Published online Jul 16, 2020. doi: 10.4253/wjge.v12.i7.198
Peer-review started: March 10, 2020
First decision: April 2, 2020
Revised: May 28, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: July 16, 2020
Processing time: 119 Days and 8.1 Hours
Endoscopic mucosal resection (EMR) is an effective and minimally invasive alternative to surgery for large polyps and laterally spreading lesions. Gross morphology and surface characteristics may help predict submucosal invasion of the lesion (SMIL) during endoscopic evaluation. This is one of the largest single-center studies reporting endoscopic mucosal resection for larger (≥ 20 mm) colorectal lesions in the United States.
To determine the recurrence rate of adenomas and endoscopic features that may predict submucosal invasion of colonic mucosal neoplasia.
This is a retrospective cohort study of all the patients referred for endoscopic mucosal resection for lesions ≥ 20 mm, spanning a period from January 2013 to February 2017. The main outcome measure was identifying features that may predict submucosal invasion of mucosal lesions and predict recurrence of adenomas on follow-up surveillance colonoscopy performed at 4-6 mo.
A total of 480 patients with 500 lesions were included in the study. The median age was 68 (Inter quantile range: 14) with 52% males. The most common lesion location was ascending colon (161; 32%). Paris classification 0-IIa (Flat elevation of mucosa - 316; 63.2%); Kudo Pit Pattern IIIs (192; 38%) and Granular surface morphology (260; 52%) were most prevalent. Submucosal invasion was present in 23 (4.6%) out of 500 lesions. The independent risk factors for SMIL were Kudo Pit Pattern IIIL + IV and V (Odds ratio: 4.5; P value < 0.004) and Paris classification 0-IIc (Odds ratio: 18.2; P value < 0.01). Out of 500, 354 post-endoscopic mucosal resection scars were examined at surveillance colonoscopy. Recurrence was noted in 21.8% (77 cases).
There was overall low prevalence of SMIL in our study. Kudo pit pattern (IIIL + IV and V) and Paris classification 0-IIc were the only factors identified as an independent risk factor for submucosal invasion. The independent risk factor for recurrence was adenoma size (> 40 mm). Almost all recurrences (98.8%) were treated endoscopically.
Core tip: Endoscopic mucosal resection is an effective and minimally invasive alternative to surgery for large polyps and laterally spreading lesions. Endoscopic features can also help identify the high-risk features for submucosal invasion and recurrence of adenomas. Our study conducted review of 480 patients with 500 lesions. We found endoscopic mucosal resection to be an effective treatment for large colon lesions. We were also able to identify independent risk factors for submucosal invasion (Kudo Pit Pattern IIIL + IV and V and Paris classification 0-IIc) and recurrence.
- Citation: Rashid MU, Khetpal N, Zafar H, Ali S, Idrisov E, Du Y, Stein A, Jain D, Hasan MK. Colon mucosal neoplasia referred for endoscopic mucosal resection: Recurrence of adenomas and prediction of submucosal invasion. World J Gastrointest Endosc 2020; 12(7): 198-211
- URL: https://www.wjgnet.com/1948-5190/full/v12/i7/198.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i7.198
Colonoscopic detection and removal of precursor adenoma helps prevent colorectal cancer[1]. Small (up to 10 mm) and pedunculated lesions (constituting up to 90% of lesions) can be resected easily during colonoscopy, but larger lesions are increasingly being identified and are associated with a significant risk of cancer[2,3]. Serrated polyps can account for up to 15%-30% of colorectal cancer and have a high malignancy potential. Similarly, laterally spreading lesions may have high-grade dysplasia or invasive carcinoma in more than 30% cases[4]. These high-risk lesions must be evaluated carefully to guide the appropriate intervention and minimize the risk of recurrence[5].
Until recently, large colon polyps have been treated most commonly with either open or laparoscopic surgical resection. But surgery has an inherent risk of significant morbidity and cost and the average length of hospitalization after surgical intervention could be up to 5 d[6]. On the other hand, endoscopic mucosal resection (EMR) and endoscopic submucosal dissection are two minimally invasive techniques that are used to resect large polyps. EMR involves submucosal injection to elevate the lesion and then perform en-bloc or piecemeal resection of the lesions[7]. EMR is an outpatient procedure; most patients are discharged on the same day. EMR is three times more cost-effective than surgical intervention and is associated with less morbidity, an effect that particularly benefits elderly patients with multiple comorbidities[8].
The gross characteristics and surface morphology of large colonic lesions may predict submucosal invasion, and this correlation can be used to guide the intervention. Narrow band imaging, chromoendoscopy, and surface pit patterns by Kudo classification have been studied to predict the submucosal invasion of colorectal lesions[9,10].
Overt submucosal invasion often is recognized by a depression or ulceration of the lesions. Other factors associated with submucosal invasion include size of the lesion, location, and non-granular surface pattern. Gross morphological features should be inspected carefully during endoscopy to select an appropriate resection technique that can minimize morbidity and optimize clinical outcomes[5].
The aim of our study was to determine the recurrence rate of adenomas and endoscopic features that may predict submucosal invasion of colonic mucosal neoplasia.
This retrospective cohort study was conducted at our advanced endoscopy unit of Advent Health Hospital. A total of 480 patients with 500 lesions were included in the study. The study period spanned from January 2013 through April 2017. Inclusion criteria were set to include polyps with size ≥ 20 mm. The hospital’s Institutional Review Board approved this study.
A primary endoscopist performed the initial colonoscopies and identified the lesions. Afterward, the patients were referred to our center for endoscopic resection of these lesions. We thoroughly reviewed their medical records and considered their comorbidities in determining the therapeutic process.
