom 20% are symptomatic[1]. Laparoscopic cholecystectomy is the gold standard treatment for symptomatic gallstone disease and one of the most commonly performed general surgical procedures[1]. This minimally invasive procedure results in a shorter length of hospital stay and quicker overall recovery compared with the traditional open approach[2].
Traditionally, laparoscopic cholecystectomy is carried out under general anesthesia (GA). Some argue the endotracheal intubation is required to prevent aspiration or respiratory complications secondary to the induction of pneumoperitoneum[3]. Furthermore, GA is associated with rapid onset of action and reduces the procedure related stress[4].
In an effort to further reduce the morbidity and mortality profile of laparoscopic cholecystectomy, the outcomes of such procedure under regional anesthesia (RA) have been evaluated[5]. RA, including spinal anesthesia (SA) and epidural anesthesia (EA), confers the advantages of avoidance of both paralytic agents and endotracheal intubation[6]. Although combining a minimally invasive surgical procedure with a minimally invasive anesthetic technique would appear attractive, it’s use is currently limited[7]. Nevertheless, it has been demonstrated that the use of neuraxial anesthetics decreases postoperative thromboembolic events, myocardial infarction as well as overall mortality[8]. Moreover, RA has been demonstrated to be associated with less postoperative pain and earlier ambulation in patients undergoing laparoscopic cholecystectomy[7].
The purpose of our study was to conduct a comprehensive review of the current literature and conduct a meta-analysis of randomized trials to evaluate comparative outcomes of RA and GA in patients undergoing laparoscopic cholecystectomy. Furthermore, we aimed to conduct a trial sequential analysis to assess the robustness of our meta-analysis findings.
We highlighted our eligibility criteria, methods, and evaluated outcomes in a review protocol. Our study was carried out in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement standards[9].
(1) Randomized controlled trials (RCTs); (2) Including patients aged > 18 years old of any gender; (3) Including patients undergoing laparoscopic cholecystectomy under RA; and (4) Comparing laparoscopic cholecystectomy under GA.
(1) Observational studies, case series, case reports, and letters; (2) Including patients undergoing open cholecystectomy; and (3) Including patients undergoing laparoscopic intraoperative cholangiogram with or without common bile duct exploration.
Primary outcome measures were defined as the post-operative pain intensity assessed on a 10 mm visual analogue scale (VAS) at 4 h, 6 h, 12 h and 24 h. The pain intensity data described by other means than a 10 mm VAS were standardized to such a scale. Operative time, total operative and anesthetic time, urinary retention (defined as inability to urinate spontaneously during the early postoperative period requiring application of heat or urinary catheterization), nausea and vomiting, headache, and hypotension (defined as a reduction of > 30% in mean arterial pressure or systolic blood pressure < 90 mmHg) were the secondary outcome parameters.
Three authors independently searched the following electronic databases: MEDLINE, EMBASE, CINAHL, and the Cochrane Central Register of Controlled Trials (CENTRAL). The literature search was performed on 08 March 2019. Our search strategy was adapted according to thesaurus headings, search operators and limits in the aforementioned databases (Supplementary Table 1). Furthermore, we searched World Health Organization International Clinical Trials Registry (http://apps.who.int/trialsearch/), ClinicalTrials.gov (http://clinicaltrials.gov/), and ISRCTN Register (http://www.isrctn.com/) to identify ongoing and unpublished studies. Moreover, the reference lists of identified articles were screened for further potentially eligible trials.
The yielded search results were evaluated by two reviewers. Following evaluation of their titles, abstracts and full-texts of identified articles, those studies that met the inclusion criteria of our study were selected for inclusion in data synthesis. Disagreements in selection of studies were resolved by discussion between the reviewers. However, if the discrepancies remained unresolved, a third reviewer was involved.
We created an electronic data extraction spreadsheet according to the Cochrane's recommendations for intervention reviews. The data extraction spreadsheet was pilot-tested in randomly selected articles and adjusted accordingly. The following information were extracted from the included studies by two independent authors: (1) Study-related data (first author, publication year, country of origin of the corresponding author, journal in which the study was published, study design, and study size); (2) Baseline demographic and clinical information of the study populations (age, gender, weight, height, body mass index, American Society of Anesthesiologists classification); (3) Type of anesthetic agent used in the RA group or any additional medications used, conversion from SA to GA; (4) Primary and secondary outcome data; and (5) Disagreements during data extraction and management were resolved following consultation with a third independent author.
The methodological quality and risk of bias assessment were carried out by two authors using the Cochrane's tool[10]. The Cochrane’s tool classifies studies into low, unclear and high risk of bias following evaluating and determining the risk of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other sources of bias. We resolved discrepancies in risk of bias assessment by discussion between the assessing authors. Nevertheless, if no agreement could be reached, a third reviewer was involved as an adjudicator.
For urinary retention, nausea and vomiting, and headache we calculated the risk ratio (RR) as the summary measures. The RR is the risk of an adverse event in the RA group compared to the GA group. An RR of less than one would favor the SA group. For VAS score at 4 h, 6 h, 12 h and 24 h, operative time, and total operative and anesthetic time we calculated the mean difference (MD) between the two groups.
The number of individual patients was used as the unit of analysis for all outcome parameters. Information with regards to dropouts, withdrawals and any other missing data were recorded. We planned to contact authors of the included studies where information about our outcome of interest was not reported. Our final analysis respected the intention-to-treat concept.
One independent review author entered the extracted data into Review Manager 5.3 software for data synthesis[10]. The entered data were subsequently checked by a second independent review author. Random-effects or fixed-effect modelling were used, as appropriate, for analysis. Only when significant between-study heterogeneity existed, random-effects models were applied. This has previously been defined by Higgins et al[10]. We reported the results of our analysis for each outcome parameter in a forest plot with 95% confidence intervals (CIs).
Heterogeneity among the studies was assessed using the Cochran Q test (χ2). We quantified inconsistency by calculating I2 and interpreted it using the following guide: 0% to 25% might not be important; 25% to 75%: may represent moderate heterogeneity; 75% to 100% may represent substantial heterogeneity. Moreover, where more than 10 studies were available in analysis of an outcome parameter, funnel plots were planned to be constructed in order to assess their symmetry to visually evaluate publication bias.
We conducted sensitivity analyses to explore potential sources of heterogeneity and assess the robustness of our results. For each outcome parameter, we repeated the primary analysis using random-effects or fixed-effect models. Moreover, for each of our defined dichotomous variable, we calculated the pooled odds ratio or risk difference. Finally, we evaluated the effect of each study on the overall effect size and heterogeneity by repeating the analysis following excluding one study at a time.
