Published online Nov 16, 2021. doi: 10.4253/wjge.v13.i11.565
Peer-review started: June 29, 2021
First decision: July 27, 2021
Revised: August 4, 2021
Accepted: October 15, 2021
Article in press: October 15, 2021
Published online: November 16, 2021
Processing time: 133 Days and 17.2 Hours
Fistula between the esophagus and bronchial artery is an extremely rare and potentially life-threatening cause of acute upper gastrointestinal bleeding. Here, we report a case of fistula formation between the esophagus and a nonaneurysmal right bronchial artery (RBA).
An 80-year-old woman with previous left pneumonectomy and recent placement of an uncovered self-expandable metallic stent for esophageal adenocarcinoma was admitted due to hematemesis. Emergent computed tomography showed indirect signs of fistulization between the esophagus and a nonaneurysmal RBA, in the absence of active bleeding. Endoscopy revealed the esophageal stent correctly placed and a moderate amount of red blood within the stomach, in the absence of active bleeding or tumor ingrowth/overgrowth. After prompt multidisciplinary evaluation, a step-up approach was planned. The bleeding was successfully controlled by esophageal restenting followed by RBA embolization. No signs of rebleeding were observed and the patient was discharged home with stable hemoglobin level on postoperative day 7.
This was a previously unreported case of an esophageal RBA fistula successfully managed by esophageal restenting followed by RBA embolization.
Core Tip: Esophageal bronchial artery fistula is an extremely rare cause of upper gastrointestinal bleeding. Here, we describe a previously unreported case of fistula formation between the esophagus and a nonaneurysmal right bronchial artery (RBA), in the setting of palliative esophageal metallic stenting and previous left pneumonectomy. Hemostasis was achieved by the use of esophageal restenting followed by RBA embolization.
- Citation: Martino A, Oliva G, Zito FP, Silvestre M, Bennato R, Orsini L, Niola R, Romano L, Lombardi G. Acute upper gastrointestinal bleeding caused by esophageal right bronchial artery fistula: A case report. World J Gastrointest Endosc 2021; 13(11): 565-570
- URL: https://www.wjgnet.com/1948-5190/full/v13/i11/565.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i11.565
Acute upper gastrointestinal bleeding (UGIB) is a potentially life-threatening emergency with a reported incidence of about 100 per 100000 persons per year[1,2]. Its etiology has been divided into variceal and nonvariceal bleeding. The most common causes of acute UGIB include peptic ulcer disease and esophageal varices, followed by Mallory–Weiss syndrome and neoplasms[1-3]. Acute UGIB caused by esophageal bronchial artery fistula is extremely rare. To date, only a few cases of fistula formation between the esophagus and the right bronchial artery (RBA) have been reported worldwide. Here, we describe a previously unreported case of a fistula between the esophagus and a nonaneurysmal RBA, in the setting of palliative esophageal metallic stenting and previous left pneumonectomy.
An 80-year-old woman was admitted to our bleeding unit due to severe anemia (hemoglobin 7.1 g/dL) and hematemesis with signs of hemodynamic instability.
One episode of hematemesis with presyncope occurred 1 h prior to hospital admission.
The patient underwent left pneumonectomy with adjuvant chemoradiotherapy for lung cancer 6 years before. An uncovered self-expandable metallic stent (SEMS) had been placed 3 mo prior at another institution for the palliation of a locally advanced esophageal adenocarcinoma.
The patient denied further medical history. There was no family history of GI cancer.
On presentation, the patient was hemodynamically unstable (pulse 115 bpm, blood pressure 90/60 mmHg). She was afebrile, with respiratory rate 17 breaths/min and oxygen saturation 94%. On general physical examination, she looked pale and dehydrated. Abdominal examination revealed nondistended, nontender abdomen with normal bowel sounds. The rectal examination exhibited melena.
Complete blood count analysis was notable for hemoglobin of 7.1 g/dL and hematocrit of 23.6%. All remaining laboratory examinations, including liver enzymes, coagulation studies and renal function tests, were within normal limits.
