Published online Sep 16, 2020. doi: 10.4253/wjge.v12.i9.304
Peer-review started: May 27, 2020
First decision: July 4, 2020
Revised: July 16, 2020
Accepted: August 24, 2020
Article in press: August 24, 2020
Published online: September 16, 2020
Processing time: 106 Days and 0.1 Hours
Endocytoscopy is a next-generation endoscopic system that facilitates real-time histopathologic endoscopic diagnosis of colorectal lesions by virtue of its 520 × maximum magnification.
We present the case of a 63-year-old man with sigmoid colon cancer who was regularly referred for follow-up colonoscopy after endoscopic resection of T1 rectal cancer. Colonoscopy revealed a 12 mm reddish polyp, including a depression and a flat area in the sigmoid colon. Endocytoscopic observation showed unclear gland formation and agglomeration of distorted nuclei (depression), suggesting a submucosal invasive (T1) cancer. In the flat area, slit-like smooth lumens and regular pattern of fusiform nuclei were found, suggesting an adenoma. On the basis of these endocytoscopic findings, we predicted this lesion as T1 cancer (depression) with adenoma (flat area) and performed endoscopic resection corresponding to the final histopathological diagnosis.
We could perform an optical diagnosis of T1 sigmoid cancer with adenoma by using endocytoscopy before treatment.
Core Tip: Endocytoscopy is a next-generation endoscopic system. Endocytoscopic observation suggested that a 12 mm reddish polyp, including a depression and a flat area in the sigmoid colon was T1 cancer (depression) with adenoma (flat area). We could perform an optical diagnosis of the lesion by using endocytoscopy before treatment.
- Citation: Akimoto Y, Kudo SE, Ichimasa K, Kouyama Y, Misawa M, Hisayuki T, Kudo T, Nemoto T. Small invasive colon cancer with adenoma observed by endocytoscopy: A case report. World J Gastrointest Endosc 2020; 12(9): 304-309
- URL: https://www.wjgnet.com/1948-5190/full/v12/i9/304.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i9.304
Integrated-type endocytoscopy (CF-H290ECI; Olympus Corp, Tokyo, Japan) is a next-generation endoscopy commercially available in Europe, the Middle East, Hong Kong, and Japan. An endocytoscope comprises a contact light microscopy system (magnification, × 520; focusing depth, 35 μm) integrated into the distal tip of a colonoscope. It enables in vivo microvascular evaluation with the narrow band imaging mode and cellular visualization after staining with 0.05% crystal violet (1% methylene blue may be added if necessary)[1-3]. In the diagnosis of colorectal lesions, endocytoscopy is particularly useful not only for differentiating neoplastic and non-neoplastic lesions but also for diagnosing the depth of invasion of colorectal cancer[4,5]. In addition, endocytoscopy has recently been reported to be an effective modality for the differential diagnosis of low-grade colorectal adenomas[6]. In the present case, we accurately predicted the histopathological findings of a two-component lesion by using endocytoscopy before treatment.
A 63-year-old man was regularly referred for follow-up colonoscopy after endoscopic resection of T1 rectal cancer.
The patient consumed alcohol twice a week but denied smoking cigarettes. His family history was negative for any gastrointestinal cancer.
The patient was treated for T1 rectal cancer via endoscopic resection 4 years previously. The result of the histological diagnosis was adenocarcinoma (well to moderately differentiated), with a depth of invasion of 9000 μm (T1), negative lymphovascular invasion, budding grade 1, and negative horizontal and vertical margins. We considered the risk of lymph node metastasis (LNM), and recommended that he undergo additional surgery. However, he refused to undergo surgery and was consequently regularly referred for follow-up colonoscopy. In addition, he had high blood pressure and diabetes.
