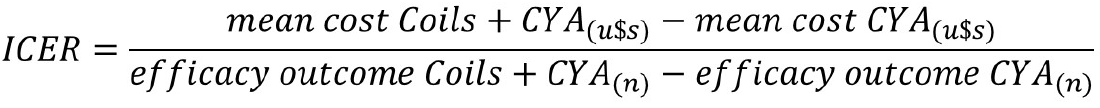Published online Jan 16, 2021. doi: 10.4253/wjge.v13.i1.13
Peer-review started: October 6, 2020
First decision: December 1, 2020
Revised: December 9, 2020
Accepted: December 16, 2020
Article in press: December 16, 2020
Published online: January 16, 2021
Processing time: 95 Days and 16.9 Hours
Cyanoacrylate (CYA) injection can be performed using a standard upper endoscopy technique or under endoscopic ultrasound (EUS) guidance alone or in combination with coils. There is little information available on the economic impact of these treatment methods.
To compare the cost-effectiveness of treating gastric varices by CYA injection via upper endoscopy vs coils plus CYA guided by EUS.
This was an observational, descriptive, and retrospective study. Patients were allocated into two groups: A CYA group and coils plus CYA group. The baseline characteristics were compared, and a cost analysis was performed.
Overall, 36 patients were included (19 in the CYA group and 17 in the coils + CYA group). All patients in the CYA group had acute bleeding. They underwent a higher mean number of procedures (1.47 vs 1, P = 0.025), and the mean volume of glue used was 2.15 vs 1.65 mL, P = 0.133. The coils + CYA group showed a higher technical success rate (100% vs 84.2%), with a complication rate similar to the CYA group. The majority of CYA patients required hospitalization, and although the mean total per procedure cost was lower (United States $ 1350.29 vs United States $ 2978), the mean total treatment cost was significantly different (United States $ 11060.89 for CYA vs United States $ 3007.13 for coils + CYA, P = 0.03).
The use of EUS-guided coils plus cyanoacrylate is more cost-effective than cyanoacrylate injection when the total costs are evaluated. Larger, randomized trials are needed to validate the cost-effectiveness of the EUS-guided approach to treat gastric varices.
Core Tip: There is little evidence regarding the economic impact of standard endoscopic cyanoacrylate therapy vs endoscopic ultrasound (EUS)-guided endovascular therapy in the management of gastric varices. In this retrospective study, we found that patients treated with endoscopic cyanoacrylate injection required hospitalization and had a significantly higher total treatment cost in comparison to those treated with an EUS-guided therapy. The incremental cost-effectiveness ratio analysis shows that in endoscopic therapy, each early rebleeding, adverse events, and day of hospitalization increased health-related costs on United States $ 2670.80, United States $ 8012.40, United States $ 127.18 per presented event, respectively, when comparing with coils + cyanoacrylate group cost and presented events. Each inevitable death on the endoscopic group represented a health-related cost increase on United States $ 8012.40 in comparison with EUS-guided therapy.
- Citation: Robles-Medranda C, Nebel JA, Puga-Tejada M, Oleas R, Baquerizo-Burgos J, Ospina-Arboleda J, Valero M, Pitanga-Lukashok H. Cost-effectiveness of endoscopic ultrasound-guided coils plus cyanoacrylate injection compared to endoscopic cyanoacrylate injection in the management of gastric varices. World J Gastrointest Endosc 2021; 13(1): 13-23
- URL: https://www.wjgnet.com/1948-5190/full/v13/i1/13.htm
- DOI: https://dx.doi.org/10.4253/wjge.v13.i1.13
Variceal bleeding is the most expensive of all digestive diseases in terms of hospitalization charges[1]. Although the prevalence of gastric varices (GV) is lower than esophageal varices (5% to 33%), and the risk of bleeding is also lower for GV than esophageal varices, the bleeding from GV can be severe, and the associated mortality rate is high[1]. The incidence of bleeding was reported to be 25%, with re-bleeding rates as high as 40% and mortality rates of 50%[2].
Endoscopy sclerotherapy with cyanoacrylate glue (CYA) has demonstrated higher hemostasis (> 90%) and lower rebleeding rates compared to band ligation or sclerotherapy with alcohol products for the management of GV[3]. However, this procedure has been shown to be associated with significant adverse events. For example, pulmonary embolism due to CYA injection is a serious and sometimes fatal complication, which is seen in 4.3% of cases and is dependent on the volume of glue injected[3]. Other related complications may include hemorrhage from post-injection ulcers, fever, abdominal pain, and needle impaction. In addition, the injection material can cause serious damage to the endoscope[4].
Currently, endoscopic treatments with CYA injection can be performed under direct visualization using a standard gastroscope or under endoscopic ultrasound (EUS) guidance with the injection of CYA alone or in combination with coils[5]. There is little information available in the current literature on the economic impact of these treatment methods for GV.
The aim of this study was to compare the cost-effectiveness of GV treatment with two different techniques, CYA glue injections using a standard gastroscope vs the use of coils plus CYA guided by EUS.
