Published online Jun 16, 2020. doi: 10.4253/wjge.v12.i6.172
Peer-review started: February 27, 2020
First decision: April 22, 2020
Revised: May 9, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 16, 2020
Processing time: 110 Days and 14.6 Hours
Endoscopic procedures hold a basal risk of bleeding that depends on the type of procedure and patients’ comorbidities. Moreover, they are often performed in patients taking antiplatelet and anticoagulants agents, increasing the potential risk of intraprocedural and delayed bleeding. Even if the interruption of antithrombotic therapies is undoubtful effective in reducing the risk of bleeding, the thromboembolic risk that follows their suspension should not be underestimated. Therefore, it is fundamental for each endoscopist to be aware of the bleeding risk for every procedure, in order to measure the risk-benefit ratio for each patient. Moreover, knowledge of the proper management of antithrombotic agents before endoscopy, as well as the adequate timing for their resumption is essential.
This review aims to analyze current evidence from literature assessing, for each procedure, the basal risk of bleeding and the risk of bleeding in patients taking antithrombotic therapy, as well as to review the recommendation of American society for gastrointestinal endoscopy, European society of gastrointestinal endoscopy, British society of gastroenterology, Asian pacific association of gastroenterology and Asian pacific society for digestive endoscopy guidelines for the management of antithrombotic agents in urgent and elective endoscopic procedures.
Core tip: Endoscopic procedures hold a basal risk of bleeding, and they are often performed in patients taking antiplatelet and anticoagulant agents, increasing the potential risk of intraprocedural and delayed bleeding. This review aims to analyze current evidence from literature assessing, for each procedure, the basal bleeding risk and the risk of bleeding in patients taking antithrombotic therapy, as well as to review the recommendation of international guidelines for the management of these agents in urgent and elective endoscopic procedures.
- Citation: Maida M, Sferrazza S, Maida C, Morreale GC, Vitello A, Longo G, Garofalo V, Sinagra E. Management of antiplatelet or anticoagulant therapy in endoscopy: A review of literature. World J Gastrointest Endosc 2020; 12(6): 172-192
- URL: https://www.wjgnet.com/1948-5190/full/v12/i6/172.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i6.172
Endoscopic procedures are commonly performed in patients taking antiplatelet and anticoagulants agents. These antithrombotic medications reduce thromboembolic events by inhibiting platelet aggregation and coagulation and are the most widely prescribed agents in both primary and secondary care in many patients[1]. In addition, a growing number of subjects have an indication for combination therapy which increases the overall risk of bleeding, in particular, that from the gastrointestinal tract[2].
This review aims to analyze current evidence from literature assessing, for each procedure, the basal bleeding risk and the risk of bleeding during antithrombotic therapy, as well as to review the recommendation of American society for gastrointestinal endoscopy (ASGE)[3], European society of gastrointestinal endoscopy, British society of gastroenterology (ESGE/BSG)[4], Asian pacific association of gastroenterology and Asian pacific society for digestive endoscopy (APAGE/APSDE)[5] for the management of antithrombotic agents in urgent and elective endoscopic procedures.
Antithrombotic therapies may be classified in antiplatelet agents (APA) and anticoagulants.
APA interfere in specific steps of platelets activation process and include several agents namely aspirin [acetylsalicylic acid (ASA)], nonsteroidal anti-inflammatory drugs (NSAIDs), P2Y12 platelet receptor blockers and other agents such as glycoprotein IIb/IIIa antibodies and receptor antagonists and the competitive and selective inhibitors of protease-activated receptor-1.