Trial sequential analysis was performed for the outcomes reported by at least 5 trials using the trial sequential analysis software 0.9.5.5 Beta (Copenhagen Trial Unit, Copenhagen, Denmark). In order to control the risk of type 1 error, we planned to adjust the thresholds for the Z values using O’Brien-Fleming α-spending function; allowing the type I error risk to be restored to the desired maximum risk. Crossing the O’Brien-Fleming α-spending boundaries by a Z-curve would indicate statistical significance. Moreover, we penalised the Z values according to the strength of the available evidence and the number of repeated significance tests as defined by the law of the iterated logarithm. The risk of type 2 error was controlled using the β-spending function and futility boundaries. Crossing the futility boundaries by a Z-curve would indicate that the two interventions do not differ more than the anticipated intervention effect. Random or fixed effects modelling were applied as appropriate for the analyses. We handled the zero event trials by constant continuity correction which involved adding a continuity correction factor to the number of events and non-events in each intervention group. A two-sided CI with 95% confidence level was used to indicate statistical significance. We estimated the information size for the analyses based on achievement of 80% power and 10% relative risk reduction between the two groups.
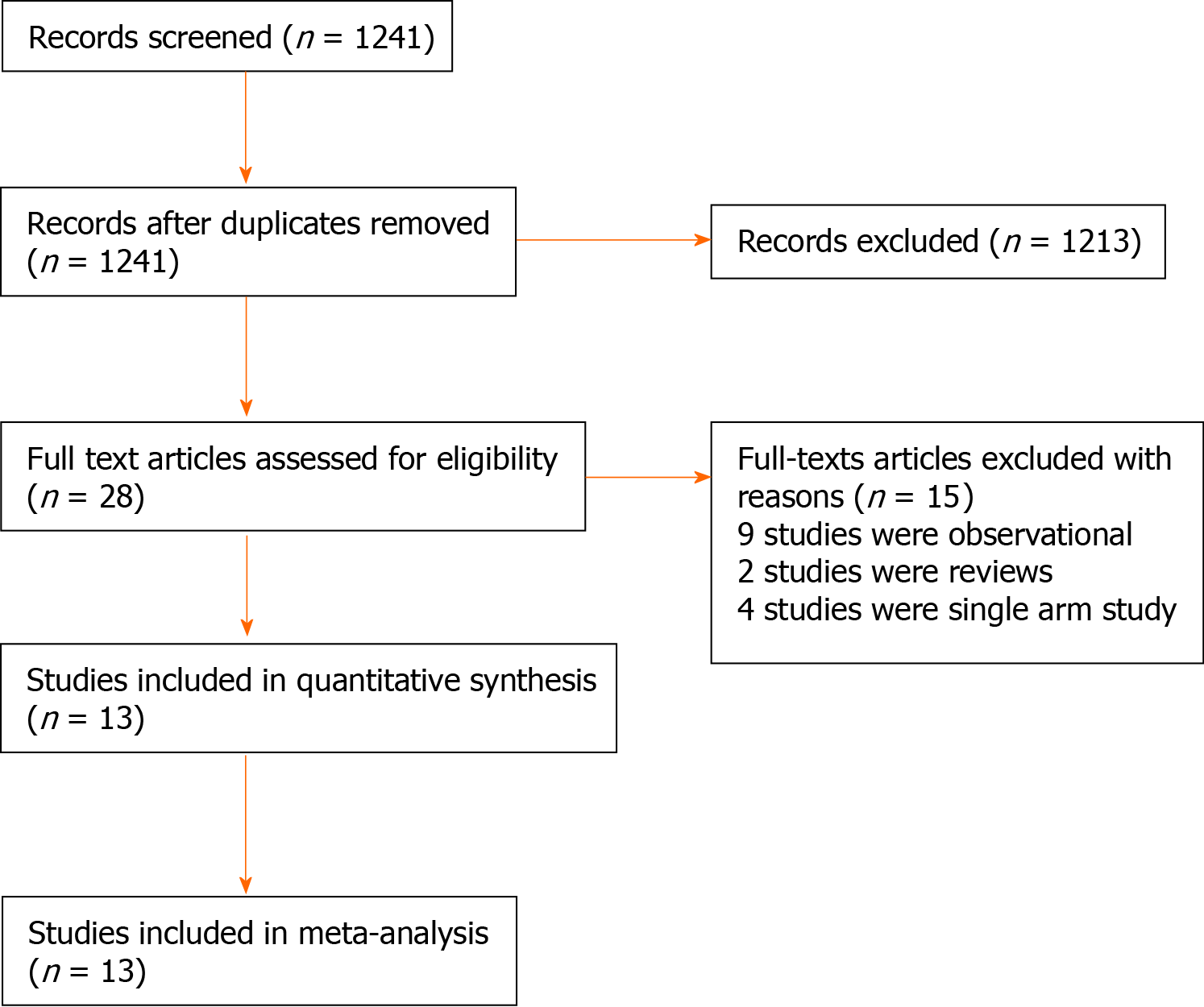
The literature search identified 1267 articles. After further evaluation of the identified articles, 13 RCTs[4,5,11-21] met our inclusion criteria (Figure 1). The included studies reported the outcomes of 1111 patients of whom 554 patients underwent laparoscopic cholecystectomy under RA and the remaining 557 patients had laparoscopic cholecystectomy under GA.