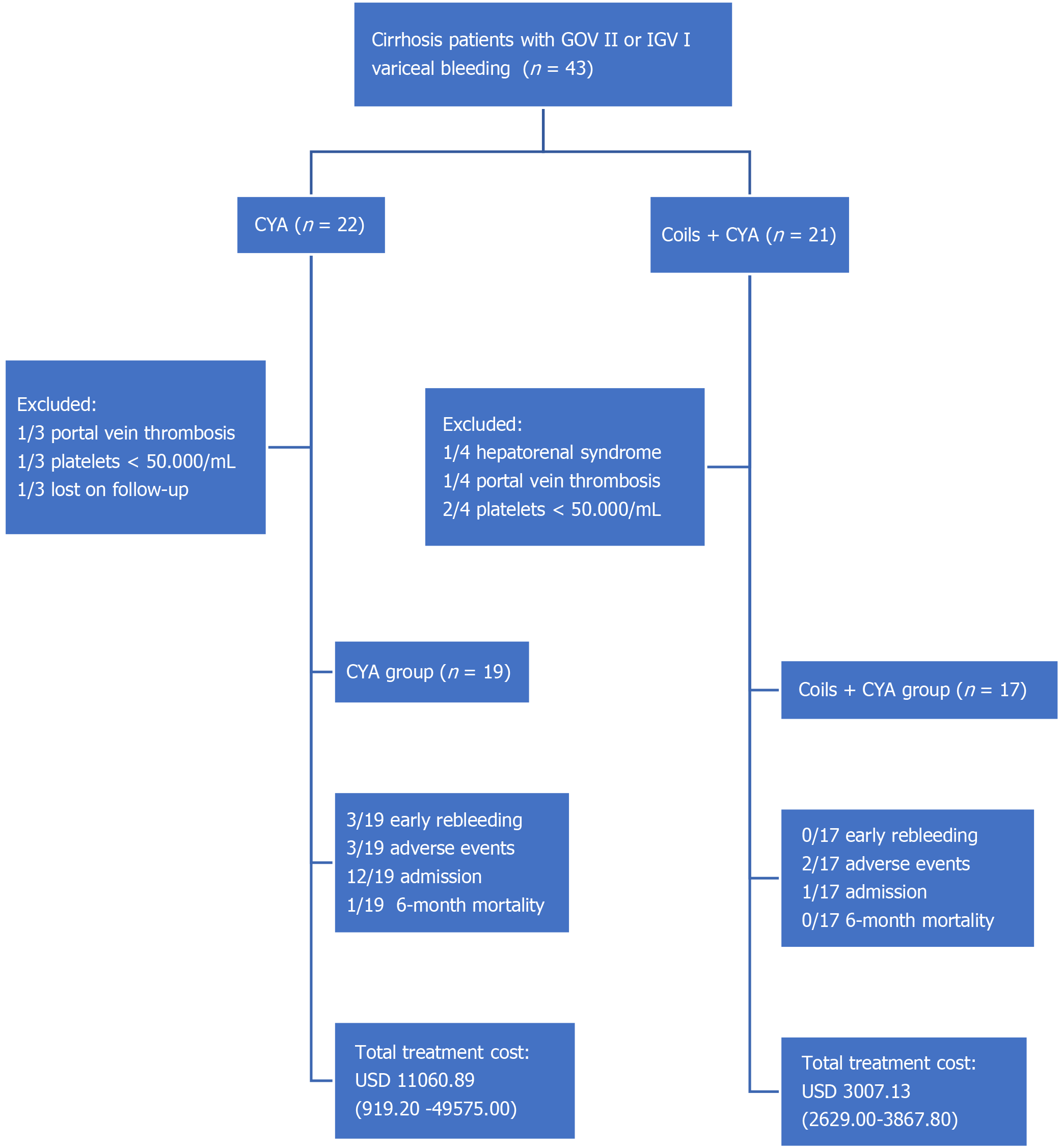
This was an observational, analytic, retrospective cohort study conducted in patients with cirrhosis and variceal bleeding, attended at an academic tertiary center in Guayaquil, Ecuador from November 2014 to March 2016 (Figure 1). The patients were categorized into two groups: One treated with only CYA injection by the standard upper endoscopy technique (CYA group) and the other treated by the EUS-guided insertion of coils + CYA injection (Coils + CYA group). The protocol of the study and consent form were approved by the Institutional Review Board, and the study was conducted according to the Declaration of Helsinki. All patients provided written informed consent for attendance purposes.
For the study analysis, we considered ≥ 18 years old patients with gastroesophageal varices type II (GOV II, fundal varices communicating with esophageal varices) and isolated gastric varices type I (IGV I, fundal varices within a few centimeters of the gastric cardia) according to the classification described by Sarin and Kumar[6]. The study included patients with acute bleeding or a history of previous bleeding due to GV (secondary prophylaxis).
We did not include patients with concurrent hepatorenal syndrome and/or multi-organ failure; esophageal stricture; splenic or portal vein thrombosis; a platelets count less than 50.000/mL or an international normalized ratio > 2; pregnancy[7]; as well as patients with incomplete medical reports, or those without 6-mo follow-up.