Adenomas were classified into the following types using white light and narrow band imaging: (1) Paris classification; (2) Kudo pit patterns I-V; and (3) Surface morphology (granular, mixed and non-granular). Snares with known dimensions were used to assess the lesion size carefully.
One of the three advanced endoscopists involved in the study performed the colonoscopy with EMR. A split-dose regimen was preferred for colonoscopy preparation, and the majority of the procedures were performed under monitored anesthesia care. Carbon dioxide (CO2) gas was used for colonic insufflation.
Initially, polyps were inspected carefully with both white light and narrow band imaging, and findings were recorded. The resection was performed in three steps repetitively: (1) Injection; (2) Snare resection; and (3) Inspection. Injection was used to elevate the lesion towards the lumen. Indigo carmine or methylene blue dye mixed with Voluven® solution (6% hydroxyethyl starch 130/0.4 in 0.9% sodium chloride) was injected into the submucosa. The use of epinephrine diluted to 1:100000 was added to the injection at the discretion of the endoscopist. Different snares were used for resection, including Oval snare (Cook Medicals), Spiral snare (Olympus), Captivator snares (Boston Scientific), and Histolock (United States endoscopy). In addition to resecting the polyp, the surrounding 1-3 mm margin around the lesion also was excised. We used endocut 2-1-4 or coag with an effect of 2 and 18 WATTs (ERBE, VIO 300) cautery settings for polyp resection.
Following the procedures, the resected and collected tissues were sent for histopathology evaluation. We recorded all the specifics of the procedure, including procedure time, intra-procedure complications, morphological characteristics of lesions, and the use of additional therapeutic modalities.
Patients were kept under observation for a few hours and were counseled for further care. In the absence of any obvious immediate complications, patients were discharged on the same day with necessary instructions about post-EMR procedural care.
For a regular follow-up as per our endoscopy unit protocol, we telephoned patients within a week and recorded any delayed adverse events. If the pathology did not show lesions with submucosal invasion, a surveillance colonoscopy (SC) was performed at 4-6 mo. We used the same follow-up protocol (phone call within a week) for the patients receiving SCs. Any recurrent or residual adenomas that were identified during SC were treated endoscopically at the endoscopist’s discretion.
If patients had evidence of submucosal Invasion cancer on initial histopathology, they were contacted and referred for surgical intervention.
The resected tissue was collected and sent for microscopic examination. Special attention was given to vascular and lymphatic invasion, the depth of invasion, and basal resection margin and differentiation. The resection was marked complete when the examined basal margins did not have evidence of tumor, and incomplete when basal margins had tumor involvement or were not evaluable due to coagulation necrosis. Lateral margins were not included in the analysis due to the piecemeal nature of majority of the resections.
Submucosal mucosal invasion: When tumor cells penetrate through the muscularis mucosa into the submucosa.
Recurrence/residual: Histological confirmation of adenoma at 4-6 mo (SC).
Sustained intra-procedural bleeding: Bleeding that lasts for more than 30 s and requires intervention to stop it.
Delayed bleeding: Bleeding that develops post-procedurally and requires an emergency department visit, hospital admission, or colonoscopic intervention for hemostasis.
Immediate perforation: Perforations that develop during the procedure or immediately after the procedure. It was defined as full-thickness defect in the colonic wall.
Delayed perforation: Perforation that develops after patients are discharged from the hospital, and patients presenting again to the hospital with abdominal pain, distension and signs/symptoms of peritonitis.
Median and Inter quantile range (IQR) were used to summarize results for continuous variables. Frequencies and percentages (%) were used for categorical variables. Mann-Whitney or Student’s-t tests were used to compare the distribution of continuous variables by the outcome. χ2 or Fisher's exact tests were used to test for association between categorical variables and outcome. We used 95% confidence interval (CI) and odds ratios (ORs) for association between variables. Significance level of 5% was used to test two-tailed hypothesis throughout. For submucosal invasion (SMI), we used multiple logistic regression analysis, adjusted by confounding factors. Lesion size was one of the continuous variables and was grouped into categories. For statistical analysis, we used SAS 9.4 (SAS Institute, Cary, NC, United States).
A total of 500 lesions in 480 patients were included in the study. The median age was 68 years (IQR: 14), with 52 % males. The median lesion size was 30 mm (IQR: 15). The majority of adenomas were resected with piecemeal resection (372) with en-bloc resection performed in 96 cases. The remaining 32 patients did not undergo complete endoscopic resection for various reasons (unable to engage in snare 16, submucosal fibrosis 9, suspected cancer on endoscopic evaluation 7). At SC, 354 post-EMR scars were examined, and the remaining did not undergo due to carcinoma, incomplete or partial resection of adenoma at initial EMR, no follow-up available, or other reason (Figure 1).
The majority of polyps were in the ascending colon 161 (32%) and cecum 102 (20%). When grouped together, rectosigmoid colon had only 80 lesions (16%). The most prevalent lesions were flat elevation of mucosa (Paris classification 0-IIa 316; 63.2%), Kudo pit pattern IIIs (192; 38%), and granular surface morphology (260; 52%). The majority of the patients were American Society of Anesthesiology (ASA) class 2 (350, 70%). The most common lesion size group was 21-30 mm (198; 40%) (Table 1).