ASA is used to inhibit platelet aggregation by irreversibly blocking the cyclooxygenase pathway, resulting in the suppression of prostaglandin and thromboxane biosynthesis from arachidonic acid in platelets. After cessation of ASA, 7 to 9 d are required to fully recover the platelet function. Dipyridamole reversibly prevents platelet activation by multiple mechanisms, including the inhibitions of cyclic nucleotide phosphodiesterase and the blocking of the adenosine uptake. Dipyridamole has an elimination half-life of 12 h and a duration of action of about two days after discontinuation. Thienopyridine agents represent the most common antiplatelet drugs used following ASA. These agents selectively inhibit adenosine diphosphate-induced platelet aggregation, with no direct effects on the metabolism of arachidonic acid[6]. The class of P2Y12 platelet receptor blockers is broad and include ticlopidine, clopidogrel and the most recent third-generation thienopyridine agents such as prasugrel and ticagrelor. Ticlopidine, clopidogrel and prasugrel are prodrugs which achieve their antiplatelet effects through active metabolites irreversibly inactivating the P2Y12 receptor. The hepatic metabolism of prasugrel is more rapid (occurs in a single hepatic step) and less influenced by cytochrome P450 polymorphisms than clopidogrel[7,8]. Prasugrel and ticagrelor induce a more rapid and pronounced inhibition of platelet aggregation compared to clopidogrel. Unlike the other thienopyridine which requires discontinuation of at least 5 to 7 d to recover adequate platelet function, ticagrelor induce reversible inhibition of the P2Y12 receptor which permits a shorter interval of interruption (3 to 5 d)[9]. Other antiplatelet medications include the antagonists of the platelet glycoprotein (GP)IIb/IIIa (e.g. abciximab, roxifiban, eptifibatide, tirofiban, orbofiban, sibrafiban) which play their antiplatelet effects by blocking the final common pathway of platelet aggregation. Current guidelines recommend antiplatelet agents for the secondary prevention of cardiovascular disease[10]. Conversely, these drugs are not recommended for the primary prevention of cardiovascular disease, even if some evidence weakly supports the advantage of aspirin in patients with hypertension and impaired renal function or who are at high cardiovascular risk (10-year risk > 20%)[11,12].
Anticoagulants are prescribed in several clinical setting such as the prevention of stroke in patients with atrial fibrillation and as prophylaxis and treatment of venous thromboembolism. These drugs prevent the clotting of blood by inhibiting one or more steps in the coagulation cascade through several mechanisms of actions including both direct and indirect enzymatic blocking, the antagonism of vitamin K–dependent clotting factors and the binding to antithrombin. Available drugs include unfractionated heparin, low molecular weight heparins, fondaparinux, vitamin K antagonists (e.g. warfarin), direct factor Xa inhibitors (e.g. rivaroxaban, apixaban, edoxaban) and direct thrombin inhibitors which prevent thrombin from cleaving fibrinogen to fibrin and include both parenteral agents (bivalirudin, argatroban and desirudin) and oral agent (dabigatran etexilate). The efficacy and the bleeding risk related to the use of anticoagulants depends on the drug used and the clinical setting[13].
Data from the pivotal clinical trials (RE-LY, ROCKET AF, ARISTOTLE, and ENGAGE AF-TIMI 48) suggest that direct oral anticoagulants (DOACs) are not inferior to warfarin for the prevention of stroke or systemic embolism in subjects with non-valvular atrial fibrillation and that these drugs also had significant reductions in hemorrhagic stroke and intracranial hemorrhage compared to warfarin, resulting in a lower risk of stroke and mortality[14]. Furthermore, DOACs were associated with less severe major bleedings than those related to warfarin. Nevertheless, the rates of gastrointestinal (GI) bleeding are increased in non-valvular atrial fibrillation patients treated with DOACs and in particular with dabigatran 150 mg bid, rivaroxaban, and edoxaban, but it seems to occur the least in patients receiving apixaban compared with vitamin K antagonists therapy[15]. In this regard, Abraham et al[16] reported that the rates of GI bleeding were significantly increased with rivaroxaban than dabigatran (HR 1.20: 95%CI: 1.00-1.45), whereas apixaban was associated to a lower risk of GI bleeding than dabigatran (HR 0.39: 95%CI: 0.27-0.58; P < 0.001) or rivaroxaban (HR 0.33: 95%CI: 0.22-0.49; P < 0.001). However, despite the risk of bleeding associated with DOACs, this risk is counterbalanced by their effectiveness in preventing the risk of stroke that is considered by patients as a “fate worse than death"[17]. A specific antidote is available for dabigatran (idarucizumab)[18], but not for the others DOACs. Recently, a direct factor Xa inhibitor has shown promising results against rivaroxaban, apixaban and edoxaban (andexanet alfa)[19].