One expert endoscopist (Robles-Medranda C) performed all endoscopic procedures in a hospital-based interventional endoscopy suite, where EUS and fluoroscopy were available. Endoscopic procedures were performed under general anesthesia and with antibiotic prophylaxis. After the procedure, the patients in both groups were observed for 2 h in the recovery room before being discharged. Patients were hospitalized if they had active bleeding or if they had early post-treatment bleeding according to the Baveno VI consensus[8]. All patients with acute upper GI bleeding admitted to receive a standard assessment and were given resuscitation fluid, antibiotics, blood components if necessary, and intravenous octreotide (50 μg bolus plus 50 μg/h) for at least 72 h. Upper endoscopy was performed within 24 h of hospital admission.
A 3.2-mm forward-view endoscope (EG29-i10 and EG 2990-I series, Pentax Medical, Hoya Corp, Japan) was used to perform the standard endoscopic technique. EUS was performed using a 3.8-mm working channel linear-array therapeutic echoendoscope (EG 3870UTK; Pentax Medical, Hoya Corp, Japan) attached to an ultrasound console (Avius Hitachi, Tokyo, Japan). Active flow within the GFV was confirmed by color Doppler and fine flow Doppler color before and after the treatment.
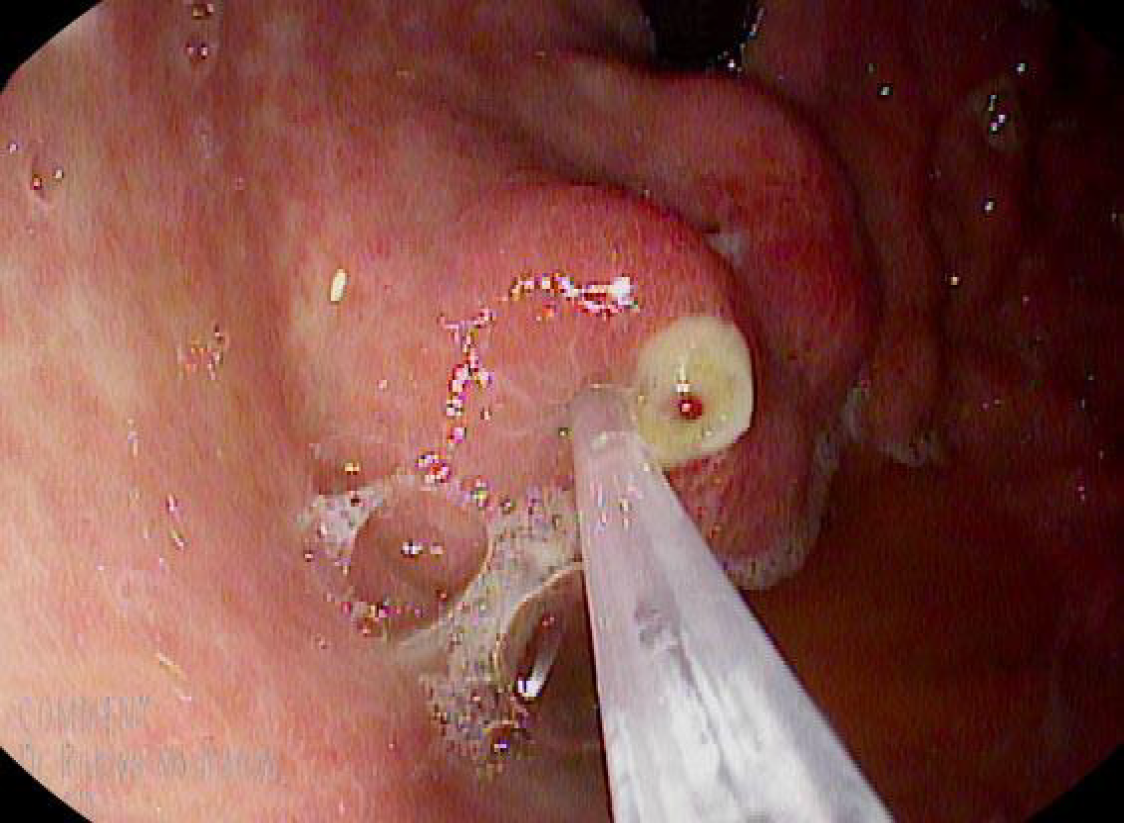
CYA injection by upper endoscopy: The 2-Octyl-CYA (Dermabond; Ethicon, Piscataway, NJ, United States) was injected through a 21 or 22 G needle. This type of CYA precludes the need for a diluent, such as lipiodol. After puncturing varix and injecting the CYA, the needle was rinsed with saline solution. A proper dosage has not been established, and it is usually decided by the endoscopist at the time of intervention, taking into account gastric varix size and the initial success in arresting bleeding, considering that larger doses can increase the risk of embolism to distal organs. However, no more than 2.5 mL of CYA was injected per session per our institution’s protocol for this technique (Figure 2).