The date of publication and country of origin, journal, and study design of the included studies are presented in Table 1. Table 2 presents baseline demographic and clinical characteristics of the study populations. There was no significant difference in mean age (P = 0.41), gender (P = 0.98) and body mass index (P = 0.24) between two groups. There were 13 conversion from RA to GA. Table 3 demonstrates details of anesthetic agent used in the RA group in the included studies
| Ref. | Year | Country | Journal | Design | Total number of patients | GA | RA |
| Majedi et al[15] | 2019 | Iran | Advanced Biomedical Research | RCT | 80 | 40 | 40 |
| Sharaf et al[19] | 2018 | Pakistan | Anaesthesia, Pain and Intensive Care | RCT | 120 | 60 | 60 |
| Donmez et al[11] | 2017 | Turkey | Annals of Surgical Treatment and Research | RCT | 49 | 25 | 24 |
| Kalaivani et al[14] | 2014 | India | Journal of Clinical and Diagnostic Research | RCT | 50 | 25 | 25 |
| Prasad et al[17] | 2014 | India | Journal of Evolution of Medical and Dental Sciences | RCT | 60 | 30 | 30 |
| Ellakany et al[12] | 2013 | Egypt | Egyptian Journal of Anaesthesia | RCT | 40 | 20 | 20 |
| Tiwari et al[20] | 2013 | India | Journal of Minimal Access Surgery | RCT | 235 | 114 | 110 |
| Bessa et al[5] | 2012 | Egypt | Journal of Laparoendoscopic and Advanced Surgical Techniques | RCT | 180 | 90 | 90 |
| Ross et al[18] | 2012 | United States | Surgical Endoscopy | RCT | 20 | 10 | 10 |
| Mehta et al[16] | 2010 | India | Anesthesia, Essays and Researches | RCT | 60 | 30 | 30 |
| Imbelloni et al[13] | 2010 | Brazil | Revista Brasileira de Anestesiologia | RCT | 68 | 33 | 35 |
| Bessa et al[21] | 2010 | Egypt | Journal of Laparoendoscopic and Advanced Surgical Techniques | RCT | 60 | 30 | 30 |
| Tzovaras et al[4] | 2008 | Greece | Archives of Surgery | RCT | 100 | 50 | 50 |
| Ref. | Age | Male:female ratio | BMI | ASA I: II: III | ||||
| GA | RA | GA | RA | GA | RA | GA | RA | |
| Majedi et al[15] | 50.1 ± 9.78 | 52.06 ± 15.03 | 14:26 | 16:24 | NR | NR | NR | NR |
| Sharaf et al[19] | 44.07 ± 5.62 | 42.57 ± 5.77 | 0:60 | 0:60 | 25.41 ± 2.36 | 26 ± 2.31 | 14:46:0 | 22:38:0 |
| Donmez et al[11] | 45 ± 13 | 45 ± 14 | 18:07 | 18:6 | 28.75 ± 4.5 | 30.63 ± 3.6 | 18:7:0 | 16:6:2 |
| Kalaivani et al[14] | 47.84 ± 10.49 | 45 ± 11.73 | 08:17 | 10:15 | NR | NR | NR | NR |
| Prasad et al[17] | 38.5 ± 9.83 | 35.06 ± 7.5 | 25:5 | 17:13 | 23.5 ± 1.98 | 22.96 ± 2.98 | 23:7:0 | 22:8:0 |
| Ellakany et al[12] | 44.3 ± 13.2 | 45.9 ± 13.6 | 07:13 | 8:12 | 30 ± 3.9 | 29.8 ± 4.1 | NR | NR |
| Tiwari et al[20] | 46.1 ± 12.9 | 45.07 ±13.19 | 16:98 | 13:96 | NR | NR | NR | NR |