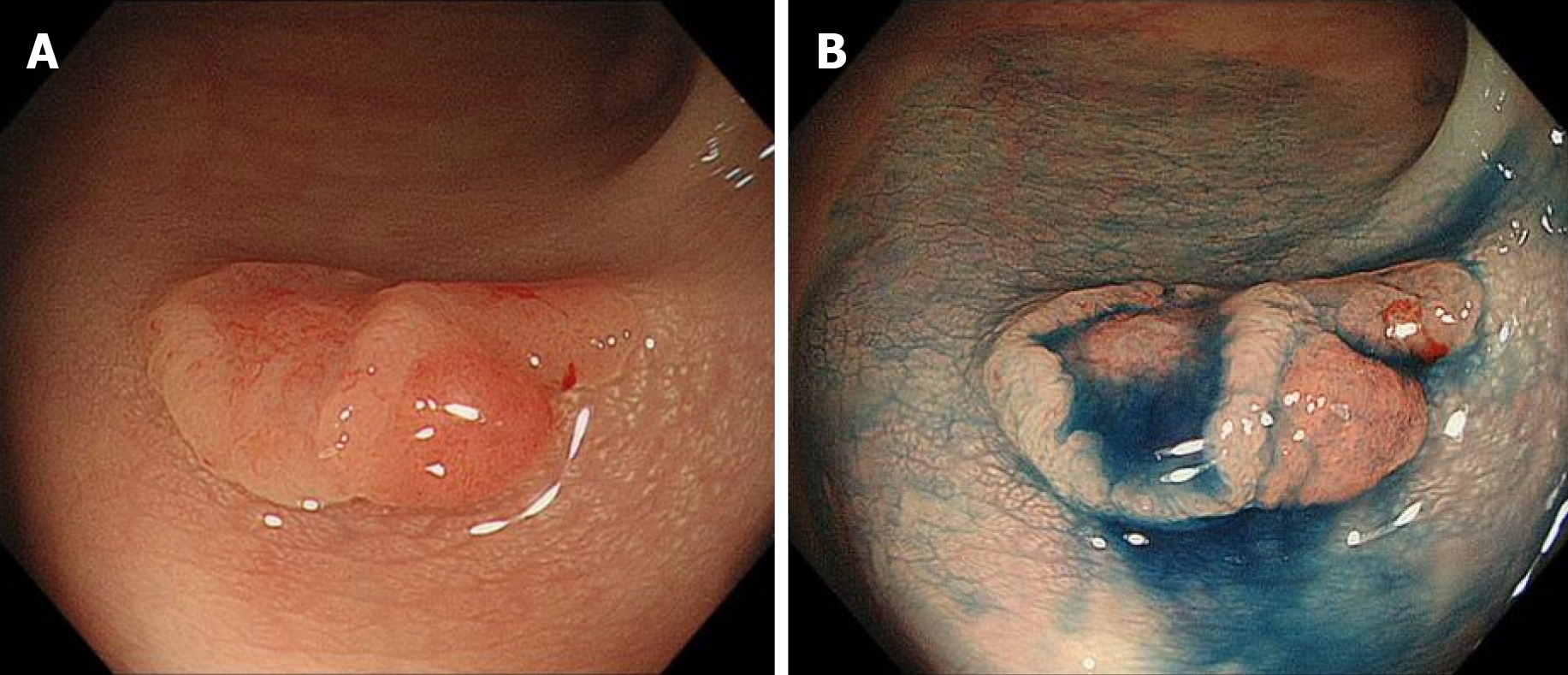
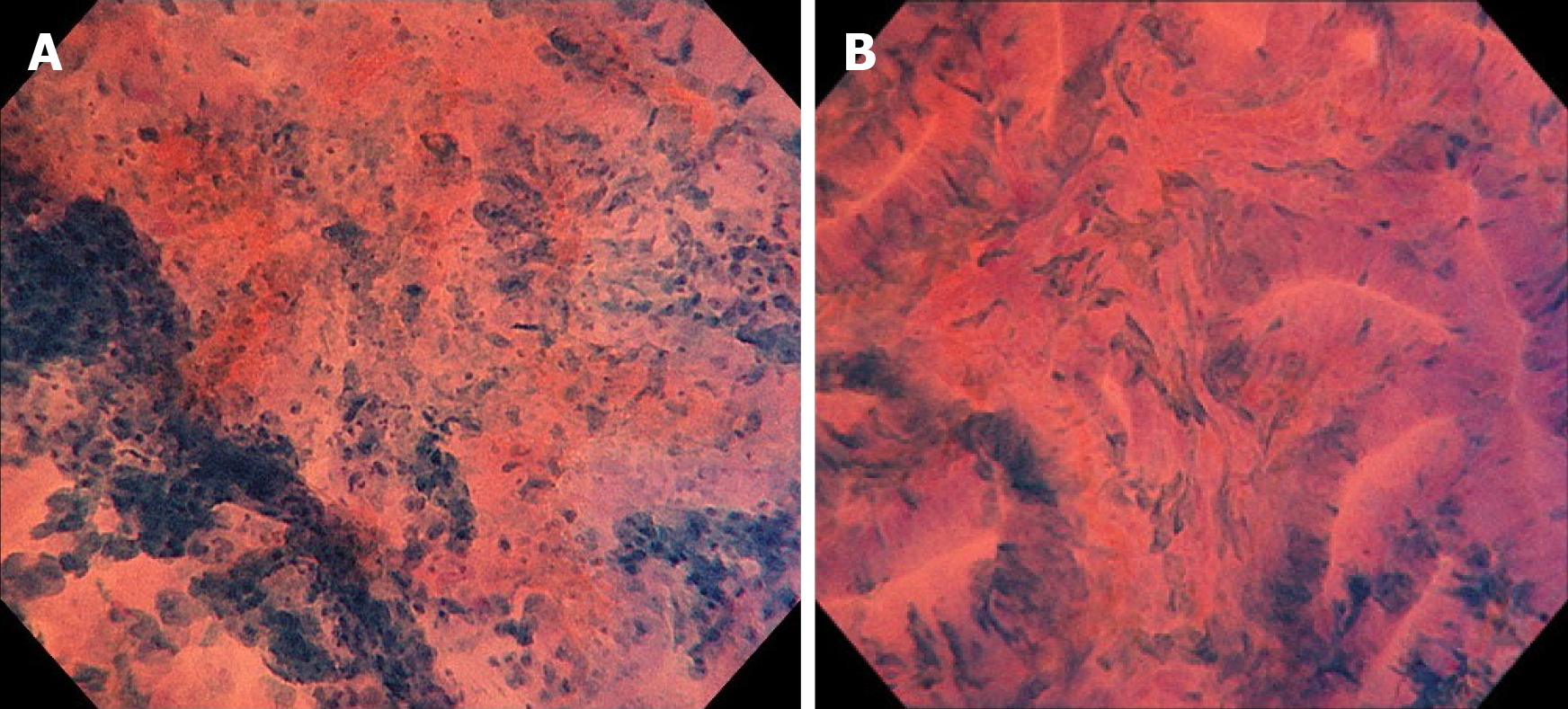
Colonoscopy revealed a 12 mm reddish polyp in the sigmoid colon (Figure 1A). We could recognize a depression on the left side and a flat area on the right side with indigo carmine staining (Figure 1B). On the left side of the lesion (depression), unclear gland formation and agglomeration of distorted nuclei strongly stained by methylene blue were visible with endocytoscopy, suggesting a submucosal invasive cancer (Figure 2A). On the right side of the lesion (flat area), slit-like smooth lumens and a regular pattern of fusiform nuclei were found, suggesting an adenoma (Figure 2B).