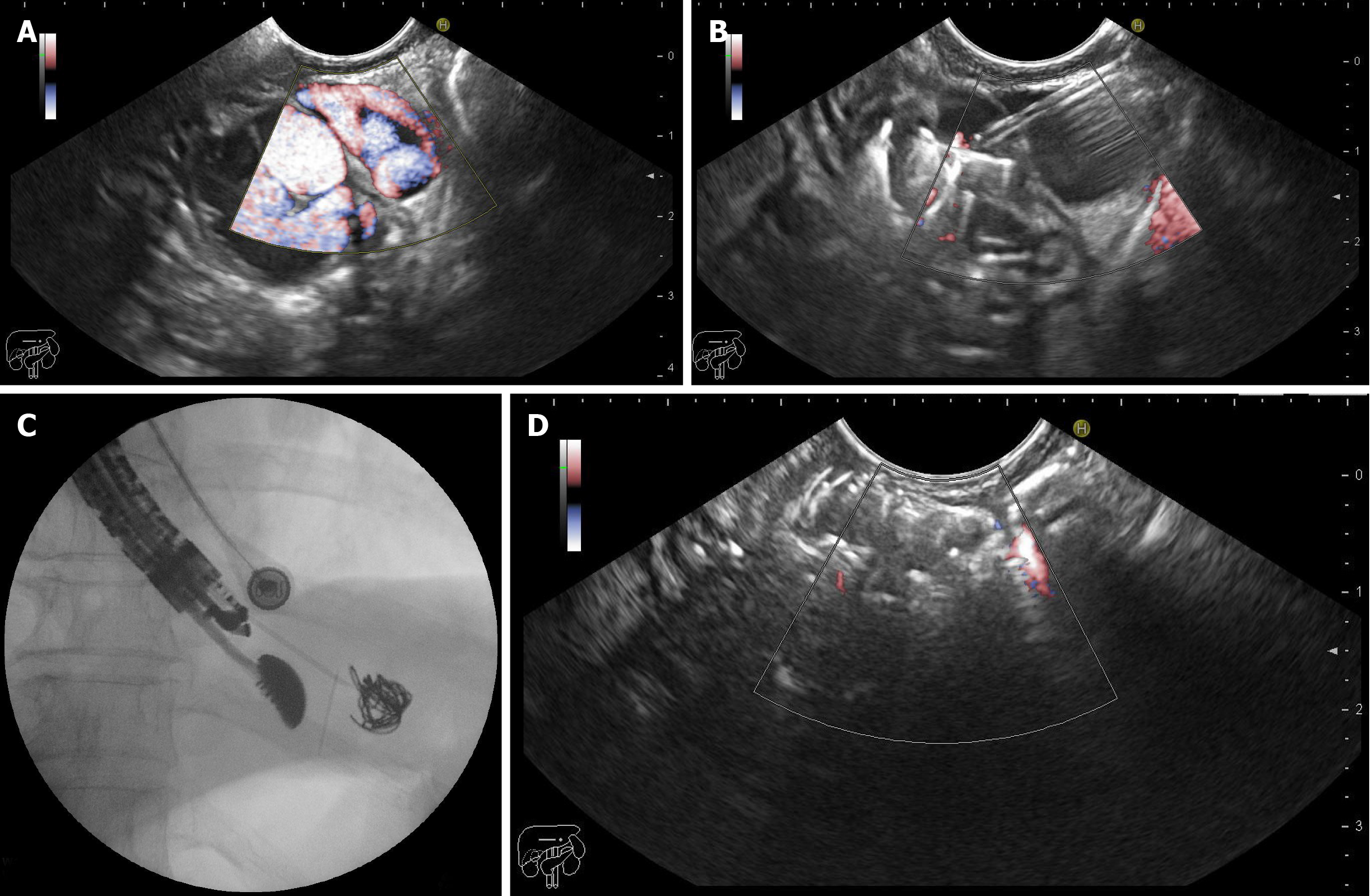
EUS-guided deployment of coil(s) plus CYA injection: First, a standard diagnostic upper endoscopy was performed to classify the varices according to the classification described by Sarin and Kumar[6]. Then, an echoendoscope was positioned in the distal esophagus (anterograde transesophageal, transcrural approach) to endosonographically evaluate the gastric fundus, intramural varices, and gastric varices feeder vessels. Once positioned, water was instilled in order to fill the gastric fundus, improving the acoustic coupling and visualization of the GFV. EUS color Doppler imaging was used to allow direct visualization of the variceal flow. Then, a 19-gauge EUS-FNA needle (Expect flexible; Boston Scientific, United States) was used to puncture the vessel, the stylet was withdrawn, and a syringe with negative pressure was used to evaluate the blood return and therefore the intravascular location. Once the location was confirmed, 1 mL of saline solution was instilled to prevent blood clotting in the needle lumen, and then 2 mL of water-soluble contrast agent (Ultravist, Bayer, Ecuador) was injected under fluoroscopic evaluation to further ensure the intravascular location and to determine varix flow direction (afferent or efferent), as has been reported in a previous study[9]. Then, coils were delivered, and the 2-Octyl-CYA was injected. The coils used were intravascular embolization coils (10-16 mm coiled diameter, 12-20 cm straight lengths, 0.035 inches in diameter, Nester Embolization Coil; Cook Medical) and were delivered into the vessel through the FNA needle using the stylet as a pusher. Special attention was paid to not place the needle tip at the counter wall because of the risk of perforation, bleeding, and coil extrusion and to allow enough space for the coil to curl. The 2-Octyl-CYA (Dermabond; Ethicon, Piscataway, NJ, United States) was injected using the same needle, and then 1 mL of normal saline solution was injected to rinse the needle. The diameter and number of coils (10 to 16 mm) and the volume of 2-Octyl-CYA injected were calculated according to the diameter of the vessel measured on EUS. After 90 to 120 s, the CYA was solidified, the risk of bleeding due to the puncture decreased, and the needle was withdrawn. The final obliteration of the vessel was evaluated using Doppler imaging 5 min after withdrawal (Figure 3).