| Bessa et al[5] | 44 (19-50) | 40 (16-50) | 8:82 | 11:79 | 29.1 (23.4-33.1) | 28.7 (22.8-34) | NR | NR |
| Ross et al[18] | 39.4 ± 11.7 | 44.9 ± 12.5 | 3:7 | 2:8 | 25.1 ± 4.6 | 26.1 ± 5.5 | 1:6:3 | 3:5:2 |
| Mehta et al[16] | 38.3 | 39.1 | 10:20 | 14:16 | NR | NR | NR | NR |
| Imbelloni et al[13] | 45.2 ± 12.1 | 41.1 ± 12.4 | 10:23 | 9:26 | NR | NR | NR | NR |
| Bessa et al[21] | 40.9 ± 11 | 41.4 ± 11.1 | 6:24 | 5:25 | 30.8 ± 6.6 | 31.3 ± 4.1 | NR | NR |
| Tzovaras et al[4] | 46 (26-65) | 44 (23-65) | 18:30 | 20:29 | 26 (19-30) | 25 (18-30) | 37:11:0 | 40:9:0 |
| Ref. | Anesthetic agent used |
| Majedi et al[15] | 18 mL of lidocaine 2% plus epinephrine (1:200000) plus 2 mL of sodium bicarbonate 8.4% and fentanyl 50 µg |
| Sharaf et al[19] | 15 mg of hyperbaric bupivicaine and 25 µg fentanyl |
| Donmez et al[11] | hyperbaric bupivicaine 16mg and fentanyl 10 micrograms |
| Kalaivani et al[14] | 15 mg of hyperbaric bupivicaine and 20 µg fentanyl |
| Prasad et al[17] | 15 mg of heavy bupivicaine and 25 µg fentanyl |
| Ellakany et al[12] | 5 mg plain bupivicaine and 25 µg fentanyl |
| Tiwari et al[20] | 12.5 mg to 17.5 mg of hyperbaric bupivicaine |
| Bessa et al[5] | 15 mg of hyperbaric bupivicaine and 20 mcg fentanyl |
| Ross et al[18] | 20-25 mL of lidocaine 2% |
| Mehta et al[16] | 0.3 mg/kg of hyperbaric bupivicaine 0.5% |
| Imbelloni et al[13] | 15 mg of hyperbaric bupivicaine and 20 µg fentanyl |
| Bessa et al[21] | 15 mg of hyperbaric bupivicaine and 20 µg fentanyl |
| Tzovaras et al[4] | 15 mg of hyperbaric bupivicaine, 0.25 mg morphine and 20 µg fentanyl |
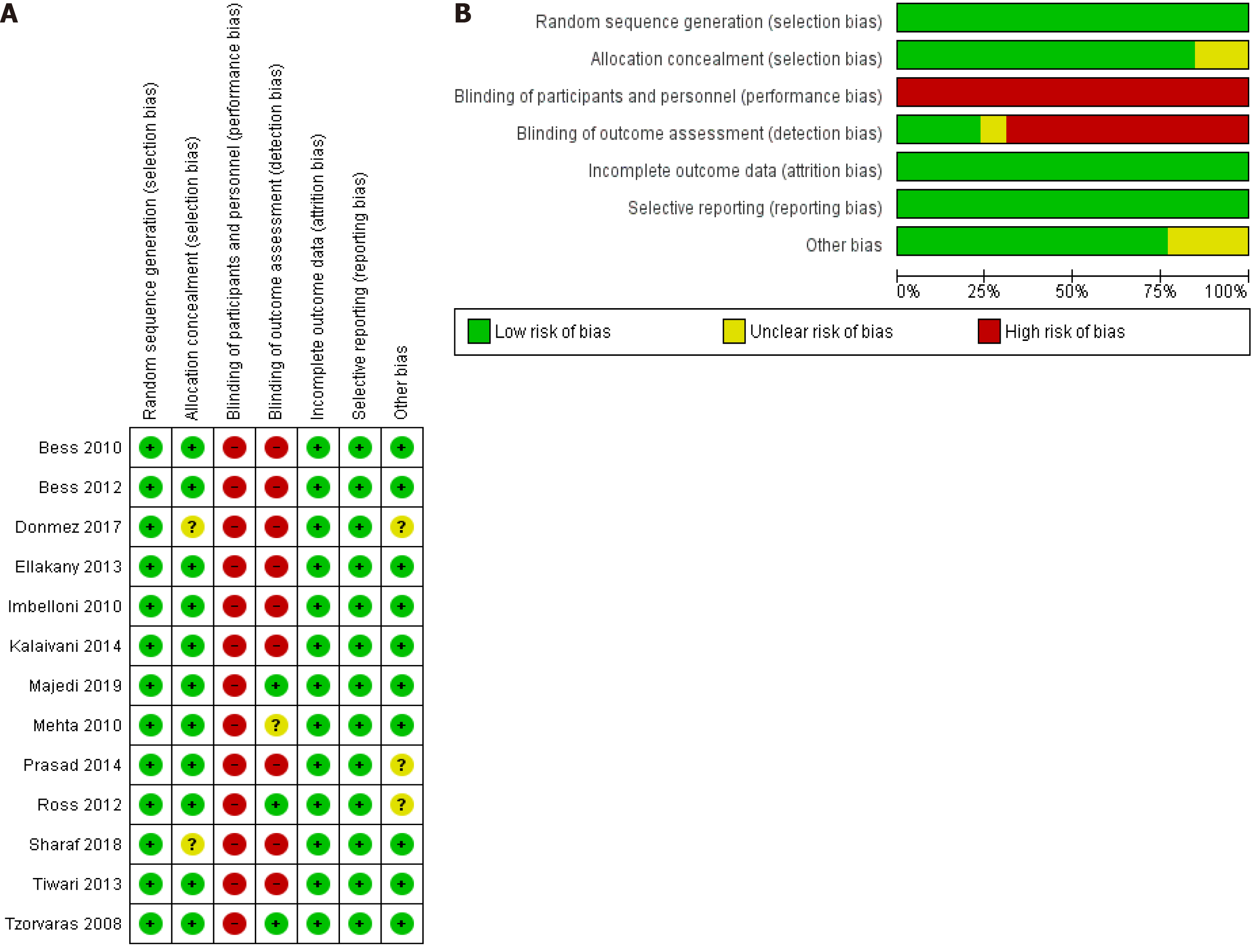
Figure 2 presents the risk of bias assessment of the included RCT. Eleven studies had low risk of selection bias and the remaining two had unclear risk of selection bias due to not providing information about the allocation concealment. All included studies had high risk of performance bias due to lack of blinding. Three studies had low risk of detection bias as they blinded the outcome assessor. However, 9 studies had high risk of such bias. All included studies had low risk of attrition and reporting bias.
Outcomes are summarized in Figure 3.
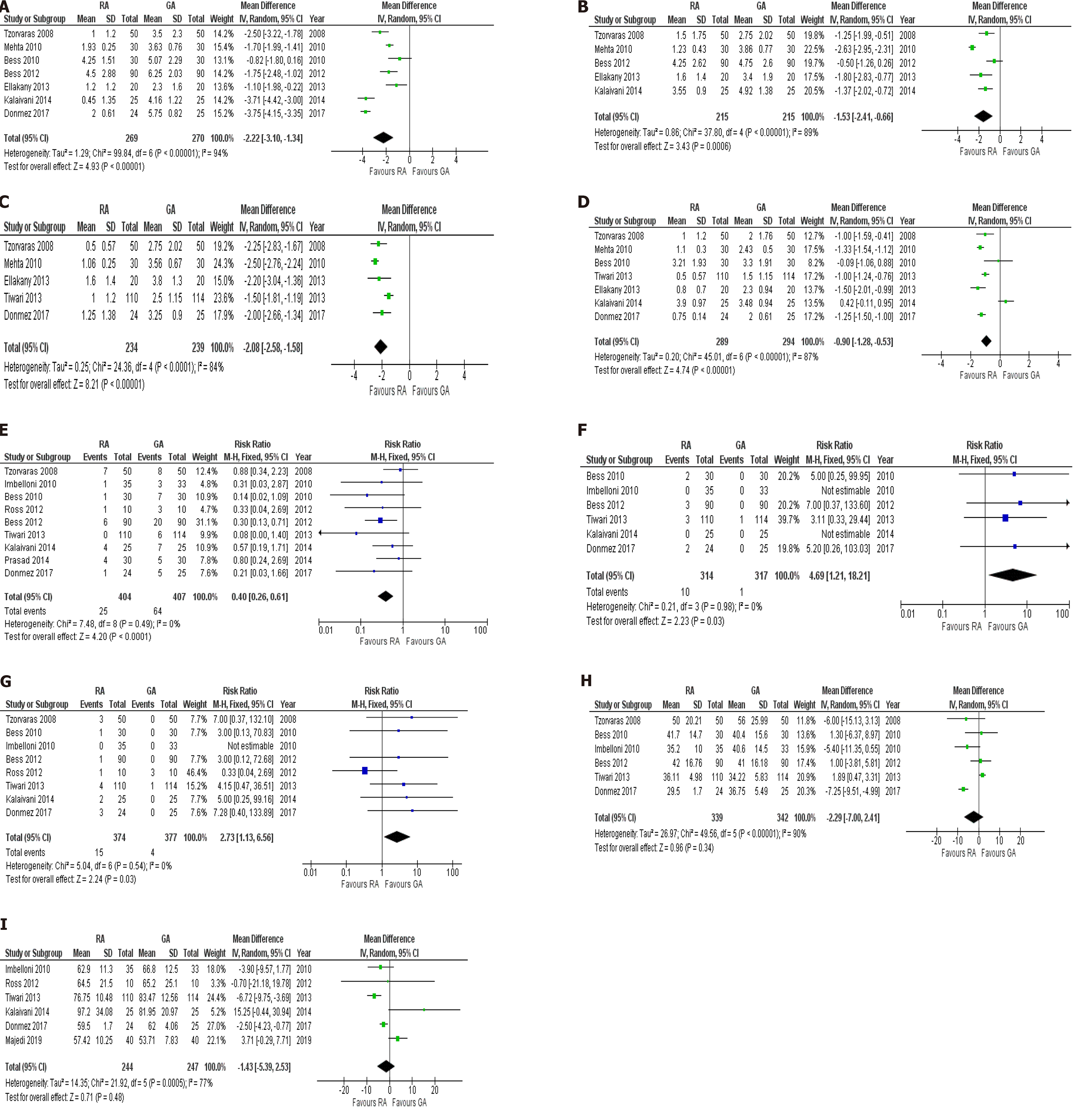
VAS score at 4 h: Seven studies (539 patients) reported the VAS score at 4 h postoperatively as one of their outcomes. The pooled analysis demonstrated that RA was associated with significantly less postoperative pain at 4 h following surgery (MD: -2.22, 95%CI: -3.10 to -1.34, P < 0.00001). The heterogeneity among the studies was significant (I2= 94%, P < 0.00001).