The risk of bleeding associated with endoscopic procedures is variable and depends mainly on the type of procedure performed (diagnostic, low-risk or high-risk operative), on the type of antiplatelet or anticoagulant therapy and patient’s comorbidities. In the following paragraphs, we will review and discuss, for each procedure, the evidence from literature assessing the risk of basal bleeding and the risk of bleeding in patients taking antithrombotic agents.
Diagnostic procedures as esophagogastroduodenoscopy (EGD), colonoscopy or sigmoidoscopy, including mucosal biopsy, present a low-risk of procedural bleeding, as shown by several studies[20-21].
Similarly, the risk of bleeding during diagnostic endoscopy is not increased in patients assuming ASA, clopidogrel or warfarin. In this regard, a prospective, single-blind, randomized study in healthy volunteers, showed that on a sample of 405 antral biopsies and 225 duodenal biopsies performed during 90 EGD in 45 subjects receiving aspirin or clopidogrel, no bleeding events were noted in the clopidogrel group after 350 biopsies, while in the aspirin group, only one minor endoscopic bleeding event was reported, in the absence of clinical events[22].
Moreover, a prospective single-arm study including 112 Japanese outpatients receiving antithrombotic agents showed that after 101 biopsies performed during EGD or colonoscopy, no patients complained of any bleeding symptoms in the following 2 wk observation period. In addition, the authors didn’t find significant differences between patients receiving single and multiple antithrombotic agents, as well as between patients not receiving and receiving warfarin[23].
Conversely, no data is currently available on the risk of bleeding after biopsies in patients taking DOACs.
A retrospective multicenter study of double-balloon enteroscopy complications performed in the United States reported a risk of gastrointestinal bleeding of 0.2% associated with the procedure[24]. Unfortunately, no studies assessing the risk of bleeding during double-balloon enteroscopy in patients on antiaggregant or anticoagulant therapy have been performed up to date. In the absence of data, the risk is uncertain, and it may be assimilated to that of other endoscopic diagnostic procedures.
Polypectomy may be complicated by intraprocedural or immediate bleeding (IPB) and by post-procedural bleeding (PPB). While IPB is often self-limited and may be controlled during the procedure, conversely, PPB may be worrisome, since it arises after the procedure when the patient has already been discharged.
Data from large series show a PPB ranging from 0.07% to 2.2%[25-28]. A large report from the English National Health Service Bowel Cancer Screening Programme shows an overall bleeding rate of 0.65 %, a rate of severe bleeding requiring transfusion of 0.04 %, and an increased bleeding risk attributable to polypectomy of 11.1-fold[29].
Exploring factors associated with PPG, a prospective, cross-sectional study of 5152 patients undergoing polypectomy, showed that age ≥ 65 years, cardiovascular or chronic renal disease, anticoagulants use, polyp size > 1 cm, pedunculated polyp or laterally spreading tumors, poor bowel preparation and use of pure cutting current were risk factors for PPB[30]. Another case-control study confirmed that polyp size was associated with PPG, with an increased risk of hemorrhage of 9% for every 1 mm increase in polyp diameter (OR 1.09: 95%CI 1.0-1.2; P = 0.008)[31].
Post-polypectomy bleeding in patients on antithrombotic therapy has a different impact. The risk in patients on ASA or NSAID is generally considered low.
One of the first studies performed in this field showed as the risk of bleeding did not seem to be affected by NSAID use. Although the use of NSAIDs increased the incidence of minor self-limited bleeding, an increase in the rate of major bleeding was not observed[32]. Besides, a revision of a cohort of 5593 patients and 1657 polypectomies clearly showed that the use of antiplatelet agents during polypectomy was not associated with an increase in post-polypectomy bleeding[33].