Efficacy was measured by technical success, defined as successful technique performance, and functional success, defined as the complete obliteration of varix by endoscopy and/or by the absence of Doppler flow on EUS. Safety was determined based on the development of adverse events related to the procedure within and 30 d after the procedure.
Follow-up was performed in accordance with our institution’s protocol for these kinds of procedures by standard endoscopy in the CYA group and by EUS and upper endoscopy at 1, 3, and 6 mo post-procedure. Hemostasis, early post-treatment bleeding, and late post-treatment bleeding were considered according to the Baveno VI consensus[7].
Demographic data, endoscopic procedure records, cost variables [both endoscopic procedure and hospitalization; currency: United States of America dollar, United States dollar (USD); ISO 4217 code: USD] and clinical follow-up were obtained from institutional database register (SIAM V2.0, MD Consulting Group, Guayaquil, Ecuador). A 6-mo mortality was confirmed through the Ecuadorian Civil Registration database.
Technical considerations: The data analysis was reviewed by the institutional biostatistician (M.P-T.). Statistical analysis was performed using R v3.6.3 (R Foundation for Statistical Computing; Vienna, Austria). A P value < 0.05 was considered to be statistically significant.
Sample size: A sample of 15 participants per study group was calculated using corresponding formula to compare two means (two-samples, one-sided), on the basis of a 5% α error, a 20% β error, κ = 2, and a 3-mo post-bleeding mean charges (standard deviation, ± SD) between CYA-treated cases (USD: 42.450 ± 43.916) and controls (USD: 78.165 ± 47.857), as described by Greenwald et al[10].
Baseline characteristics: Demographic and clinical data were described by mean ± SD or median (minimum–maximum range) in accordance with statistical distribution (Shapiro–Wilk test), for quantitative variables, and frequency (percentage) for qualitative variables. Hospitalization length was described in a range of days. Cost variables were described as means considering it properly for economic data in terms of further cost analyses[10] but using the maximum-minimum range for easier comprehension of corresponding distribution. Data were also compared among CYA vs coils + CYA groups using Welch Two Sample t-test for normal-distributed and cost data, Mann–Whitney U test for skewed-distributed data, Pearson’s Chi-squared or Fisher’s Exact test for qualitative data, and Gray’s test for the length of hospitalization.
Cost analysis: The incremental cost-effectiveness ratio (ICER) is a proportion of the difference in the mean cost of procedures between groups and the number of episodes of a specific outcome between groups, such as the number of deaths, adverse events, or days of hospitalization. This ratio represents the amount of money saved to prevent the aforementioned outcomes[11]. The ICER in the present study was established in terms of the following efficacy outcomes: Early re-bleeding, adverse effects, length of hospitalization, and 6-mo mortality. This corresponded to the difference between CYA vs coils + CYA in terms of the mean total treatment cost, divided by the difference between the numbers of events in each efficacy outcome, per the corresponding study group (Figure 4).
We enrolled 36 patients in the study (19 in the CYA group and 17 in the coils + CYA group. The overall mean age was 63.06 years old, and 20 (55.5%) patients were men. The baseline data are shown in Table 1.
| Total (n = 36) | CYA (n = 19) | Coils + CYA (n = 17) | P value | |
| Age (yr), mean ± SD | 63.06 ± 10.1 | 62.83 ± 11.5 | 63.29 ± 8.8 | 0.8951 |
| Gender (female), n (%) | 16 (44.4) | 9 (47.4) | 7 (41.2) | 0.9702 |
| Indication, n (%) | < 0.0012 | |||
| Acute bleeding | 26 (72.2) | 19 (100.0) | 7 (41.2) | |
| Secondary prophylaxis | 10 (27.7) | - | 10 (58.8) | |
| Type of GV, n (%) | 0.9062 | |||
| GOV II | 24 (66.7) | 12 (63.1) | 12 (70.5) | |
| IGV I | 12 (33.3) | 7 (36.9) | 5 (29.5) | |
| Varix size (mm), mean ± SD | 21.8 ± 7.8 | 21.1 ± 8.7 | 22.6 ± 6.8 | 0.5781 |
| Technical success (n of events), n (%) | 33/36 (91.6) | 16/19 (84.2) | 17/17 (100) | 0.2313 |
| Volume of CYA (mL), median (range) | 1.8 (0.6–6.6) | 1.8 (0.6–6.6) | 1.8 (1.2–2.4) | 0.136 |
| No of coils, median (range) | 2 (1–3) | 0 | 2 (1–3) | N/A |
Regarding the indications for the procedure, all 19 (100%) patients in the CYA group had a history of acute bleeding, while in the coils + CYA group, ten (58.8%) patients underwent the procedure for secondary prophylaxis.