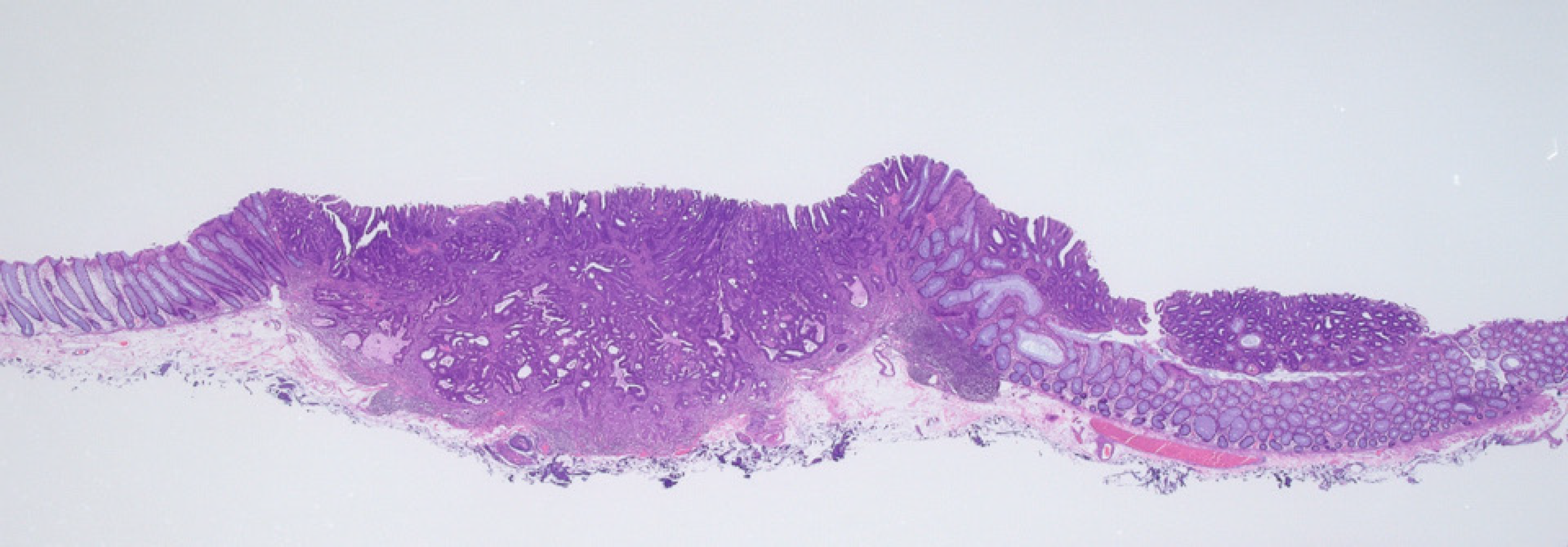
T1 sigmoid colon cancer with adenoma. The left side of the lesion corresponded to T1 cancer and the right side corresponded to adenoma (Figure 3).
Although we predicted this lesion to show massive submucosal invasion based on the optical diagnosis using endocytoscopy, we performed endoscopic mucosal resection as the first-line treatment. We decided to determine the need for additional surgery on the basis of the final histopathological diagnosis of the resected specimens.
Histopathological findings revealed tubular adenocarcinoma (depression) with adenoma (flat area), which was consistent with the results of the endocytoscopic diagnosis. Non-neoplastic glands were observed between the left and right sides. This lesion may have two different origins, that is, the depression and flat areas may correspond to submucosal invasive (T1) cancer and adenoma, respectively. The final histological diagnosis was as follows: Adenocarcinoma (well to moderately differentiated tubular adenocarcinoma) with adenoma, with a depth of invasion of 2407 μm (T1), negative for lymphovascular invasion, budding grade 1, and negative horizontal and vertical margins. He did not wish to undergo additional surgery. In conformity with the Japanese guidelines for the treatment of colorectal cancer, he was followed up using CT, colonoscopy and tumor marker[7]. There has not been any evidence of recurrence to date.