A prospective multicenter trial, including a total of 1015 polyps < 10 mm removed by cold snare in 823 patients, 15% of them taking low dose aspirin or ticlopidine, reported a higher PPB in patients taking APA (6.2 % vs 1.4 %; P < 0.001). Nevertheless, all bleeding episodes were intraprocedural and successfully treated, while no delayed PPB occurred[34].
Although low in patients receiving aspirin or NSAID, the risk of post-polypectomy bleeding is higher in patients receiving thienopyridine or warfarin.
Despite some studies advocate a low-risk of post-polypectomy bleeding in patients taking thienopyridine[35], a meta-analysis of 5 observational studies including 574 subjects on clopidogrel therapy and 6169 controls showed an overall higher risk of PPB on continued clopidogrel (RR 2.54, 95%CI: 1.68-3.84, P < 0.00001), with a non-significant risk of immediate bleeding (RR of 1.76, 95%CI: 0.90-3.46, P = 0.10) and a significantly higher risk of delayed bleeding (RR of 4.66, 95%CI: 2.37-9.17, P < 0.00001)[36].
While patients on anticoagulation therapy may safely undergo colonoscopy, current practice guidelines consider polypectomy a high-risk procedure for which anticoagulation must temporarily be discontinued. Despite this, the risk estimate of bleeding is difficult to quantify with accuracy, since it depends on many variables, especially by the size of the polyp to be removed and International Normalized Ratio (INR) values.
A small single-center retrospective study performed on 21 patients receiving long-term anticoagulation with warfarin with an average INR of 2.3 and undergoing polypectomy, reported no episodes of PPB[37].
Another retrospective study supported the safety of polypectomy of small polyps < 10 mm without interruption of anticoagulation showing that, in a sample of 225 polypectomies with subsequent prophylactic placement of hemoclips, the rate of severe PPB requiring transfusion and of minor PPB not requiring treatment were 0.8% and 1.6%, respectively[38].
Similarly, a Japanese prospective controlled trial of 70 patients randomized to cold snare or traditional polypectomy of lesions up to 10 mm without interruption of warfarin, confirmed that the incidence of PPB after cold snare resection was acceptable reporting a lower incidence of immediate (5.7% vs 23.0%) and delayed (0% vs 14%) bleeding compared to traditional polypectomy, even without interruption of anticoagulant therapy[39]. Despite this, further data are needed in order to properly assess the setting in which a polypectomy on warfarin therapy could be performed safely.
To date, current guidelines recommend discontinuing warfarin 5 d before high-risk endoscopic procedures in patients at low thrombotic risk and discontinuing warfarin 5 d before high-risk endoscopic procedures with low molecular weight heparin (LMWH) bridging in patients at high thrombotic risk. In this regard, also the role of bridging need to be better assessed, as recent data suggest that patients undergoing bridging with LMWH are at higher risk of procedural-related bleeding compared to patients not undergoing bridging with LMWH or continuing warfarin therapy[40,41]. On the same line, a recent study showed as patients discontinuing anticoagulant with LMWH bridging, as suggested by guidelines, had a higher PPB rate compared to patients continuing anticoagulants (19.6% vs 10.8%, P = 0.087)[42]. Based on these observations, recommendation on bridging therapy should be revised, and the choice should be individualized, taking into account the hemorrhagic and thromboembolic risk of each patient.
With regard to DOACs, the risk of PPB is not well known. The aforementioned study, comparing post-polypectomy complication rates in 218 patients receiving oral anticoagulants (73 DOACs, 145 warfarin) and 218 patients not receiving anticoagulant therapy, showed that the PPB was similar between DOACs and warfarin and higher for both compared with controls (13.7% vs 13.7% vs 0.9%, P < 0.001)[42].