VAS score at 8 h: Five studies reported the VAS score at 8 h as an outcome. The pooled analysis which included 430 patients demonstrated that RA was associated with significantly lower pain 8 h following laparoscopic cholecystectomy (MD: -1.53, 95%CI: -2.41 to -0.66), P = 0.0006). The between-studies heterogeneity was significant (
VAS score at 12 h: Five studies including 473 patients reported this outcome. The meta-analysis demonstrated RA was associated with significantly lower postoperative pain at 12 h following surgery when compared to GA (MD: -2.08, 95%CI: -2.58 to -1.58, P < 0.00001). Significant heterogeneity existed among the included studies (I2= 84%, P < 0.0001).
VAS score at 24 h: Seven studies (583 patients) reported postoperative VAS score at 24 h in their study groups. The pooled analysis demonstrated that there was a significantly lower postoperative pain at 24 h in favor of RA (MD: -0.90, 95%CI: -1.28 to -0.53, P < 0.00001). The heterogeneity among the included studies was considerable (I2= 87%, P < 0.00001).
Nausea and vomiting: Nine studies (811 patients) reported postoperative nausea and vomiting as an outcome in their intervention groups. The nausea and vomiting rates in the RA and GA groups were 6.2% and 15.7%, respectively. There was a significantly lower rate of nausea and vomiting in favor of RA compared to GA (RR: 0.40, 95%CI: 0.26-0.61, P < 0.0001). Low heterogeneity existed among the included studies (I2= 0%, P = 0.49).
Headache: Four studies (631 patients) reported post-operative headache as one of their outcomes. The rate of headache in the RA group was 3.2% while it was only 0.3% in the GA group. The pooled analysis demonstrated that RA was associated with significantly higher rate of postoperative headaches compared to GA (RR: 4.69, 95%CI: 1.21-18.21, P = 0.03). The between-study heterogeneity was low (I2= 0%, P = 0.98).
Urinary retention: Seven studies reported postoperative urinary retention as an outcome. The urinary retention rates in the RA and GA groups were 4.1% and 1.1%, respectively. The pooled analysis of 751 patients demonstrated that RA was associated with significantly higher postoperative urinary retention when compared to GA (RR: 2.73, 95%CI: 1.13-6.56), P = 0.03). There was low between-study heterogeneity (I2= 0%, P = 0.54).
Operative time: Six studies reported the operative time as one of their outcomes. The pooled analysis included 681 patients and demonstrated that there was no significant difference in operative time between RA and GA (MD: -2.29, 95%CI: -7.00-2.41, P = 0.34). The heterogeneity among the included studies was significant (I2= 90%, P < 0.00001).
Total operative and anesthetic time: Six studies (491 patients) reported the total operative and anesthetic time as one of their outcomes. The meta-analysis demonstrated that there was no significant difference in total operative and anesthetic time between two groups (MD: -1.43, 95%CI: -5.39-2.53, P = 0.48). The heterogeneity between studies was high (I2= 77%, P = 0.0005).
Considering the data provided by the included studies, it was not possible to conduct analysis on hypotension which was one of our secondary outcomes.
Using random-effects fixed-effect models did not affect the pooled effect size in analysis of any of the reported outcomes, except urinary retention where the increased rate of urinary retention in the RA group became insignificant. Nevertheless, considering heterogeneity of 0%, fixed-effect model was deemed more appropriate. The direction of pooled effect size remained unchanged when odds ratio, RR, or risk difference were calculated for dichotomous variables.
As two of our included studies, Bessa et al[21] and Bessa et al[5] were conducted by the same group, in order to ensure that potential overlapping patients are not included, we repeated all analyses with exclusion of Bessa et al[5] which did not change the direction of pooled effect size in any of our outcomes
Outcomes are summarised in Figure 4.
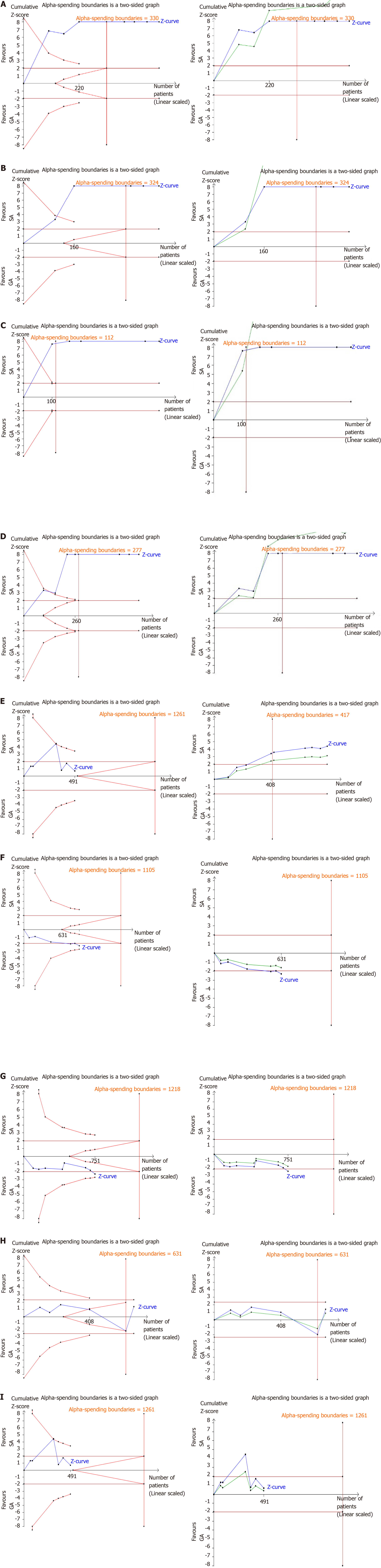
VAS score at 4 h: The information size was calculated at 330 patients. The Z-curve crossed the conventional boundaries and alpha-spending boundaries in favor of RA before and after the information size was reached and the penalized Z value remained greater than 1.96; therefore, the meta-analysis was conclusive and the risk of type 1 error was minimal.
VAS score at 8 h: The information size was calculated at 324 patients. The Z-curve crossed the conventional boundaries and alpha-spending boundaries in favor of RA before and after the information size was reached and the penalized Z value remained greater than 1.96; therefore, the meta-analysis was conclusive and the risk of type 1 error was minimal.
VAS score at 12 h: The information size was calculated at 112 patients. The Z-curve crossed the conventional boundaries and alpha-spending boundaries in favor of RA before and after the information size was reached and the penalized Z value remained greater than 1.96; therefore, the meta-analysis was conclusive and the risk of type 1 error was minimal.