| Variable | Median | IQR | Frequency | Percent, % |
| Number of > 20 mm adenomas | 1 | 0 | ||
| Polyp number | 1 | 0 | ||
| Size (mm) | 30 | 15 | ||
| Age (yr) | 68 | 14 | ||
| Height (cm) | 170 | 15 | ||
| Weight (kg) | 80 | 25 | ||
| BMI | 28 | 7.35 | ||
| Colonoscopy duration (min) | 44 | 30 | ||
| If blood transfusion, number of units | 0 | 0 | ||
| Sex | ||||
| Male | 260 | 52 | ||
| Female | 240 | 48 | ||
| ASA | ||||
| Class 1 | 14 | 3 | ||
| Class 2 | 350 | 70 | ||
| Class 3 | 136 | 27 | ||
| Paris classification | ||||
| Ip (pedunculated) | 34 | 6.8 | ||
| 0-IIa (flat elevation of mucosa) | 316 | 63.2 | ||
| 0-IIb (flat mucosal change) | 68 | 13.6 | ||
| 0-IIc (flat mucosal depression) | 3 | 0.6 | ||
| 0-IIa + c (flat elevation with central depression) | 26 | 5.2 | ||
| 0-IIa + Is (flat elevation with raised broad based nodule) | 53 | 10.6 | ||
| Morphology | ||||
| Granular | 260 | 52 | ||
| Non-granular | 206 | 41 | ||
| Mixed | 34 | 7 | ||
| Kudo Pit Pattern | ||||
| I | 3 | 1 | ||
| II | 10 | 2 | ||
| IIIs | 192 | 38 | ||
| IIIL | 172 | 34 | ||
| IV | 62 | 12 | ||
| V | 19 | 4 | ||
| IIIs + IIIL | 20 | 4 | ||
| IIIs + IV | 4 | 1 | ||
| IIIL + IV | 18 | 4 | ||
| Location | ||||
| Rectum (< 5 cm from anus) | 4 | 1 | ||
| Rectum (> 5 cm from anus) | 31 | 6 | ||
| Sigmoid | 45 | 9 | ||
| Descending colon | 25 | 5 | ||
| Splenic flexure | 6 | 1 | ||
| Transverse | 65 | 13 | ||
| Hepatic flexure | 39 | 8 | ||
| Ascending colon | 161 | 32 | ||
| Cecum | 102 | 20 | ||
| Ileocecal valve involved | 18 | 4 | ||
| Appendiceal orifice involved | 4 | 1 | ||
| Lesion size | ||||
| 20 mm | 89 | 18 | ||
| 21-30 mm | 198 | 40 | ||
| 31-40 mm | 107 | 21 | ||
| > 40 mm | 106 | 21 |
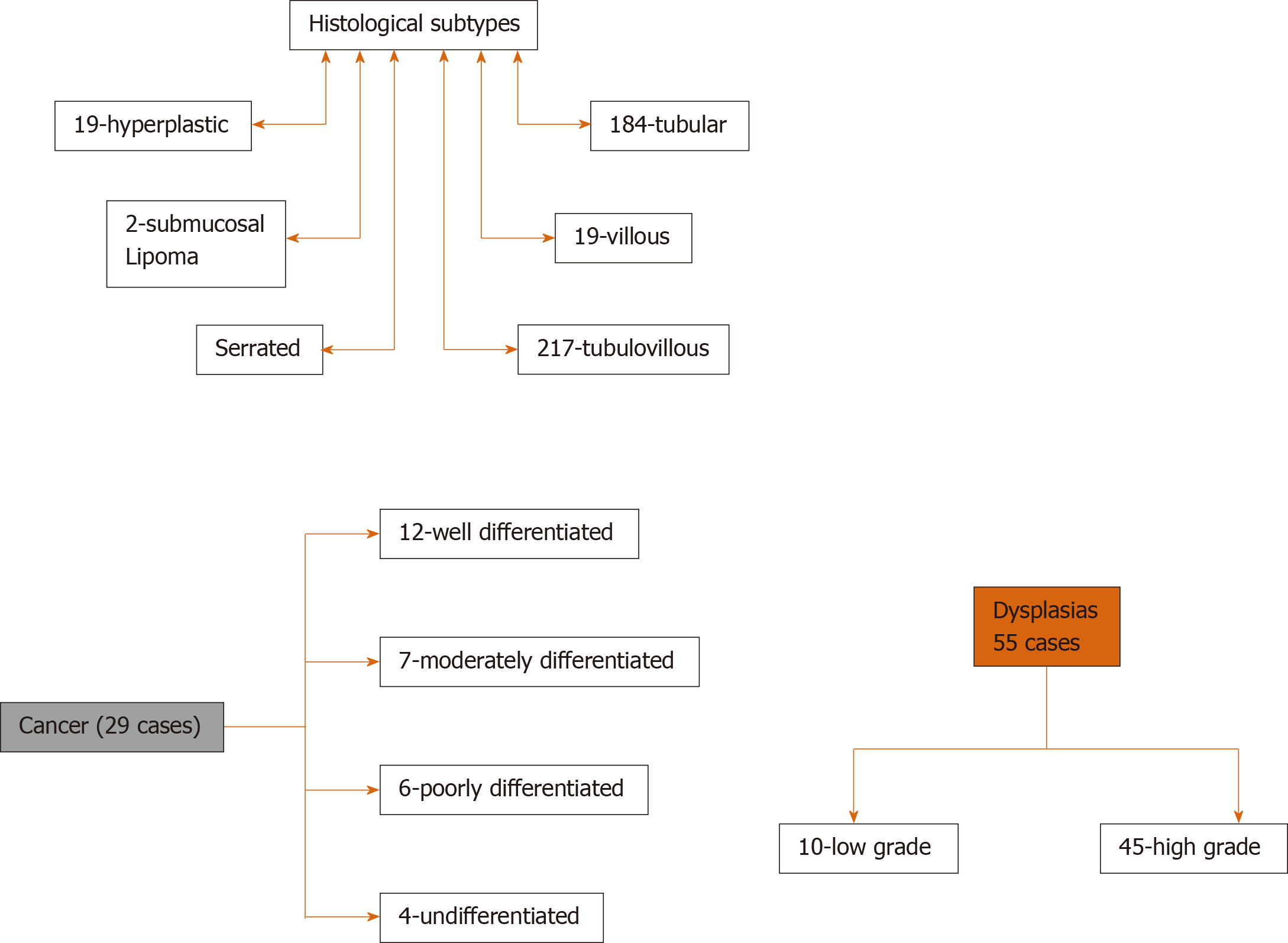
Tubulo-villous adenoma (216 cases) was the most common histological subtype followed by tubular adenoma (184 cases), and least were submucosal lipoma (2 cases). High-grade dysplasia was noted in 45 cases, and cancer was found in 29 cases. SMI was found in 23/29 cases of the cancerous lesions and intramucosal cancer in 6/29 cases. Lesion characteristics are detailed in Figure 2.