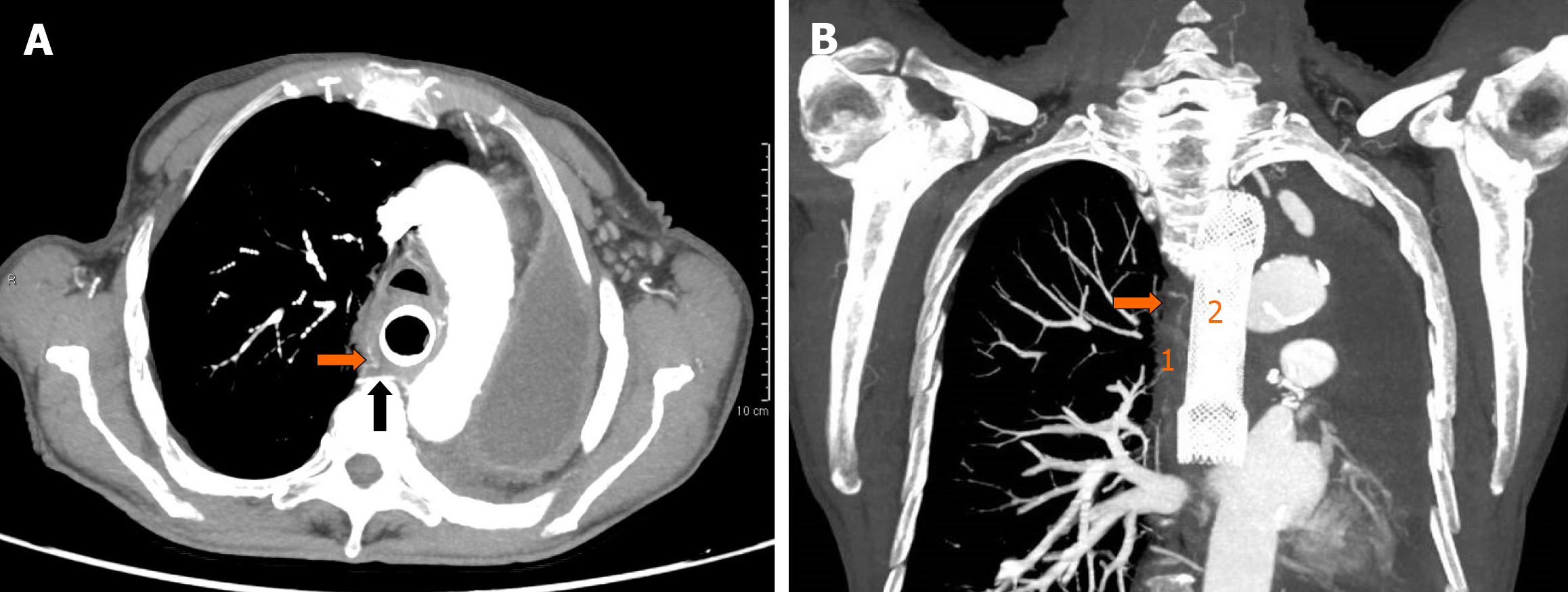
After blood transfusion and hemodynamic stabilization, emergent computed tomography (CT) angiography was performed showing no active GI bleeding with the esophageal stent correctly placed. The RBA appeared tortuous, dilated and tightly adherent to the thickened middle esophagus wall. Although no contrast extravasation was noted, the tissue planes between the RBA and the esophagus appeared obliterated (Figure 1).
After prompt multidisciplinary evaluation, involving a GI endoscopist, surgeon, and a diagnostic and interventional radiologist, a minimally invasive step-up approach with esophageal restenting followed, if necessary, by RBA embolization was planned.
Fistula formation between the esophagus and a nonaneurysmal RBA, in the setting of palliative esophageal metallic stenting and previous left pneumonectomy.