Similarly, the second mentioned analysis of 11504 comparing patients on antithrombotic therapy (1590 DOACs, 3471 warfarin, and 6443 clopidogrel) and 599983 control undergoing colonoscopy with polypectomy or endoscopic mucosal resection, showed that subjects undergoing DOACs did not have a statistically significant increased risk gastrointestinal bleeding, as well as cerebrovascular accident or myocardial infarction and hospital admissions compared with controls. On the contrary, clopidogrel and warfarin were associated with increased odds of PPB, cerebrovascular accident or myocardial infarction and hospital admissions compared with controls[43].
The overall risk of intraprocedural and delayed bleeding after endoscopic mucosal resection (EMR) has been estimated between 3.7%-11.3 % and 0.6%-6.2 %, respectively, and it is, therefore, higher compared with polypectomy[4].
The risk of bleeding after EMR is associated with the location and the size of the lesion. Esophageal EMR has a greater risk of bleeding ranging from 4% to 20%[44-47]. Moreover, a retrospective study showed as esophageal EMR (OR = 2.5, 95%CI: 1.2-5, P = 0.0009) and lesion size (OR = 1.24, 95%CI: 1.1–1.5, P = 0.003) were independently associated with a higher risk of early bleeding in EMR, when controlled for age, gender and NSAIDs or clopidogrel therapy[48]. Besides, duodenal EMR presents a risk of delayed bleeding between 6.3 and 12.3[47,48]. The risk of hemorrhages seems to be lower for EMR of lesions smaller than 1 cm[49].
A delayed bleeding prediction model (GSEED-RE2), taking into account several variables including lesion size, proximal location, comorbidity, and antiplatelet or anticoagulant therapy, has been recently proposed showing higher values of area under the curve (0.69-0.73; 95%CI: 0.59-0.80) compared to previous models[50].
Prophylactic measures may help to reduce the risk of delayed bleeding after EMR, for instance, with the placement of hemoclips. In this regard, a retrospective study of 524 lesions 2 cm or larger resected by EMR showed that the delayed bleeding rate was 1.8% in the clipped group vs 9.7% in the not clipped group and that the absence of clipping (OR = 6.0; 95%CI: 2.0-18.5) was independently associated with delayed hemorrhage[51]. On the other hand, the other two studies did not find any significant difference in the rate of PPB between patients with and without prophylactic placement of hemoclips[52,53].
Concerning the risk of bleeding associated with antiplatelet therapy, data from two large prospective intention-to-treat studies of 302 EMR for colonic laterally spreading tumors ≥ 20 mm performed in 288 patients, showed that the use of aspirin (OR = 6.3, P = 0.005), was independently associated with the risk of bleeding at multivariate analysis[54]. On the contrary, temporary discontinuation of anticoagulants seems to be safe. A retrospective study on a cohort of 798 patients undergoing 1716 EMR, all of them stopping antiplatelets and anticoagulants 7 d before EMR and resuming clopidogrel 2 d after EMR, showed that the temporary cessation of clopidogrel before EMR and its prompt resumption was not associated with an increased risk of gastrointestinal bleeding[55]. No data is available for other anticoagulants, including DOACs, for the risk of bleeding after EMR.
Based on these data, EMR is considered a high-risk endoscopic procedure for the management of anticoagulant therapy.
Endoscopic submucosal dissection (ESD) is a more recent and more complex technique, with growing popularity worldwide. Although a more radical resection, the risk of IPB and of PPB seems to be higher compared to EMR. A meta-analysis of 15 non-randomized studies comparing ESD with EMR confirmed higher “en bloc” resection rates (OR = 13.87, 95%CI: 10.12-18.99) and higher curative resection rates for ESD compared to EMR (OR = 3.53, 95%CI: 2.57-4.84), with the disadvantage of higher procedure-related bleeding (OR = 2.20, 95%CI: 1.58)[56].
Nevertheless, the risk is lower for esophageal and colonic ESD, and higher for gastric ESD. A meta-analysis of 15 studies with a total of 776 ESD procedures performed for resection of esophageal neoplasia showed a pooled estimate of PPB of 2.1% (95%CI: 1.2-3.8)[57]. Similarly, in another meta-analysis of 22 studies with a total of 2841 colonic ESD, the pooled estimate of PPB was 2.0% (95%CI: 1.0-2.0)[58].