Out of 500 lesions removed, 23 (4.6%) lesions were diagnosed with submucosal invasion on pathology. The mean age in both groups (SMI vs non- SMI) was almost the same, 68 (non-SMI group) vs 69 (SMI group). Submucosal invasion had equal incidence in both males and females (11 vs 12 cases, respectively). The majority of adenomas with submucosal invasions had a size greater than 40 mm (9; 39%), Kudo Pit pattern IIIL (8; 34%), and had Paris classification 0-IIa (8; 26%). On univariate analysis for factors associated with SMI, P value was found significant (P < 0.05) for Kudo Pit pattern, Paris Classification and size of the lesion. A large number of lesions with SMI appeared granular (13; 56%) and were found in ascending colon (6, 26%), followed by rectum, sigmoid, and cecum (4, 17%). No statistical significance on univariate analysis was found for locations of the tumor, morphology, gender, BMI, and age. Factors associated with SMI were shown in Table 2.
| Factors | No SMI (%) | SMI (%) | P value |
| n = 477 | n = 23 | ||
| Size (mm) | 30 (12.38) | 40 (18.14) | aP < 0.05 |
| Age (yr) | 68 (9.7) | 69 (10.6) | 0.5288 |
| Height (cm) | 170 (10.38) | 170 (11.04) | 0.9185 |
| Weight (kg) | 80 (22.32) | 87 (30.74) | 0.518 |
| BMI | 28 (6.77) | 30 (8.3) | 0.31 |
| Colonoscopy duration (min) | 43 (25.03) | 56 (21.49) | 0.063 |
| Gender | 0.6816 | ||
| Male | 249 (52.2) | 11 (47.83) | |
| Female | 228 (47.8) | 12 (52.17) | |
| ASA | 0.6912 | ||
| 1 | 14 (2.94) | 0 (0) | |
| 2 | 333 (69.81) | 17 (73.91) | |
| 3 | 130 (27.25) | 6 (26.09) | |
| Size group | 0.1663 | ||
| 20 mm | 86 (18.03) | 3 (13.04) | |
| 21-30 mm | 192 (40.25) | 6 (26.09) | |
| 31-40 mm | 102 (21.38) | 5 (21.74) | |
| > 40 mm | 97 (20.34) | 9 (39.13) | |
| Paris classification | bP < 0.01 | ||
| Ip (pedunculated) | 32 (6.7) | 2 (8.7) | |
| 0-IIa (flat elevation of mucosa) | 308 (64.57) | 8 (26.09) | |
| 0-IIb (flat mucosal change) | 68 (14.25) | 0 (0) | |
| 0-IIc (flat mucosal depression) | 1 (0.21) | 2 (0) | |
| 0-IIa + c (flat elevation with central depression) | 22 (4.61) | 4 (17.39) | |
| 0-IIa + Is (flat elevation with raised broad based nodule) | 46 (9.64) | 7 (30.43) | |
| Kudo Pit Pattern | cP < 0.01 | ||
| I | 3 (0.63) | 0 (0) | |
| II | 10 (2.1) | 0 (0) | |
| IIIs | 190 (39.83) | 2(8.7) | |
| IIIL | 164 (34.38) | 8 (34.78) | |
| IV | 58 (12.16) | 4 (17.39) | |
| V | 15 (3.14) | 4 (17.39) | |
| IIIs + IIIL | 18 (3.77) | 2 (8.7) | |
| IIIs + IV | 4 (0.84) | 0 (0) | |
| IIIL + IV, Vn | 15 (3.14) | 3 (13.04) | |
| Morphology | 0.3404 | ||
| Granular | 247 (51.78) | 13 (56.52) | |
| Non granular | 199 (41.72) | 7 (30.43) | |
| Mixed | 31 (6.5) | 3 (13.04) | |
| Location | 0.6017 | ||
| Rectum (< 5 cm from anus) | 4 (0.84) | 0 (0) | |
| Rectum (> 5 cm from anus) | 27 (5.66) | 4 (17.39) | |
| Sigmoid | 41 (8.6) | 4 (17.39) | |
| Descending colon | 24 (5.03) | 1 (4.35) | |
| Splenic flexure | 6 (1.26) | 0 (0) | |
| Transverse | 62 (13) | 3 (13.04) | |
| Hepatic flexure | 38 (7.97) | 1 (4.35) | |
| Ascending colon | 155 (32.49) | 6 (26.09) | |
| Cecum | 98 (20.55) | 4 (17.39) | |
| Ileocecal valve involved | 18 (3.77) | 0 (0) | |
| Appendiceal orifice involved | 4 (0.84) | 0 (0) |
We studied the association of submucosal invasion with age, gender, ASA, size, Paris classification, Kudo Pit pattern, morphology, and location. No statistically significant association was found with age, gender, and ASA classification. Patients with adenoma size greater than 40 mm had increased OR of 1.54 for submucosal lesion, but this relationship was not statistically significant (P value 0.30). Similarly, adenomas classified as mixed morphology had increased OR, but the relationship was not statistically significant. Kudo pit pattern (IIIL + IV and V) and Paris classification 0-IIc were the only variables that showed a statistically significant association with SMI (P value < 0.05) with OR of more than 4.5 and 18.2, respectively (Table 3).