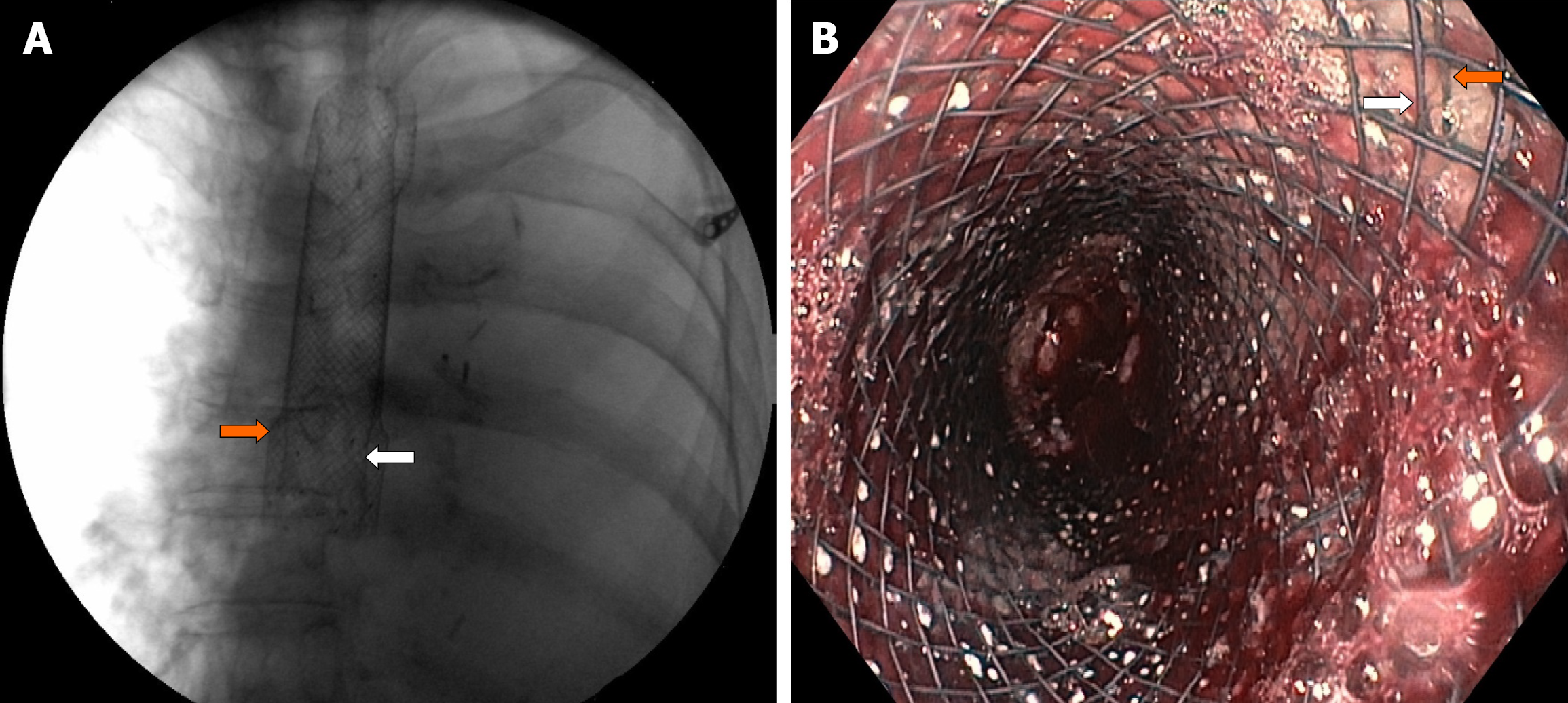
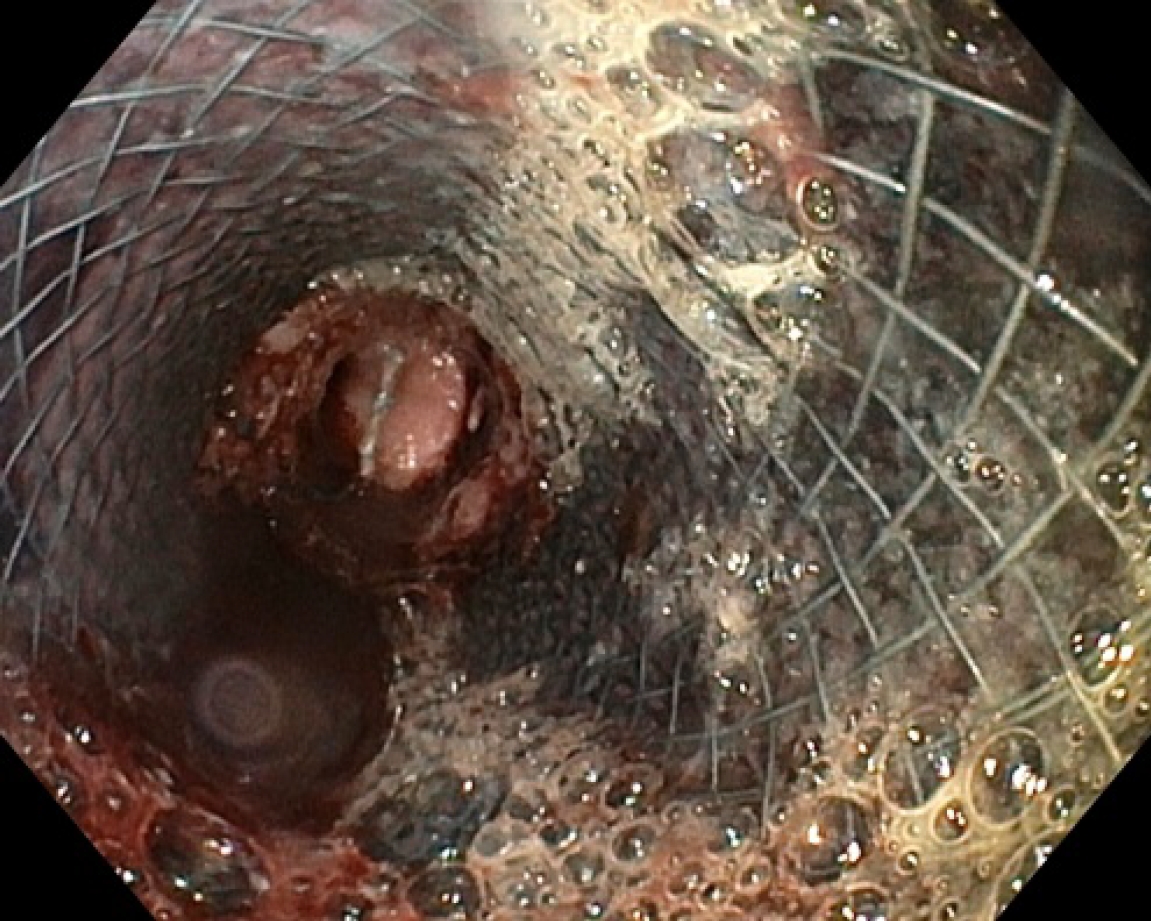
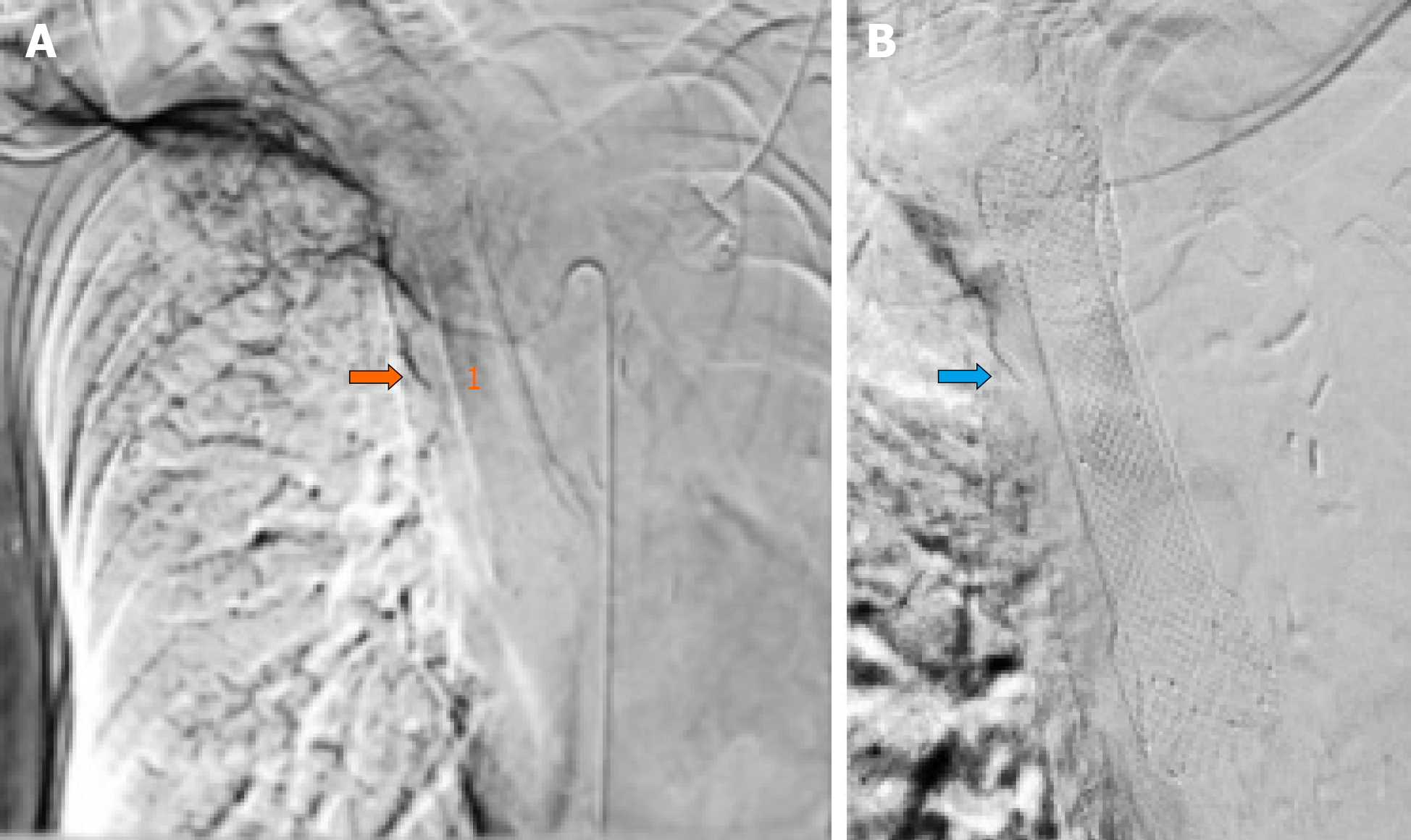
Under fluoroscopic and direct endoscopic guidance, an over-the-guidewire partially covered SEMS was placed through the previously inserted uncovered SEMS. Immediately thereafter, diffuse esophageal bleeding controlled by the partially covered SEMS was endoscopically noted (Figure 2). On postoperative day (POD) 1, hematemesis with severe anemization (hemoglobin 5.7 g/dL) and hemodynamic instability occurred. After blood transfusion and hemodynamic stabilization, emergent CT angiography was repeated, showing the esophageal stents correctly placed with unmodified previous findings and no GI active bleeding. Esophagogastroduodenoscopy (EGD) revealed fresh blood within the esophagus and a large amount of dark blood under the partially covered SEMS, in the absence of identifiable active bleeding sites (Figure 3). Thus, operative angiography was performed. Selective RBA arteriography showed contrast extravasation within the esophagus and RBA was successfully embolized with microcoils (Figure 4).
Postoperative stay was complicated by the occurrence of pulmonary edema responsive to medical therapy. No rebleeding was observed and the patient was discharged home with stable hemoglobin level (9.1 g/dL) on POD 7. The patient died at home 1 mo postoperatively, in the absence of overt GI rebleeding or anemization.