GOV II type varices were predominant in both groups, being present in 12 (63.1%) and 12 (70.5%) patients in the CYA group and coils + CYA group, respectively. The mean varix size was 21.1 ± 8.7 mm in the CYA group and 22.6 ± 6.8 in the coils + CYA group.
The patients in the CYA group underwent a total of 28 procedures, with a mean of 1.47 procedures per patient. In this group, the mean volume of CYA used was 2.15 (0.6-2.4) mL. Conversely, in the coils + CYA, 17 procedures were performed (with a mean of 1 procedure per patient) using a mean volume of 1.65 (1.2-2.4) mL CYA and a mean of 2.1 (1-3) coils per patient. Technical success was achieved in 16 of the 19 (84.2%) patients in the CYA group, with 3 (15.8%) patients showing early rebleeding and with 3 (15.8%) adverse events, represented by 2 cases of pulmonary embolism and one death. In the coils + CYA group, technical success was achieved in all 17 (100%) patients, with no cases of early rebleeding and 2 (7.1%) adverse events (1 episode of fever and 1 of transient abdominal pain).
In relation to treatment modality, 13 (68.4%) patients in the CYA group were hospitalized for a mean of 3.36 (0–14) d, with most of the time spent in the Intensive Care Unit. Nevertheless, only 1 (5.9%) patient was hospitalized in the coils + CYA group, and this patient remained in the Emergency Department.
Concerning the financial aspects of the procedures, the cost per procedure with endoscopic CYA injection was USD 816.70 [mean of 1 203.56 (816.70-3266.80)], while it was USD 2247.00 (mean of 2247.00) with the EUS-guided approach. The mean total procedure costs were USD 1350.29 (857.70-3717.80) in the CYA group and USD 2978.00 (2629.00-3270.00) in the coils + CYA group. The hospitalization and mean total treatment costs were much higher in the CYA group, in which patients spent USD 9 710.60 (0-45857.20) and USD 11060.89 (912.20-49575.00), respectively. ICERs analysis lets us to estimate that in CYA group, each early rebleeding, adverse events, and day of hospitalization increased health-related costs on USD 2670.80, USD 8012.40, USD 127.18 per presented event, respectively, when comparing with coils + CYA group cost and presented events (Table 2). Each inevitable death on CYA group represented a health-related cost increase on USD 8012.40 in comparison with coils + CYA group (Table 3).
| Total (n = 36) | CYA (n = 19) | Coils + CYA (n = 17) | P value | |
| Early rebleeding (n of events), n (%) | 3/36 (8.3) | 3/19 (15.8) | 0 | 0.2311 |
| Adverse events (n of events), n (%) | 5/36 (13.8) | 3/19 (15.8) | 2/17 (11.8) | 1.0001 |
| Treatment modality, n (%) | 0.0012 | |||
| Ambulatory | 23 (63.1) | 7 (36.8) | 16 (94.1) | |
| Hospitalization | 13 (36.1) | 12 (63.2) | 1 (5.9) | |
| No of endoscopic procedures, total | 45 | 28 | 17 | N/A |
| No. of endoscopic procedures per patient, median (range) | 1 (1-4) | 1 (1-4) | 1 | 0.0143 |
| Length of hospitalization (d), range | 0–14 | 0-14 | 0–1 | < 0.0014 |
| Intensive care unit | 0-11 | 0-11 | - | 0.0124 |
| Intermediate care unit | 0-14 | 0-14 | - | 0.0014 |
| Emergency Department | 0–1 | - | 0–1 | 0.3034 |
| Cost per procedure (USD) | N/A | 816.70 | 2247.00 | N/A |
| Cost per procedure (USD), mean (range) | 1696.29 (816.70-3266.80) | 1203.56 (816.70-3266.80) | 2247.00 | < 0.0015 |
| Coil cost (1 coil = $ 300, USD), mean (range) | 291.67 (0-900.00) | 0 | 617.65 (300.00-900.00) | < 0.0015 |
| CYA cost (1 vial × 0.3 mL = $ 20.5, USD), mean (range) | 130.97.00 (41.00-451.00) | 146.74 (41.00-451.00) | 113.35 (82.00-164.00) | 0.1415 |
| Total procedure cost (USD), mean (range) | 2118.93 (857.70-3717.80) | 1350.29 (857.70-3717.80) | 2978.00 (2629.00-3270.00) | < 0.0015 |
| Hospitalization cost (USD), mean (range) | 5158.31 (0-45857.20) | 9710.60 (0-45857.20) | 70.46 (0-1197.80) | 0.0105 |
| Total treatment cost (procedure + hospitalization, USD) mean (range) | 7277.20 (919.20-49575.00) | 11060.89 (919.20-49575.00) | 3007.13 (2629.00-3867.80) | 0.0305 |
| Efficacy outcome | ICER analysis |
| Early rebleeding (n of events) | (USD 3048.50) - (USD 11060.90)/(0) - (3) = US$ 2670.80 |
| Adverse events (n of events) | (USD 3048.50) - (USD 11060.90)/(2) - (3) = US$ 8012.40 |
| Length of hospitalization (total days) | (USD 3048.50) - (USD 11060.90)/(1) - (64) = US$ 127.18 |
| 6-mo mortality (n of events) | (USD 3048.50) - (USD 11060.90)/(0) - (1) = US$ 8012.40 |