On the contrary, gastric ESD presents a risk of PPB ranging from 3.6% to 6.9% as reported in 7 studies, including > 11000 procedures[59-65].
With regard to the risk of bleeding associated with antiplatelet therapy, most of the data come from gastric setting. In patients who do not discontinue aspirin before gastric ESD, aspirin use is independently associated with PPB (RR 4.49; 95%CI: 1.09-18.38)[66]. The risk of bleeding in patients who discontinue aspirin before gastric ESD is controversial. Some studies show that the risk of bleeding is increased even after its temporary withdrawal[67,68], while other studies showed that its discontinuation is not independently associated with delayed bleeding after the procedure[69,70]. Similarly, in colorectal ESD, discontinuation of antiplatelet therapy is not associated with post-procedural bleeding[71,72]. Beyond aspirin, no consistent data is available on the effect of other agents, such as clopidogrel, ticagrelor and DOACs, on the risk of bleeding associated with ESD.
According to current literature data, no significant risk of bleeding is reported neither in esophageal dilations[73-77] nor in those of the colon[78-84]. Besides, no study evaluated so far the risk of bleeding due to endoscopic dilatation in patients under APA or anticoagulant therapy.
The risk of bleeding after endoscopic stenting is still controversial, and it is difficult to assess precisely, due to heterogeneity of the type of stent, the anatomical site and the indication (benign vs malignant).
With regard to esophageal stenting, several studies report a risk of bleeding ranging between 1% and 8%[85,86].
Besides, in the setting of gastroduodenal stenting, a systematic review of 32 case series of stent placement in 606 patients with malignant symptomatic gastroduodenal obstruction showed an incidence of bleeding of 0.5%[87].
Moreover, two systematic reviews assessed the incidence of bleeding in colonic stenting. The first one, evaluating 29 case series and 598 stent placements, showed a 4.5% bleeding rate[88]. The second, including 54 non-randomized studies with the use of stents in a total of 1198 patients, did not report any case of bleeding[89].
Also in this case, no study evaluated so far the risk of bleeding due to endoscopic stenting in patients taking APA or anticoagulants.
Percutaneous endoscopic gastrostomy (PEG) is performed through the perforation of the abdominal and the anterior stomach walls. Therefore, bleeding from some vessel placed in the path of the puncture or, rarely, a hematoma of the wall, may occur. A recent multicenter prospective cohort study, including 950 patients undergoing PEG placement or replacement, showed a 1% bleeding rate[90].
In another retrospective, single-center study of 990 patients undergoing PEG placement, the incidence of bleeding was 1.6%, and multivariate analysis demonstrated no association between periprocedural use of aspirin (at any dose) or clopidogrel and post-PEG bleeding[91].
Also in this setting, no data is available on the risk of bleeding after PEG placement in patients taking prasugrel, ticagrelor or DOACs.
Band ligation of esophageal varices is generally performed as an emergency treatment for active upper variceal bleeding and, in this setting, measures for the management of anticoagulants in urgent endoscopy should be applied. The band ligation can also be performed as an elective procedure for primary prophylaxis of varices that have never previously bleed. A case-control study showed an incidence of delayed bleeding around 3.4%. In the same study, aspirin or anticoagulation were not independently associated with the risk of bleeding, even though they were taken by a small minority (1.3%) of patients[92].
No data regarding the risk of bleeding in patients taking other agents, such as thienopyridines, ticagrelor or DOACs, is available.
The risk of bleeding during endoscopic retrograde cholangiopancreatography (ERCP) is reported in about 0.1% to 2% of procedures with sphincterotomy[93].
In this regard, a meta-analysis showed that, even if sphincterotomy before stent placement reduces the risk of post ERCP pancreatitis (OR = 0.34, 95%CI: 0.12-0.93, P = 0.04), it is associated with a higher risk of post-ERCP bleeding (OR = 9.70, 95%CI: 1.21-77.75, P = 0.03)[94].