Arterioesophageal fistulas (AEFs) are pathological communications between an arterial system and the esophagus, which may lead to exsanguination from massive UGIB if not recognized promptly. They develop most commonly due to aortic fistulization caused by foreign bodies, aortic aneurysm, or esophageal neoplasms[4-6]. Nonaortic AEFs have been less frequently reported, with the bronchial artery being the most commonly involved vessel. Etiology includes foreign bodies, vascular surgery and thoracic arterial malformations, and chemoradiotherapy in esophageal cancer patients with invasion of the aorta[7,8].
Although extremely rare, an esophageal RBA fistula is a potentially life-threatening condition. To date, only a few cases of fistula formation between the esophagus and a bronchial artery aneurysm have been reported. Shaer and Bashist[9] first reported a fatal case of massive UGIB due to a bronchial artery aneurysm with an esophageal fistula (BAAEF). Later on, two cases of BAAEFs successfully treated with RBA coil embolization have been reported[10,11]. In 2018, Nakada et al[12] reported a case of BAAEF caused by bronchial arterial embolization. Due to the unfeasibility of transcatheter coil embolization, hemostasis was achieved by emergent thoracic endovascular aortic repair. Subsequently, aneurysmotomy, debridement and pedicled omental flap repair were successfully performed. Finally, a case of fistula between the esophagus and a RBA pseudoaneurysm secondary to an endobronchial ultrasound-guided transbronchial needle aspiration has been recently reported. This was successfully managed by endoscopic clipping followed by transcatheter coil embolization[13].
Moreover, only four cases of esophageal fistulas with a nonaneurysmal RBA have been reported, including three patients with locally advanced esophageal cancer and one with Mallory–Weiss tear refractory to endoscopic hemostasis. In all cases, the esophageal bleeding was successfully controlled by means of transcatheter arterial embolization[14-16].
However, to our knowledge, this is the first reported case of a fistula between the esophagus and a nonaneurysmal RBA, in the setting of palliative esophageal metallic stenting and previous left pneumonectomy.
In our case, emergent CT showed no active GI bleeding with the esophageal stent correctly placed. Although no direct signs of fistulization were observed, the RBA appeared tortuous, dilated and tightly adherent to the thickened middle esophagus wall, with obliteration of the tissue planes between the RBA and the esophagus. Subsequent emergent EGD confirmed the absence of active bleeding without identifiable bleeding sources. After prompt multidisciplinary evaluation, a minimally invasive step-up approach with esophageal restenting followed, if necessary, by RBA embolization was planned. However, after esophageal restenting, rebleeding occurred. Thus, operative angiography was performed. Selective RBA arteriography showed contrast extravasation within the esophageal lumen and RBA embolization was performed.