| Factor | Estimate | OR | 95%CI | P value |
| Age | -0.0155 | 0.985 | 0.943-1.029 | 0.4553 |
| Gender | ||||
| Male | (reference) | |||
| Female | 0.2196 | 1.551 | 0.634-3.947 | 0.3149 |
| Size group | ||||
| 20 mm | (reference) | |||
| 21-30 mm | -0.2904 | 0.852 | 0.227-3.753 | 0.5141 |
| 31-40 mm | 0.0314 | 1.033 | 0.25-4.766 | 0.9082 |
| > 40 mm | 0.3512 | 1.545 | 0.42-6.813 | 0.3037 |
| Paris classification | ||||
| Paris classification 1p | (reference) | |||
| Paris classification (IIa, IIa + c, IIa + Is) vs 1p | -0.2608 | 0.98 | 0.279-5.193 | 0.6221 |
| Paris classification IIb vs 1p | -2.1639 | 0.146 | 0.001-1.943 | 0.0406 |
| Paris classification IIc vs 1p | 2.6652 | 18.281 | 1.366-340.689 | aP < 0.05 |
| Kudo Pit Pattern | ||||
| Others | (reference) | |||
| Pattern (IIIL + IV and V) | 0.7626 | 4.596 | 1.506-12.997 | bP < 0.001 |
| Morphology | ||||
| Granular | (reference) | |||
| Non granular | -0.2178 | 0.761 | 0.263-2.018 | 0.5423 |
| Mixed | 0.1624 | 1.113 | 0.21-4.336 | 0.7352 |
| Location | ||||
| Recto sigmoid | (reference) | |||
| Proximal colon (Cecum- Descending Colon) | -0.4555 | 0.402 | 0.161-1.056 | 0.0458 |
Out of 354 lesions examined at SC, the majority of the lesions (277; 78.2%) did not have evidence of recurrent/residual adenoma. Recurrence was present in 77 patients (21.8%) (Figure 1). All the recurrent adenomas were treated endoscopically in 76 patients (98.8%) except for one patient who was referred to surgery because of advanced endoscopic appearance and inability to resect endoscopically.
Multiple logistic regression analysis is described in Table 4. Compared to lesion size < 20 mm, lesion size > 40 mm had an OR: 15.412 for recurrence of adenoma at SC, and the relation was statistically significant (P < 0.0001). Lesions with size 31-40 mm and 21-30 mm had OR of 4.509 (P = 0.5467) and 3.081 (P = 0.386), respectively, but no statistically significant association with recurrence was found. Lesions with mixed morphology and non-granular morphology had OR of 2.423 (P = 0.05) and 1.087 (P = 0.26), respectively, as compared to granular morphology, but this association was also not statistically significant for recurrence at SC.
| Risk factor for recurrence | Estimate | OR | P value | |
| Lesion size | 21-30 mm (reference) | -0.2162 | 3.081 (0.875-10.849) | 0.386 |
| Lesion size | 31-40 mm vs Reference | 0.1644 | 4.509 (1.227-16.572) | 0.5467 |
| Lesion size | > 40 mm vs Reference | 1.3935 | 15.412 (4.292-55.338) | aP < 0.0001 |
| Morphology | Non-Granular vs Granular (reference) | -0.2396 | 1.087 (0.599-1.97) | 0.2681 |
| Morphology | Mixed vs Granular (reference) | 0.5623 | 2.423 (0.978-6.002) | 0.0573 |
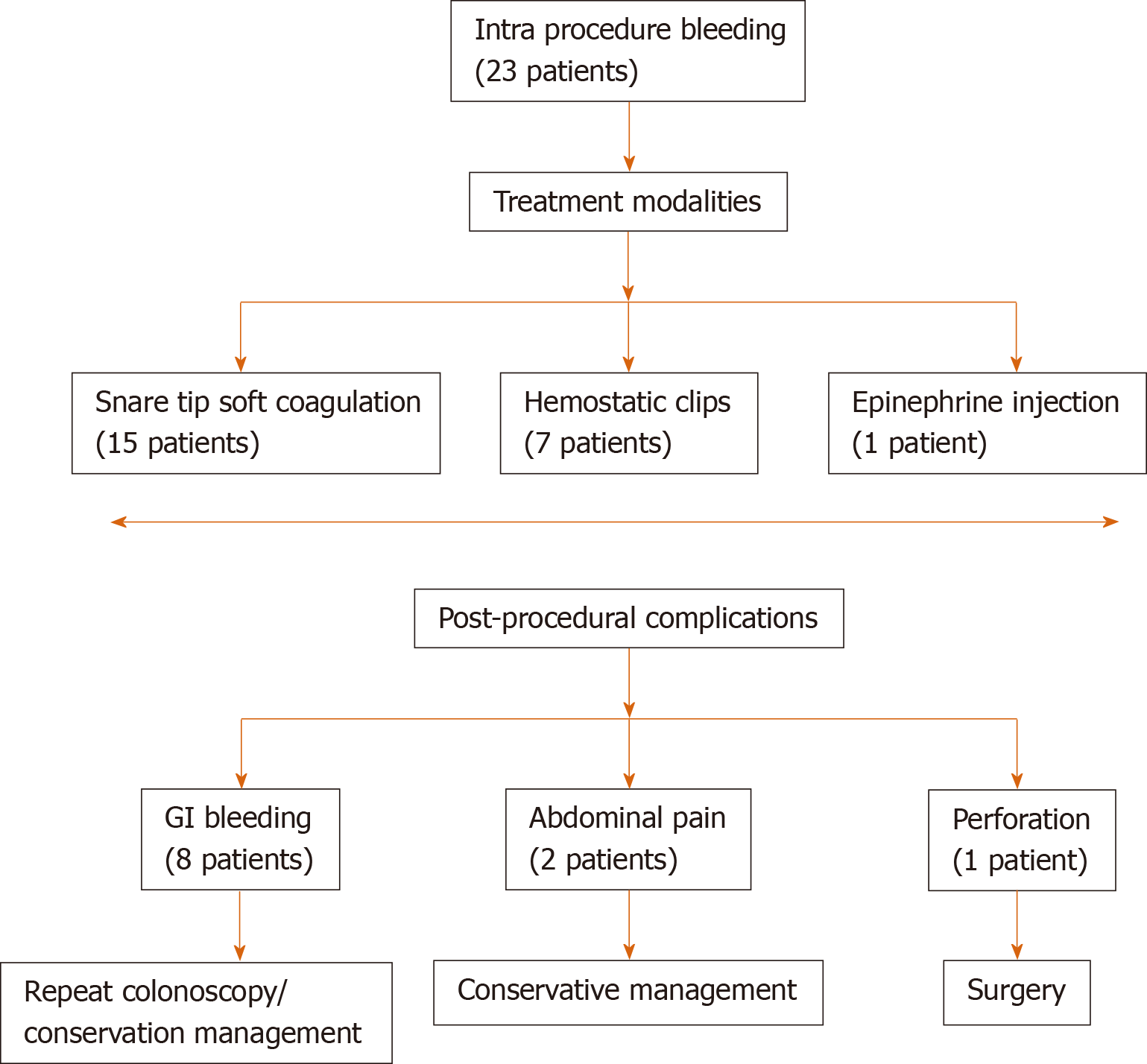
Intra-procedural complications: Sustained intra-procedural bleeding occurred in 23 patients. Snare tip soft coagulation, hemostatic clips, and epinephrine injection were used to control the bleeding (Figure 3).
Post-procedural complications: It occurred in a total of 11 patients with delayed gastrointestinal bleeding in eight patients, abdominal pain in two patients, and perforation in one patient.
(1) For patients with bleeding, seven out of eight patients were treated in the inpatient setting. Repeat colonoscopy was done in five patients and conservative treatment in two of them; (2) Abdominal pain patients were managed conservatively; and (3) One patient developed perforation, which was not evident at the end of the procedure. The patient had post-procedural abdominal pain and distention and underwent surgery requiring seven days of post-operative admission. Afterward, the patient was discharged without any further sequelae.