VAS score at 24 h: The information size was calculated at 277 patients. The Z-curve crossed the conventional boundaries and alpha-spending boundaries in favour of RA before and after the information size was reached and the penalized Z value remained greater than 1.96; therefore, the meta-analysis was conclusive and the risk of type 1 error was minimal.
Nausea and vomiting: The information size was calculated at 417 patients. The Z-curve crossed the conventional boundaries and alpha-spending boundaries in favor of RA before and after the information size was reached and the penalized Z value remained greater than 1.96; therefore, the meta-analysis was conclusive and the risk of type 1 error was minimal.
Headache: The information size was calculated at 1105 patients. The Z-curve crossed the conventional boundaries in favor of GA before the information size is reached. However, the Z-curve did not cross the α-spending boundaries and the futility boundaries before the information size is reached and the absolute number for penalized Z value remained smaller than 1.96; therefore, the meta-analysis was not conclusive and the results for this outcome were subject to type 1 error.
Urinary retention: The information size was calculated at 1218 patients. The Z-curve crossed the conventional boundaries in favor of GA before the information size is reached. However, the Z-curve did not cross the α-spending boundaries and the futility boundaries before the information size is reached and the absolute number for penalized Z value remained smaller than 1.96; therefore, the meta-analysis was not conclusive and the results for this outcome were subject to type 1 error.
Operative time: The information size was calculated at 631 patients. The Z-curve did not cross the conventional boundaries and the absolute number for penalized Z value remained smaller than 1.96 in both sides after the information size is reached; therefore, the meta-analysis was conclusive and the risk of type 2 error was minimal.
Total operative and anesthetic time: The information size was calculated at 1261 patients. The Z-curve did not cross the α-spending boundaries and the futility boundaries before the information size is reached and the absolute number for penalized Z value remained smaller than 1.96; therefore, the meta-analysis was not conclusive and the results for this outcome were subject to type 2 error.
We have conducted a comprehensive literature review and meta-analysis of the best available evidence to evaluate the comparative outcomes of RA and GA in laparoscopic cholecystectomy. We identified 13 RCTs[4,5,11-21] reporting on a total of 1111 patients who underwent laparoscopic cholecystectomy under RA (n = 557) and GA (n = 554). Our subsequent analysis of outcomes demonstrated that RA was associated with significantly lower postoperative pain within 24 h following the surgery, and lower nausea and vomiting compared to GA. However, it was associated with significantly higher rates of urinary retention and headache. Moreover, there was no significant difference in operative and total procedural (surgical and anesthetic) time between two groups. The heterogeneity between studies for post-operative nausea and vomiting, headaches, and urinary retention were all low, demonstrating the robustness of these results. The between-study heterogeneity in analysis of VAS score was high indicating that our findings on these outcomes may be less robust.
We also conducted a trial sequential analysis to assess for risk of Type 1 and Type 2 errors in our meta-analysis. Overall, we found that the meta-analysis is conclusive for most of the outcomes. The exceptions to this are headache and urinary retention, which have a risk of a type 1 error, and total procedure time, which has a risk of a type 2 error.
There have been two previous systematic reviews and meta-analyses analysing the outcomes between GA and RA for laparoscopic cholecystectomy[7,22]. Yu et al[22] in 2015 included 7 RCTs and Wang et al[7] in 2016 included 8 RCTs in their meta-analysis, whilst our meta-analysis included 13 RCTs. Yu et al[22] found that postoperative pain was significantly lower at 12 h in favor of RA but they did not find any difference in postoperative pain at 24 h between RA and GA. Consistent with our findings, Wang et al[7] found significantly lower postoperative pain in favor of RA in the first 24 h of postoperative period. Moreover, Yu et al[22] reported that there was no difference in operative time between RA and GA which is in agreement with our findings on operative time. Considering the potential impact of the type of anesthesia on overall procedure time, we analysed total operative and anesthetic time independently and demonstrated that there was no significant difference between two groups. This was not considered by previous meta-analyses. Both studies reported a significant reduction in postoperative nausea and vomiting associated with RA, but an increase in risk of postoperative urinary retention. These results are similar to our findings. Considering that dural puncture is believed to induce distension of intracranial vessels and an increase in brain blood flow playing a primary role in post-dural pain headache formation[23], unlike other meta-analyses, we evaluated the headache as an outcome and found that the use of RA was associated with significantly higher postoperative headache than GA. This has previously been demonstrated in other laparoscopic procedures carried out under RA[24].
The growing evidence in favour of use of RA in laparoscopic cholecystectomy with regards to postoperative pain convinced us to not only meta-analyse the outcomes but also to evaluate the robustness of the findings of the meta-analysis by a trial sequential analysis. This is the first meta-analysis of the best available evidence complemented by a trial sequential analysis which demonstrated that the findings of our meta-analysis with regard to the postoperative pain are robust.
Postoperative pain is the most common complaint after surgery[22]. It has a unique pathophysiology and is believed to be due to peripheral and central sensitisation, as well as other humoral factors[22]. In day-case surgery, postoperative pain is problematic even when oral analgesia is optimised, as ongoing pain can lead to delayed discharges. In our analysis of the best available evidence, patients undergoing laparoscopic cholecystectomy under RA, have had significantly less postoperative pain when assessed at 4, 8, 12, and 24 h. Only 2.3% of patients had conversions from RA to GA showing that performing laparoscopic cholecystectomy under RA was well-tolerated. Furthermore, the type of anesthetic did not increase the anesthetic time or the surgical time. This further supports the argument that the use of RA for day-case laparoscopic cholecystectomy is feasible.
The second most common complaint after surgery is post-operative nausea and vomiting[25]. It is another cause of delayed discharges following day-case surgery. It has a complex pathophysiological mechanism and is influenced by multiple pre-operative, intraoperative, and postoperative factors, as well as general patient factors. Cholecystectomies in particular are known to have a high incidence of postoperative nausea and vomiting[25]. According to our meta-analysis, there is clear robust evidence that the use of RA for laparoscopic cholecystectomy has led to a significant reduction in postoperative nausea and vomiting. In turn, this should lead to a larger number of patients being successfully discharged on the day of surgery.