Digestive endoscopists should be aware of this critical, albeit extremely rare, cause of UGIB, in order to provide prompt diagnosis and treatment. In our opinion, early diagnosis, multidisciplinary evaluation and prompt tailored treatment seem to be crucial for the proper management of an esophageal RBA fistula.
| 1. | van Leerdam ME. Epidemiology of acute upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol. 2008;22:209-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 2. | Hearnshaw SA, Logan RF, Lowe D, Travis SP, Murphy MF, Palmer KR. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut. 2011;60:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 445] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 3. | Longstreth GF. Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population-based study. Am J Gastroenterol. 1995;90:206-210. [PubMed] |
| 4. | Kokatnur L, Rudrappa M. Primary aorto-esophageal fistula: Great masquerader of esophageal variceal bleeding. Indian J Crit Care Med. 2015;19:119-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Heckstall RL, Hollander JE. Aortoesophageal fistula: recognition and diagnosis in the emergency department. Ann Emerg Med. 1998;32:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Hollander JE, Quick G. Aortoesophageal fistula: a comprehensive review of the literature. Am J Med. 1991;91:279-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 240] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Yang KC, Lin MY, Huang LT, Chang WL. Massive upper gastrointestinal bleeding caused by an intercostal arterio-esophageal fistula: A rare case report. Radiol Case Rep. 2020;15:2026-2030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Tajima T, Haruki S, Usui S, Ito K, Matsumoto A, Matsuhisa A, Takiguchi N. Transcatheter arterial embolization for intercostal arterio-esophageal fistula in esophageal cancer. Surg Case Rep. 2017;3:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Shaer AH, Bashist B. Computed tomography of bronchial artery aneurysm with erosion into the esophagus. J Comput Assist Tomogr. 1989;13:1069-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Kim JS, Lee SY, Son KH, Kim KW, Choi CH, Lee JI, Park KY, Park CH. Bronchial Artery Aneurysm Presenting as Hematemesis and Mediastinal Hemorrhage. Korean J Thorac Cardiovasc Surg. 2015;48:298-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Fukunaga A, Okushiba S, Ohno K, Kitashiro S, Kawarada Y, Shitinohe T, Kondo S, Katoh H. Mediastinal bronchial artery aneurysm with hematemesis. Dis Esophagus. 2003;16:328-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Nakada T, Takahashi K, Ito E, Fukushima S, Yamamoto S, Takahashi N, Toya N, Akiba T, Morikawa T, Ohki T. A case of bronchial artery aneurysm with an esophageal fistula as an extremely rare complication after bronchial arterial embolization. J Thorac Dis. 2018;10:E476-E480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Jadeja S, Green K, Shuja A, Malespin M, De Melo S. Esophageal Bronchial Artery Fistulaization: A Complication of an Endobronchial Ultrasound. ACG Case Rep J. 2020;7:e00355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Taniguchi H, Yamazaki K, Boku N, Funakoshi T, Hamauchi S, Tsushima T, Todaka A, Sakamoto T, Tomita H, Machida N, Taku K, Fukutomi A, Onozawa Y, Tsubosa Y, Sato H, Nishimura T, Yasui H. Risk factors and clinical courses of chemoradiation-related arterio-esophageal fistula in esophageal cancer patients with clinical invasion of the aorta. Int J Clin Oncol. 2011;16:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Aoki M, Tokue H, Koyama Y, Tsushima Y, Oshima K. Transcatheter arterial embolization with N-butyl cyanoacrylate for arterial esophageal bleeding in esophageal cancer patients. World J Surg Oncol. 2016;14:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Kim EM, Lee JH, Sung JK, Kang SH, Kim JI, Moon HS, Lee BS, Kim SH, Jeong HY. Successful bronchial artery embolization for refractory esophageal bleeding after failed endoscopic therapy. Endoscopy. 2010;42 Suppl 2:E42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: European Society of Gastrointestinal Endoscopy (ESGE); Italian Association of Hospital Gastroenterologists and Digestive Endoscopists (AIGO); Italian Society for Digestive Endoscopy (SIED).
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chi TY S-Editor: Liu M L-Editor: Kerr C P-Editor: Liu M