There were no perforations or clinically significant bleeding episodes during the treatment of recurrent/residual adenoma at SC (Figure 4).
Our single-center study included a total of 480 patients with 500 lesions. The results from our study show that EMR of larger polyps is a safe and viable alternative to surgery. Surgery itself has an inherent risk of serious complications and can have a mortality rate of up to 5% for some patients[11-13]. EMR being less invasive procedure with minimal risk of mortality may be considered as first-line therapy for superficial appearing colon polyps. Despite being effective and safe, however, the EMR procedure will not be curative for lesions with submucosal invasion. Thus, our efforts aimed to identify the characteristics of the polyps that distinguish the lesions harboring submucosal invasion and likely cancer. With the availability of technologically advanced, high-resolution endoscopes, it may now be feasible for the endoscopist to assess the invasiveness of the lesions based on endoscopic appearances such as Paris classification, granularity and Kudo pit[14-16].
Table 1 describes the basic demographics of the patients in our study. No deaths were reported as a result of EMR. The prevalence of submucosal invasive cancer was 4.6%. Although mixed morphology and size of the lesion appeared to show an increasing trend towards submucosal invasion, it was statistically insignificant.
Kudo pit pattern (IIIL + IV and V) and Paris classification 0-IIc were the only variables that showed a statistically significant association with SMI (P value < 0.05) with OR of more than 4.5 and 18.2, respectively. Moss et al[16] studied 479 patients with 514 lesions. The prevalence of submucosal invasive cancer was slightly higher (6.8%) as compared to our study. Their study also indicated that lesions with advanced kudo pit pattern, non-granular surface, and depressed component (Paris 0–IIa c) have higher chances of submucosal invasion and recommended en-bloc resection for such lesions. En-bloc resection provides curative resection for lesions with low-risk SMIC (no lympho-vascular involvement), and the margins can be accurately assessed[17]. Depending on the local expertise en-bloc removal may be done by surgery, EMR, or endoscopic submucosal dissection.
Various studies have been done to identify endoscopic appearance for lesions with submucosal invasion. Puig et al[18] studied the Narrow-Band Imaging International Colorectal Endoscopic for identifying lesions with deep invasions. They found this classification system to be effective with sensitivity of 58.4% and specificity of 96.4%, positive-predictive value of 41.6%, and negative-predictive value of 98.1% for deep invasion. The same study also showed that deep invasion was more common in rectum than colon (P < 0.001). For morphology, deep invasion was frequent in sessile polyps and pseudo-depressed lesions, compared to pedunculated polyps and elevated-type lesions (P < 0.001). Kim et al[19] studied Probe-based confocal laser endomicroscopy for in vivo histological analysis for submucosal invasion in colorectal lesions. The results were compared with pathological findings and showed that the sensitivity, specificity, and accuracy of the classification of submucosal carcinoma infiltration by two observers were 91.7%, 86.8%, and 88.0%, respectively.