Several studies have reported the effectiveness of optical diagnosis with endocytoscopy for determining the treatment course of colorectal lesions. Kudo et al[8] reported that endocytoscopy provided additional diagnostic value to conventional pit pattern classification for colorectal neoplasms. The diagnostic ability of endocytoscopy to predict neoplastic changes was excellent [sensitivity: 97.4%, 95% confidence interval (CI): 95.4%-98.6%, specificity: 89.7%, 95%CI: 78.8%-96.1%, accuracy: 96.5%, 95%CI: 94.5%-97.9%]. In addition, its ability to differentiate massively invasive submucosal colorectal cancer was also good, showing 83.1% (95%CI: 73.7%-90.2%) of sensitivity, 99.1% (95%CI: 97.6%-99.7%) and 96.3% (95%CI: 94.3%-97.8%).Thus, endocytoscopy enables the optical diagnosis of colorectal lesions with high confidence levels. In the present case, we could predict the differences in histopathology between T1 cancer and adenoma within the same lesion by using endocytoscopy in real time.
The histopathological diagnosis of this lesion was T1 sigmoid cancer with adenoma. T1 colorectal cancer accounts for approximately 10% of all LNM; hence, such lesions require additional surgery with lymph node dissection after endoscopic resection for achieving complete cure[9-12]. Although several guidelines are available for the management of T1 colorectal cancer after endoscopic resection, the criteria for additional surgery remain controversial[7,13-16]. According to the Japanese guidelines, this patient had one risk factor, namely, a depth of invasion ≥ 1000 µm, and hence, additional surgery was recommended. However, some studies reported that the depth of invasion was not a risk factor for LNM in T1 colorectal cancer[12,17,18]. Moreover, the incidence of LNM is extremely low — 1.3% (95%CI: 0%-2.4%) — in the cases with submucosal invasion depth of 1000 µm or more without associated risk factors (other than the depth of invasion) as per Japanese guidelines[7]. The risk was very low since this patient had no evidence of lymphovascular invasion, reported to have most predictive value[9], differentiation, and tumor budding. However if the lesion recurs, it can be life-threatening; therefore, a decision on additional treatment should be carefully made. This patient refused to undergo surgery 4 years previously despite receiving a clear explanation about the risk of LNM and the merits and demerits of additional surgery. Our findings suggest the need for developing a more accurate risk stratification for LNM in the future.
Endocytoscopy is an effective modality for the optical diagnosis of colorectal lesions. In the present case, we could diagnose the T1 cancer and adenoma components within the same lesion before treatment.
| 1. | Misawa M, Kudo SE, Mori Y, Nakamura H, Kataoka S, Maeda Y, Kudo T, Hayashi T, Wakamura K, Miyachi H, Katagiri A, Baba T, Ishida F, Inoue H, Nimura Y, Mori K. Characterization of Colorectal Lesions Using a Computer-Aided Diagnostic System for Narrow-Band Imaging Endocytoscopy. Gastroenterology. 2016;150:1531-1532.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 2. | Mori Y, Kudo SE, Misawa M, Saito Y, Ikematsu H, Hotta K, Ohtsuka K, Urushibara F, Kataoka S, Ogawa Y, Maeda Y, Takeda K, Nakamura H, Ichimasa K, Kudo T, Hayashi T, Wakamura K, Ishida F, Inoue H, Itoh H, Oda M, Mori K. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann Intern Med. 2018;169:357-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 376] [Article Influence: 47.0] [Reference Citation Analysis (4)] |