Despite advances in endoscopic techniques and devices, the treatment of gastric varices, particularly bleeding varices, is still a challenging issue. Several previous studies on this subject showed that there were advantages for the standard endoscopic injection of cyanoacrylate in the treatment of gastric variceal bleeding, with high success and low rebleeding rates[1,2]. Thus, cyanoacrylate injection became the first choice of treatment worldwide. Nevertheless, this approach carries a huge risk of adverse events, notably, systemic embolization[8]. To overcome this problem, recent studies suggested a new approach to gastric variceal bleeding using EUS-guided technique with coils deployment plus cyanoacrylate injection in the feeding vessels, with excellent short-term results[8].
Overall, the two groups in the present analysis did not differ in age or gender, although there were slightly more males, which is common for GV[1]. With regard to the indications for the procedure, ten (58.8%) patients in the coils + CYA group underwent the procedure for secondary prophylaxis, while all 19 (100%) patients in the CYA group had acute bleeding. In this retrospective analysis from our unit, the use of EUS-guided coils plus CYA was the preferred technique for the prevention of rebleeding.
Only fundal GOV II and IGV I varices were included in the present work because it is generally accepted that GOV I varices are best treated with endoscopic band ligation. Currently, there is no established treatment for IGV II vessels. We observed that the patients in the CYA group required significantly more procedures and a significantly larger mean amount of CYA to achieve hemostasis and variceal remission. Moreover, with the EUS approach, the coils work as a frame that retains CYA within varix, with a fewer amount of cyanoacrylate needed to achieve obliteration, thus reducing the risk of adverse events, including embolism[5]. In our study, a mean of 1.65 (1.2-2.4) mL of CYA was used in the coils + CYA group, with two adverse events, one episode of fever and one transient abdominal pain, neither requiring hospitalization.
Technical success with the EUS coils + CYA method was achieved in all 17 (100%) patients (in one session), a much better performance compared with the CYA group. The EUS-guided technique used in this trial targets the perforating vessel instead of depending on direct variceal puncturing. Perforating vessels are thought to be the source of varix, and blocking the feeder, thus effectively decreasing the blood flow in gastric varix. Moreover, the use of EUS permits direct variceal visualization, which contributes to technical success, since the visual field with the standard endoscopic method can be obscured by blood and residue in the stomach. Despite this advantage, there were no differences in the numbers of patients with early rebleeding between the two groups in this study.
Although the cost per procedure and mean total procedure cost were higher for the EUS-guided approach, the total treatment costs were much higher in the CYA group, in which patients spent USD 11060.89 (912.20-49575.00). The later may be related to the fact that most patients in the latter group were hospitalized, and most of their time was spent in the Intensive Care Unit, which greatly increased the costs.
Overall, the use of EUS-guided coils plus CYA technique was more cost-effective than the current standard endoscopic therapy. The ICER demonstrated that the EUS-guided approach was advantageous in terms of cost savings. By performing this technique, we saved USD 2670.80 by preventing one early rebleed episode and USD 8012.40 by avoiding one death.
However, this study has some limitations. First, the patients who underwent the endoscopic CYA injection were all in an acute stage, and thus had a more severe clinical impairment, which naturally required more interventions, increased the length of hospitalization, and raised costs. Second, only adverse events in patients who were already hospitalized or returned to our facility after an exam were counted. Adverse events that occurred at home probably also generate costs and should be considered in future cost analyses. Finally, this study was designed retrospectively and conducted in a single center institution with a relatively small number of patients.
In a recent study, Romero-Castro et al[7] performed a thorax computed tomography (CT) scan on all patients who underwent an EUS-guided CYA injection, and they reported a very high incidence of asymptomatic pulmonary embolism that could have been missed by a clinical evaluation after the procedure. If a thorax CT was added to our EUS technique, the final treatment costs would significantly increase.
It is important to recognize that using hospital charges to estimate the costs of treatment poses a problem, because charges are different among institutions, and the treatment costs remain unknown for other institutions.