Interestingly, submucosal invasion also has been linked to as a potential predictor for involvement of lymph nodes in pT1 Colorectal Cancers. Toh et al[20] studied 207 patients with pT1 Colorectal Cancers. They concluded that the pT1 cancers with lymph node involvement have a significantly wider area of invasion (P = 0.001) and greater area of submucosal invasion (P < 0.001) compared with pT1 cancers without lymph node metastasis[20].
The rate of adenoma recurrence in our study was 21%, the same as previously reported by Conio et al[21]. Similar results also were seen in other studies for larger adenomas[22,23]. Some studies have shown that resecting a few mm of surrounding normal mucosa can decrease the rate of recurrence. In one large Australian study (1134 patients) by Moss et al[24], a prospective intention-to-treat analysis of sessile or laterally spreading colonic lesions ≥ 20 mm in size showed that the recurrence rate at SC1 was 16 % for adenomas with wide-field EMR. Another recent study[25] showed that the rate of recurrence can be reduced significantly by snare tip soft coagulation of resection margins after adenoma resection. The slightly higher recurrence in our study might have occurred because we did not ablate the resection margins.
In our study, sustained intra-procedural bleeding was present in 23 patients (4.7%) out of 480 patients. It was significantly reduced compared to other studies[14,21]. A recent study showed a decreased risk of bleeding with prophylactic clipping of right-sided polyps after resection[26]. We did not prophylactically clip the resection site in all patients. The perforation rate in our study was 0.2%, which also is significantly less than reported in previous studies[17,27]. A study by Bronsgeest et al[28] reported perforation in (1.2%), unrelated to the size of the polyp. Two patients reported abdominal pain. Patients with abdominal pain should be closely observed for signs of peritonism to rule out serositis or perforation. Patients with persistent pain require abdominal imaging. Another important cause may be excessive air insufflation. Using CO2 insufflation is recommended for long EMR procedures[17]. We used CO2 for all our procedures.
In conclusion, from our large, single-center study on endoscopic mucosal resection for larger (≥ 20 mm) colorectal lesions, we conclude that EMR is safe for large, laterally spreading lesions. EMR has a potential rate of recurrence, but residual/recurrent tissues can be excised successfully on surveillance colonoscopy. Our study demonstrated an overall low prevalence of SMI, but Kudo pit pattern and Paris classification may serve as independent risk factor for submucosal invasion. Additional studies are needed to identify endoscopic features associated with a high risk of submucosal invasion to guide the appropriate selection of either endoscopic resection or surgical approaches.
Endoscopic techniques including endoscopic mucosal resection (EMR) and endoscopic submucosal dissection are two minimally invasive techniques for removing large colonic lesions. We did a retrospective study to evaluate the effectiveness of endoscopic mucosal resection for removal of large colon mucosal lesions. We also studied in detail the various endoscopic features predicting submucosal invasion and recurrence of lesions.
Endoscopic techniques including EMR has been increasing used in resecting large colon lesions. Our research signifies the effectiveness of EMR technique as well as describes pathology, size, location, Kudo Pit classification and Paris Classification of lesions.
Our main objective was to determine the recurrence rate of adenomas after resection and endoscopic features that may predict submucosal invasion of colonic mucosal neoplasia. This will help the clinicians to identify the advanced colon lesions and will guide management accordingly.
Our research is a retrospective study involving detailed chart review. This is one of the largest studies conducted in Central Florida as our institution serve as tertiary care referral center. The study span was nearly 3 years.
We analyzed the charts of 480 patients. The median age in our study was 68 (IQR: 14) with 52% males. The most common lesion location was ascending colon (161; 32%). Paris classification 0-IIa (Flat elevation of mucosa - 316; 63.2%); Kudo Pit Pattern IIIs (192; 38%) and Granular surface morphology (260; 52%) were most prevalent. Submucosal invasion was present in 23 (4.6%) out of 500 lesions. The independent risk factors for submucosal invasive lesion were Kudo Pit Pattern IIIL+IV and V (Odds ratio: 4.5; P value < 0.004) and Paris classification 0-IIc (Odds ratio: 18.2; P value < 0.01). Out of 500, 354 post-EMR scars were examined at surveillance colonoscopy. Recurrence was noted in 21.8% (77 cases).
We found that size of lesion was an important variable for recurrence of colon lesion. Our research showed few high-risk endoscopic features for submucosal invasion (Kudo Pit Pattern IIIL + IV and V and Paris classification 0-IIc). Our study results have been in accordance with the previous research studies as well. We can hypothesize from this research that lesion size and endoscopic features can help in identification of lesions with higher risk for recurrence and submucosal invasion. These findings will help the clinicians in early identification of these lesions and help them in further management.
Future research studies are needed to determine if recurrence rate of adenomas can be decreased by endoscopic techniques including wide filed EMR and snare tip soft coagulation of resection margins after adenoma resection.