Postoperative urinary retention is a common finding after surgery with an incidence up to 70% in some procedures[26]. It is transient in most cases. Catheterisation is the primary treatment for this. Multiple risk factors for this including increasing age, longer surgery, use of postoperative analgesia, as well as the use of RA have been described[27]. The inherent pharmacology of anesthetic drugs can cause changes in the physiology of micturition. Spinal, general and regional nerve blocks can cause postoperative urinary retention by decreasing micturition control at the pontine micturition center and peripherally by blocking neural transmission in the spinal cord[28]. GA relaxes smooth muscle and reduces bladder contractility by interfering with autonomic regulation of the detrusor muscle[29]. This is physiologically apparent given the fact that bladder capacity substantially increases when a patient is subjected to GA[30]. SA and EA affect micturition via a different mechanism. They interfere with efferent and afferent nerves of micturition and disrupt the reflex arcs peripherally. The available evidence suggests that SA is associated with highest risk for postoperative urinary retention, followed by EA followed by GA[26]. The results of our meta-analysis are in agreement with this as it showed a significant increase in urinary retention in those patients undergoing laparoscopic cholecystectomy under RA. This finding may discourage some surgeons and patients from using RA.
The use of RA in laparoscopic cholecystectomy should be seen as a “half-full glass”. It is feasible with promising potential to reduce the postoperative pain and nausea or vomiting. Nevertheless, the increased risk of urinary retention and headache associated with RA can potentially cancel-out its effectiveness in pain control in early postoperative period by prolonging the length of hospital stay or need for outpatient assessment. Moreover, the impact of RA compared with GA on surgical outcomes of laparoscopic cholecystectomy is yet to be determined. Unfortunately, the available RCTs have not provided appropriate data about the indication for procedure, procedure related difficulties, and procedure related complications. Performing a laparoscopic cholecystectomy for a gallbladder polyp would be less challenging than doing the procedure for a complex cholecystitis or gallstone pancreatitis. We encourage future randomized studies to evaluate the comparative procedure related outcomes of laparoscopic cholecystectomy under RA and GA.
It is important to consider the limitations of our meta-analysis when interpreting its results. Although we included only RCTs to ensure high quality data, we found that there remained significant between-study heterogeneity when assessing operative time, total procedure time, and post-operative VAS scores. Furthermore, although our trial sequential analysis demonstrated that our meta-analysis was conclusive for most outcomes, it demonstrated a risk of type 1 error for two outcomes: headache and urinary retention. It also demonstrated a risk of type 2 error for total procedure time. Some of the include studies reported their VAS score and procedure time as median and interquartile range. We have calculated their mean and standard deviation using the method described by Hozo et al[30]. This might have subjected our findings to some degree of bias. Moreover, some the included studies excluded patients who had failure of RA which is not consistent with intention to treat concept. This might have significantly affected the results in favor of RA and subsequently introduced bias to our findings. Finally, all the risk of performance and detection bias was high among the included studies due to lack of blinding. With regards to the performance bias, the blinding of participants and surgeons would have been impossible; however, blinding of outcome assessor would have been possible to reduce the risk of detection bias.
Our meta-analysis of the best available evidence (Level 1 evidence) demonstrated that RA may be a safe and feasible anesthetic modality for laparoscopic cholecystectomy considering its associated lower postoperative pain and nausea and vomiting compared to GA. This makes it a potentially attractive option to expedite discharge planning in day-case surgery. However, its associated risk of urinary retention and headache may not help facilitating such aim. Moreover, lack of knowledge on the impact of RA on specific procedure related outcomes may discourage surgeons from selecting RA as the first choice of anesthesia for laparoscopic cholecystectomy. Most importantly, intention-to-treat principle has been breached in some of the included studies by excluding failed RA attempts. Considering our findings and the limitations of the available evidence, we do not hesitate to highlight that available evidence does not justify using RA as the first line anesthetic choice for laparoscopic cholecystectomy although it may be an option in patients who are not fit for GA. Future research should focus on procedure related outcomes of RA and GA in laparoscopic cholecystectomy with respect to intention-to-treat concept.
In an effort to further reduce the morbidity and mortality profile of laparoscopic cholecystectomy, the outcomes of such procedure under regional anesthesia (RA) have been evaluated.
In the context of cholecystectomy, combining a minimally invasive surgical procedure with a minimally invasive anesthetic technique can potentially be associated with less postoperative pain and earlier ambulation.
The main objective of this meta-analysis was to evaluate comparative outcomes of RA and general anesthesia (GA) in patients undergoing laparoscopic cholecystectomy.
A comprehensive systematic review of randomized controlled trials (RCTs) with subsequent meta-analysis and trial sequential analysis of outcomes were conducted in line with Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement standards.
Thirteen RCTs enrolling 1111 patients were included. The study populations in the RA and GA groups were of comparable age (P = 0.41), gender (P = 0.98) and body mass index (P = 0.24). The conversion rate from RA to GA was 2.3%. RA was associated with significantly less postoperative pain at 4 h [mean difference (MD): -2.22, P < 0.00001], 8 h (MD: -1.53, P = 0.0006), 12 h (MD: -2.08, P < 0.00001), and 24 h (MD: -0.90, P < 0.00001) compared to GA. Moreover, it was associated with significantly lower rate of nausea and vomiting [risk ratio (RR): 0.40, P < 0.0001]. However, RA significantly increased postoperative headaches (RR: 4.69, P = 0.03), and urinary retention (RR: 2.73, P = 0.03). The trial sequential analysis demonstrated that the meta-analysis was conclusive for most outcomes, with the exception of a risk of type 1 error for headache and urinary retention and a risk of type 2 error for total procedure time.
Our findings indicate that RA may be an attractive anesthetic modality for day-case laparoscopic cholecystectomy considering its associated lower postoperative pain and nausea and vomiting compared to GA. However, it associated risk of urinary retention and headache and lack of knowledge on its impact on procedure-related outcomes do not justify using RA as the first line anaesthetic choice for laparoscopic cholecystectomy.
The available RCTs have not provided appropriate data about the indication for procedure, procedure related difficulties, and procedure related complications. We encourage future randomised studies to evaluate the comparative procedure related outcomes of laparoscopic cholecystectomy under LA and GA.