In conclusion, this preliminary analysis showed that the use of EUS-guided coils plus cyanoacrylate injection is more cost-effective than cyanoacrylate injection when the total costs are evaluated. Larger, multi-center studies are needed to address the cost effects of the EUS-guided approach of gastric varices.
Bleeding gastric varices implies high morbidity and mortality in cirrhotic and noncirrhotic patients. Bleeding and rebleeding episodes, as well as their management, have a high health-related cost impact.
Currently, there is insufficient data about the cost-effectiveness of available therapies, mainly endoscopic cyanoacrylate injection and endoscopic ultrasound (EUS)-guided therapy for the management of gastric varices.
The study's main objective was to evaluate the cost-effectiveness of treating gastric varices, whether by the standard endoscopic cyanoacrylate injection or by the novel EUS-guided combined coiling and cyanoacrylate injection technique.
This was an observational, descriptive, and retrospective study conducted in a single tertiary center. Patients with actively bleeding gastric varices and those with a history of bleeding were treated with either one of the two modalities. We evaluated the technical success and adverse event rates and the procedure and overall treatment costs.
We described a significantly higher number of procedures needed to achieve obliteration of gastric varices in the endoscopic cyanoacrylate group, with a higher number of admissions in this cohort. Technical and adverse events rates were not significantly different in the two groups. In terms of cost, endoscopic cyanoacrylate injection has a significantly higher mean total treatment cost, probably explained by a higher reintervention rate and hospitalization cost.
In our study, EUS-guided combined therapy with coiling and cyanoacrylate injection proved to be more cost-effective than endoscopic cyanoacrylate injection in terms of the overall treatment cost.
We encourage researchers to conduct a multicenter, randomized trial with a long-term follow-up comparing the endoscopic cyanoacrylate therapy vs the EUS-guided combined therapy with coiling and cyanoacrylate injection, in order to define formal therapeutical guidelines.
| 1. | Sarin SK, Jain AK, Jain M, Gupta R. A randomized controlled trial of cyanoacrylate versus alcohol injection in patients with isolated fundic varices. Am J Gastroenterol. 2002;97:1010-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 2. | Greig JD, Garden OJ, Anderson JR, Carter DC. Management of gastric variceal haemorrhage. Br J Surg. 1990;77:297-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Ríos Castellanos E, Seron P, Gisbert JP, Bonfill Cosp X. Endoscopic injection of cyanoacrylate glue versus other endoscopic procedures for acute bleeding gastric varices in people with portal hypertension. Cochrane Database Syst Rev. 2015: CD010180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Kok K, Bond RP, Duncan IC, Fourie PA, Ziady C, van den Bogaerde JB, van der Merwe SW. Distal embolization and local vessel wall ulceration after gastric variceal obliteration with N-butyl-2-cyanoacrylate: a case report and review of the literature. Endoscopy. 2004;36:442-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Bhat YM, Weilert F, Fredrick RT, Kane SD, Shah JN, Hamerski CM, Binmoeller KF. EUS-guided treatment of gastric fundal varices with combined injection of coils and cyanoacrylate glue: a large U.S. experience over 6 years (with video). Gastrointest Endosc. 2016;83:1164-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 6. | Sarin SK, Kumar A. Gastric varices: profile, classification, and management. Am J Gastroenterol. 1989;84:1244-1249. [PubMed] |
| 7. | Romero-Castro R, Ellrichmann M, Ortiz-Moyano C, Subtil-Inigo JC, Junquera-Florez F, Gornals JB, Repiso-Ortega A, Vila-Costas J, Marcos-Sanchez F, Muñoz-Navas M, Romero-Gomez M, Brullet-Benedi E, Romero-Vazquez J, Caunedo-Alvarez A, Pellicer-Bautista F, Herrerias-Gutierrez JM, Fritscher-Ravens A. EUS-guided coil versus cyanoacrylate therapy for the treatment of gastric varices: a multicenter study (with videos). Gastrointest Endosc. 2013;78:711-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 8. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2609] [Cited by in RCA: 2361] [Article Influence: 214.6] [Reference Citation Analysis (4)] |
| 9. | Robles-Medranda C, Valero M, Nebel JA, de Britto Junior SR, Puga-Tejada M, Ospina J, Muñoz-Jurado G, Pitanga-Lukashok H. Endoscopic-ultrasound-guided coil and cyanoacrylate embolization for gastric varices and the roles of endoscopic Doppler and endosonographic varicealography in vascular targeting. Dig Endosc. 2019;31:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Greenwald BD, Caldwell SH, Hespenheide EE, Patrie JT, Williams J, Binmoeller KF, Woodall L, Haluszka O. N-2-butyl-cyanoacrylate for bleeding gastric varices: a United States pilot study and cost analysis. Am J Gastroenterol. 2003;98:1982-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Laska EM, Meisner M, Siegel C. Statistical inference for cost-effectiveness ratios. Health Econ. 1997;6:229-242. [PubMed] [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Ecuador
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Goral V S-Editor: Zhang L L-Editor: A P-Editor: Wang LL