Patient factors affecting the risk of bleeding after sphincterotomy include anticoagulant therapy, presence of coagulopathies, and active cholangitis.
On the other side, procedural factors affecting the risk of bleeding are the operator’s experience and the type of current used during the sphincterotomy. A meta-analysis confirmed as a mixed-current was associated with a lower risk of bleeding (12.2%-95%CI, 4.1%, 20.3%) compared to pure-cut current (37.3%-95%CI, 27.3%, 47.3%), with a similar risk of pancreatitis[95].
Another meta-analysis showed that, compared to sphincterotomy, endoscopic papillary balloon dilatation was associated with a lower risk of bleeding (OR = 0.12, 95%CI: 0.04-0.34, P < 0.01), even if presented a significant higher incidence of pancreatitis (OR = 2.79, 95%CI: 1.74-4.45, P < 0.0001)[96].
The risk of bleeding after sphincterotomy in patients taking antiplatelet agents is not completely defined. Several studies did not find any significant additional risk of bleeding in patients taking antiplatelet agents[93,97-100]. Besides, a retrospective study showed a higher incidence of post-sphincterotomy bleeding in patients continuing aspirin until the day of sphincterotomy, compared to patients that had never taken aspirin (9.7% vs 3.9%, P = 0.01)[101].
No data from literature is currently available regarding the risk of bleeding after biliary lithotripsy and cholangioscopy in patients taking APA or anticoagulants.
Endoscopic ultrasound (EUS) plus fine-needle aspiration (FNA) is performed for diagnosis and locoregional staging of esophageal, gastric, rectal, and pancreatic cancers. Although the procedure includes the puncture of the tissue, the reported incidence of bleeding is low.
A large systematic review of 51 articles with a total of 10941 patients undergoing endoscopic ultrasound with fine-needle aspiration (EUS-FNA), showed bleeding in 0.17% of procedures, and most bleeding complications occurred after FNA performed on pancreatic lesions[102]. Moreover, a prospective controlled study assessed the incidence of bleeding after 222 EUS-FNA procedures in patients undergoing aspirin/NSAIDs and LMWH compared to patients didn't take these therapies. Bleeding occurred in 0% (0/26), 33.3% (2/6) and 3.7% (7/190) of the patients in the aspirin/NSAIDs, LMWH, and control groups, respectively (P = 0.023)[103]. This data confirms as EUS-FNA is a safe procedure, even if performed in patients taking aspirin or NSAIDs.
No data is available regarding the risk of bleeding after EUS-FNA in patients taking anticoagulants.
Peroral endoscopic myotomy (POEM) is a new endoscopic technique for the treatment of esophageal achalasia, with excellent results[104].
Since the view on the vasculature around the muscle fibers is optimal, and only a few vessels are encountered in the submucosal tunnel, major bleeding is not frequent during the procedure. In fact, according to a recent meta-analysis, massive hemorrhage was reported in 0.2% of POEMs[105].
To date, no data on the risk of bleeding in patients taking antiplatelets or anticoagulants is available.
Current international practice guidelines provide a specific recommendation on the management of anticoagulant therapy in the periendoscopic period.
The management of anticoagulants depends on the type of molecule, the estimated risk of bleeding due to the endoscopic procedure, and the underlying thrombotic risk deriving from cardiovascular disease.
With regard to the first parameter, ASGE, ESGE/BSG and APAGE/APSDE guidelines identify specific risk groups (Table 1). The low-risk group includes all endoscopic diagnostic procedures (including EGD, colonoscopy and enteroscopy with or without mucosal biopsies, EUS without FNA) and ERCP. The high-risk group includes polypectomy, EMR, ESD, therapeutic enteroscopy, ERCP with biliary or pancreatic sphincterotomy, ampullectomy, EUS with FNA, dilatation of strictures and PEG placement. These two groups are homogeneous between the guidelines, with the exception of enteral stents that are still controversial and classified as low-risk by ASGE and APAGE/APSDE, and as high-risk by ESGE/BSG. POEM is not classified by any of the guidelines, but it could be ascribed to high-risk procedures. Moreover, APAGE/APSDE consider a third group of ultra-high risk procedures, including ESD and EMR of polyps > 2 cm, for which specific indications are provided.