| 3. | Ichimasa K, Kudo SE, Mori Y, Wakamura K, Ikehara N, Kutsukawa M, Takeda K, Misawa M, Kudo T, Miyachi H, Yamamura F, Ohkoshi S, Hamatani S, Inoue H. Double staining with crystal violet and methylene blue is appropriate for colonic endocytoscopy: an in vivo prospective pilot study. Dig Endosc. 2014;26:403-408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Kudo SE, Wakamura K, Ikehara N, Mori Y, Inoue H, Hamatani S. Diagnosis of colorectal lesions with a novel endocytoscopic classification - a pilot study. Endoscopy. 2011;43:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Mori Y, Kudo S, Ikehara N, Wakamura K, Wada Y, Kutsukawa M, Misawa M, Kudo T, Kobayashi Y, Miyachi H, Yamamura F, Ohtsuka K, Inoue H, Hamatani S. Comprehensive diagnostic ability of endocytoscopy compared with biopsy for colorectal neoplasms: a prospective randomized noninferiority trial. Endoscopy. 2013;45:98-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Kudo T, Suzuki K, Mori Y, Misawa M, Ichimasa K, Takeda K, Nakamura H, Maeda Y, Ogawa Y, Hayashi T, Wakamura K, Ishida F, Inoue H, Kudo SE. Endocytoscopy for the differential diagnosis of colorectal low-grade adenoma: a novel possibility for the "resect and discard" strategy. Gastrointest Endosc. 2020;91:676-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1431] [Article Influence: 238.5] [Reference Citation Analysis (3)] |
| 8. | Kudo SE, Mori Y, Wakamura K, Ikehara N, Ichimasa K, Wada Y, Kutsukawa M, Misawa M, Kudo T, Hayashi T, Miyachi H, Inoue H, Hamatani S. Endocytoscopy can provide additional diagnostic ability to magnifying chromoendoscopy for colorectal neoplasms. J Gastroenterol Hepatol. 2014;29:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 10. | Yasue C, Chino A, Takamatsu M, Namikawa K, Ide D, Saito S, Igarashi M, Fujisaki J. Pathological risk factors and predictive endoscopic factors for lymph node metastasis of T1 colorectal cancer: a single-center study of 846 lesions. J Gastroenterol. 2019;54:708-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Ichimasa K, Kudo SE, Mori Y, Misawa M, Matsudaira S, Kouyama Y, Baba T, Hidaka E, Wakamura K, Hayashi T, Kudo T, Ishigaki T, Yagawa Y, Nakamura H, Takeda K, Haji A, Hamatani S, Mori K, Ishida F, Miyachi H. Artificial intelligence may help in predicting the need for additional surgery after endoscopic resection of T1 colorectal cancer. Endoscopy. 2018;50:230-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 12. | Miyachi H, Kudo SE, Ichimasa K, Hisayuki T, Oikawa H, Matsudaira S, Kouyama Y, Kimura YJ, Misawa M, Mori Y, Ogata N, Kudo T, Kodama K, Hayashi T, Wakamura K, Katagiri A, Baba T, Hidaka E, Ishida F, Kohashi K, Hamatani S. Management of T1 colorectal cancers after endoscopic treatment based on the risk stratification of lymph node metastasis. J Gastroenterol Hepatol. 2016;31:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 311] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 14. | Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, Arnold D; ESMO Guidelines Working Group. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi64-vi72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 659] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 15. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 702] [Article Influence: 100.3] [Reference Citation Analysis (1)] |
| 16. | Benson AB, Venook AP, Cederquist L, Chan E, Chen YJ, Cooper HS, Deming D, Engstrom PF, Enzinger PC, Fichera A, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wu CS, Gregory KM, Freedman-Cass D. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 576] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 17. | Kouyama Y, Kudo SE, Miyachi H, Ichimasa K, Hisayuki T, Oikawa H, Matsudaira S, Kimura YJ, Misawa M, Mori Y, Kodama K, Kudo T, Hayashi T, Wakamura K, Katagiri A, Hidaka E, Ishida F, Hamatani S. Practical problems of measuring depth of submucosal invasion in T1 colorectal carcinomas. Int J Colorectal Dis. 2016;31:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Rönnow CF, Arthursson V, Toth E, Krarup PM, Syk I, Thorlacius H. Lymphovascular Infiltration, Not Depth of Invasion, is the Critical Risk Factor of Metastases in Early Colorectal Cancer: Retrospective Population-based Cohort Study on Prospectively Collected Data, Including Validation. Ann Surg. 2020;Online ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dumoulin FL S-Editor: Ma YJ L-Editor: A P-Editor: Wang LL