| 1. | National Institute for Health and Care Excellence. Costing statement: Gallstone disease – Implementing the NICE guideline on gallstone disease (CG188). 2014 [cited 20 January 2021]. Available from: https://www.nice.org.uk/guidance/cg188. |
| 2. | Marks JM, Phillips MS, Tacchino R, Roberts K, Onders R, DeNoto G, Gecelter G, Rubach E, Rivas H, Islam A, Soper N, Paraskeva P, Rosemurgy A, Ross S, Shah S. Single-incision laparoscopic cholecystectomy is associated with improved cosmesis scoring at the cost of significantly higher hernia rates: 1-year results of a prospective randomized, multicenter, single-blinded trial of traditional multiport laparoscopic cholecystectomy vs single-incision laparoscopic cholecystectomy. J Am Coll Surg. 2013;216:1037-47; discussion 1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 3. | Capdevila X, Aveline C, Delaunay L, Bouaziz H, Zetlaoui P, Choquet O, Jouffroy L, Herman-Demars H, Bonnet F. Factors Determining the Choice of Spinal Versus General Anesthesia in Patients Undergoing Ambulatory Surgery: Results of a Multicenter Observational Study. Adv Ther. 2020;37:527-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 4. | Tzovaras G, Fafoulakis F, Pratsas K, Georgopoulou S, Stamatiou G, Hatzitheofilou C. Spinal vs general anesthesia for laparoscopic cholecystectomy: interim analysis of a controlled randomized trial. Arch Surg. 2008;143:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Bessa SS, El-Sayes IA, El-Saiedi MK, Abdel-Baki NA, Abdel-Maksoud MM. Laparoscopic cholecystectomy under spinal vs general anesthesia: a prospective, randomized study. J Laparoendosc Adv Surg Tech A. 2010;20:515-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Li L, Pang Y, Wang Y, Li Q, Meng X. Comparison of spinal anesthesia and general anesthesia in inguinal hernia repair in adult: a systematic review and meta-analysis. BMC Anesthesiol. 2020;20:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Wang XX, Zhou Q, Pan DB, Deng HW, Zhou AG, Guo HJ, Huang FR. Comparison of Postoperative Events between Spinal Anesthesia and General Anesthesia in Laparoscopic Cholecystectomy: A Systemic Review and Meta-Analysis of Randomized Controlled Trials. Biomed Res Int. 2016;2016:9480539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Rodgers A, Walker N, Schug S, McKee A, Kehlet H, van Zundert A, Sage D, Futter M, Saville G, Clark T, MacMahon S. Reduction of postoperative mortality and morbidity with epidural or spinal anaesthesia: results from overview of randomised trials. BMJ. 2000;321:1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1551] [Cited by in RCA: 1280] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 9. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13784] [Article Influence: 810.8] [Reference Citation Analysis (3)] |
| 10. | Higgins JP, Altman DG. Assessing risk of bias in included studies. In: Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons, 2019. |
| 11. | Donmez T, Erdem VM, Uzman S, Yildirim D, Avaroglu H, Ferahman S, Sunamak O. Laparoscopic cholecystectomy under spinal-epidural anesthesia vs. general anaesthesia: a prospective randomised study. Ann Surg Treat Res. 2017;92:136-142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Ellakany M. Comparative study between general and thoracic spinal anesthesia for laparoscopic cholecystectomy. Egypt J Anaesth. 2013;29:375-381. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Imbelloni LE, Fornasari M, Fialho JC, Sant'Anna R, Cordeiro JA. General anesthesia vs spinal anesthesia for laparoscopic cholecystectomy. Rev Bras Anestesiol. 2010;60:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | V, Pujari VS, R SM, Hiremath BV, Bevinaguddaiah Y. Laparoscopic Cholecystectomy Under Spinal Anaesthesia vs. General Anaesthesia: A Prospective Randomised Study. J Clin Diagn Res. 2014;8:NC01-NC04. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Majedi MA, Sarlak S, Sadeghi Y, Ahsan B. Comparison of the Effects of Thoracic Epidural Anesthesia with General Anesthesia on Hemodynamic Changes and its Complications in Patients Undergoing Laparoscopic Cholecystectomy. Adv Biomed Res. 2019;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Mehta PJ, Chavda HR, Wadhwana AP, Porecha MM. Comparative analysis of spinal vs general anesthesia for laparoscopic cholecystectomy: A controlled, prospective, randomized trial. Anesth Essays Res. 2010;4:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Prasad CGS, Mannur PP, Suresh G. Spinal Anesthesia vs General Anaesthesia for Laparoscopic Cholecystectomy – A Prospective Randomized Controlled Study. JEMDS. 2014;3:1361-1368. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Ross SB, Mangar D, Karlnoski R, Camporesi E, Downes K, Luberice K, Haines K, Rosemurgy AS. Laparo-endoscopic single-site (LESS) cholecystectomy with epidural vs. general anesthesia. Surg Endosc. 2013;27:1810-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Sharaf A, Burki AM, Saira M, Bano R. Comparison of postoperative pain relief following use of spinal anesthesia vs general anesthesia for patients undergoing laparoscopic cholecystectomy. Anaesth Pain Intensive Care. 2018;22:67-72. |
| 20. | Tiwari S, Chauhan A, Chaterjee P, Alam MT. Laparoscopic cholecystectomy under spinal anaesthesia: A prospective, randomised study. J Minim Access Surg. 2013;9:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Bessa SS, Katri KM, Abdel-Salam WN, El-Kayal el-SA, Tawfik TA. Spinal versus general anesthesia for day-case laparoscopic cholecystectomy: a prospective randomized study. J Laparoendosc Adv Surg Tech A. 2012;22:550-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Yu G, Wen Q, Qiu L, Bo L, Yu J. Laparoscopic cholecystectomy under spinal anaesthesia vs. general anaesthesia: a meta-analysis of randomized controlled trials. BMC Anesthesiol. 2015;15:176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Esmaoglu A, Akpinar H, Uğur F. Oral multidose caffeine-paracetamol combination is not effective for the prophylaxis of postdural puncture headache. J Clin Anesth. 2005;17:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Hajibandeh S, Hajibandeh S, Mobarak S, Bhattacharya P, Mobarak D, Satyadas T. Meta-Analysis of Spinal Anesthesia Versus General Anesthesia During Laparoscopic Total Extraperitoneal Repair of Inguinal Hernia. Surg Laparosc Endosc Percutan Tech. 2020;30:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Shaikh SI, Nagarekha D, Hegade G, Marutheesh M. Postoperative nausea and vomiting: A simple yet complex problem. Anesth Essays Res. 2016;10:388-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Baldini G, Bagry H, Aprikian A, Carli F. Postoperative urinary retention: anesthetic and perioperative considerations. Anesthesiology. 2009;110:1139-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 502] [Article Influence: 29.5] [Reference Citation Analysis (11)] |
| 27. | Buckley BS, Lapitan MC. Drugs for treatment of urinary retention after surgery in adults. Cochrane Database Syst Rev. 2010;CD008023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Kamphuis ET, Ionescu TI, Kuipers PW, de Gier J, van Venrooij GE, Boon TA. Recovery of storage and emptying functions of the urinary bladder after spinal anesthesia with lidocaine and with bupivacaine in men. Anesthesiology. 1998;88:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 119] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 29. | Darrah DM, Griebling TL, Silverstein JH. Postoperative urinary retention. Anesthesiol Clin. 2009;27:465-484, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 135] [Article Influence: 7.9] [Reference Citation Analysis (3)] |
| 30. | Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4895] [Cited by in RCA: 7265] [Article Influence: 346.0] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mutter D S-Editor: Zhang L L-Editor: A P-Editor: Wang LL