| 1. | Kuzmich S, Harvey CJ, Kuzmich T, Tan KL. Ultrasound detection of colonic polyps: perspective. Br J Radiol. 2012;85:e1155-e1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Rex DK. Have we defined best colonoscopic polypectomy practice in the United States? Clin Gastroenterol Hepatol. 2007;5:674-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, Matsui S, Friedland S. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 440] [Article Influence: 24.4] [Reference Citation Analysis (1)] |
| 4. | Togashi K, Utano K, Kijima S, Sato Y, Horie H, Sunada K, Lefor AT, Sugimoto H, Yasuda Y. Laterally spreading tumors: limitations of computed tomography colonography. World J Gastroenterol. 2014;20:17552-17557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, Mahajan H, McLeod D, Bourke MJ. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology. 2017;153:732-742.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 6. | Dulskas A, Kuliešius Ž, Samalavičius NE. Laparoscopic colorectal surgery for colorectal polyps: experience of ten years. Acta Med Litu. 2017;24:18-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Cruz RA, Ragupathi M, Pedraza R, Pickron TB, Le AT, Haas EM. Minimally invasive approaches for the management of "difficult" colonic polyps. Diagn Ther Endosc. 2011;2011:682793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Tavakkoli A, Law RJ, Bedi AO, Prabhu A, Hiatt T, Anderson MA, Wamsteker EJ, Elmunzer BJ, Piraka CR, Scheiman JM, Elta GH, Kwon RS. Specialist Endoscopists Are Associated with a Decreased Risk of Incomplete Polyp Resection During Endoscopic Mucosal Resection in the Colon. Dig Dis Sci. 2017;62:2464-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Wada Y, Kashida H, Kudo SE, Misawa M, Ikehara N, Hamatani S. Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc. 2010;22:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Backes Y, Moss A, Reitsma JB, Siersema PD, Moons LM. Narrow Band Imaging, Magnifying Chromoendoscopy, and Gross Morphological Features for the Optical Diagnosis of T1 Colorectal Cancer and Deep Submucosal Invasion: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Manfredi S, Piette C, Durand G, Plihon G, Mallard G, Bretagne JF. Colonoscopy results of a French regional FOBT-based colorectal cancer screening program with high compliance. Endoscopy. 2008;40:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | McNicol L, Story DA, Leslie K, Myles PS, Fink M, Shelton AC, Clavisi O, Poustie SJ. Postoperative complications and mortality in older patients having non-cardiac surgery at three Melbourne teaching hospitals. Med J Aust. 2007;186:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2383] [Cited by in RCA: 2497] [Article Influence: 108.6] [Reference Citation Analysis (0)] |
| 14. | Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 232] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 309] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 16. | Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 450] [Article Influence: 30.0] [Reference Citation Analysis (1)] |
| 17. | Alexander S, Bourke MJ, Williams SJ, Bailey A, Co J. EMR of large, sessile, sporadic nonampullary duodenal adenomas: technical aspects and long-term outcome (with videos). Gastrointest Endosc. 2009;69:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Puig I, López-Cerón M, Arnau A, Rosiñol Ò, Cuatrecasas M, Herreros-de-Tejada A, Ferrández Á, Serra-Burriel M, Nogales Ó, Vida F, de Castro L, López-Vicente J, Vega P, Álvarez-González MA, González-Santiago J, Hernández-Conde M, Díez-Redondo P, Rivero-Sánchez L, Gimeno-García AZ, Burgos A, García-Alonso FJ, Bustamante-Balén M, Martínez-Bauer E, Peñas B, Pellise M; EndoCAR group, Spanish Gastroenterological Association and the Spanish Digestive Endoscopy Society. Accuracy of the Narrow-Band Imaging International Colorectal Endoscopic Classification System in Identification of Deep Invasion in Colorectal Polyps. Gastroenterology. 2019;156:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Kim B, Kim YH, Park SJ, Cheon JH, Kim TI, Kim WH, Kim H, Hong SP. Probe-based confocal laser endomicroscopy for evaluating the submucosal invasion of colorectal neoplasms. Surg Endosc. 2017;31:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Toh EW, Brown P, Morris E, Botterill I, Quirke P. Area of submucosal invasion and width of invasion predicts lymph node metastasis in pT1 colorectal cancers. Dis Colon Rectum. 2015;58:393-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004;60:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Swan MP, Bourke MJ, Alexander S, Moss A, Williams SJ. Large refractory colonic polyps: is it time to change our practice? A prospective study of the clinical and economic impact of a tertiary referral colonic mucosal resection and polypectomy service (with videos). Gastrointest Endosc. 2009;70:1128-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 23. | Khashab M, Eid E, Rusche M, Rex DK. Incidence and predictors of "late" recurrences after endoscopic piecemeal resection of large sessile adenomas. Gastrointest Endosc. 2009;70:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Moss A, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Burgess NG, Sonson R, Byth K, Bourke MJ. Long-term adenoma recurrence following wide-field endoscopic mucosal resection (WF-EMR) for advanced colonic mucosal neoplasia is infrequent: results and risk factors in 1000 cases from the Australian Colonic EMR (ACE) study. Gut. 2015;64:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 25. | Bourke M. Current status of colonic endoscopic mucosal resection in the west and the interface with endoscopic submucosal dissection. Dig Endosc. 2009;21 Suppl 1:S22-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Pohl H, Grimm IS, Moyer MT, Hasan MK, Pleskow D, Elmunzer BJ, Khashab MA, Sanaei O, Al-Kawas FH, Gordon SR, Mathew A, Levenick JM, Aslanian HR, Antaki F, von Renteln D, Crockett SD, Rastogi A, Gill JA, Law RJ, Elias PA, Pellise M, Wallace MB, Mackenzie TA, Rex DK. Clip Closure Prevents Bleeding After Endoscopic Resection of Large Colon Polyps in a Randomized Trial. Gastroenterology. 2019;157:977-984.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 27. | Hurlstone DP, Sanders DS, Cross SS, Adam I, Shorthouse AJ, Brown S, Drew K, Lobo AJ. Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut. 2004;53:1334-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 207] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Bronsgeest K, Huisman JF, Langers A, Boonstra JJ, Schenk BE, de Vos Tot Nederveen Cappel WH, Vasen HFA, Hardwick JCH. Safety of endoscopic mucosal resection (EMR) of large non-pedunculated colorectal adenomas in the elderly. Int J Colorectal Dis. 2017;32:1711-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hu B, Ierardi E, Manes G, Zhang HZ S-Editor: Zhang L L-Editor: A E-Editor: Wu YXJ