| Procedure risk group | Practice guidelines | ||
| ASGE[3] | ESGE/BSG[4] | APAGE/APSDE[5] | |
| Low-risk | (1) Diagnostic (EGD, colonoscopy, flexible sigmoidoscopy) including mucosal biopsy; (2) ERCP with stent (biliary or pancreatic) placement or papillary balloon dilation without sphincterotomy; (3) Push enteroscopy and diagnostic balloon-assisted enteroscopy; (4) Capsule endoscopy; (5) Enteral stent deployment (controversial); (6) EUS without FNA; (7) Argon plasma coagulation and (8) Barrett’s ablation | (1) Diagnostic procedures +/– biopsy; (2) Biliary or pancreatic stenting; (3) Diagnostic EUS and (4) Device-assisted enteroscopy without polypectomy | (1) Diagnostic endoscopy with biopsy; (2) Endoscopic ultrasound without fine needle aspiration; (3) ERCP with biliary or pancreatic stenting; (4) Diagnostic push or device-assisted enteroscopy; (5) Video capsule endoscopy; (6) Oesophageal, enteral and colonic stenting and (7) Argon plasma coagulation |
| High-risk | (1) Polypectomy; (2) Biliary or pancreatic sphincterotomy; (3) Treatment of varices; (4) PEG/PEJ placement; (5) Therapeutic balloon-assisted enteroscopy; (6) EUS with FNA; (7) Endoscopic hemostasis; (8) Tumor ablation; (9) Cystgastrostomy; (10) Ampullary resection; (11) EMR; (12) Endoscopic submucosal dissection and (13) Pneumatic or bougie dilation | (1) Polypectomy; (2) ERCP with sphincterotomy; (3) Ampullectomy; (4) EMR; (5) ESD; (6) Dilation of strictures; (7) Therapy of varices; (8) PEG; (9) EUS with FNA and (10) Oesophageal, enteral or colonic stenting | (1) Polypectomy; (2) ERCP with sphincterotomy ± balloon sphincteroplasty; (3) Dilatation of strictures; (4) Injection or banding of varices; (5) PEG/PEJ placement; (6) EUS with FNA and (7) Ampullectomy |
| Ultra-high-risk | NA | NA | (1) ESD; (2) EMR of large (> 2 cm) polyps |
On the other hand, the assessment of cardiovascular risk differs between the three guidelines and is summarized in Table 2.
| Thrombotic risk category | Practice guidelines | ||
| ASGE[3] | ESGE/BSG[4] | APAGE/APSDE[5] | |
| Low-risk | Anticoagulant therapy: (1) Bileaflet aortic valve prosthesis without AF and no other risk factors for CVA; (2) VTE > 12 mo previous and no other risk factors; and (3) Atrial fibrillation with CHA2DS2-VASc score < 2 | Antithrombotic therapy: (1) Ischaemic heart disease without coronary stent; (2) Cerbrovascular disease; and (3) Peripheral vascular disease. | Antithrombotic therapy: (1) Acute coronary syndrome or percutaneous coronary intervention > 6 mo ago; and (2) Stable coronary artery disease. |
| Anticoagulant therapy: (1) Prosthetic metal heart valve in aortic position; (2) Xenograft heart valve; (3) Atrial fibrillation without valvular disease; (4) > 3 mo after venous thromboembolism; and (5) Thrombophilia syndromes | |||
| Anticoagulant therapy: (1) Non-valvular atrial fibrillation with a CHA2DS2-VASc score ≤ 5; (2) Prosthetic valve without atrial fibrillation; and (3) > 3 mo after venous thromboembolism | |||
| Moderate-risk | Anticoagulant therapy: (1) Bileaflet aortic valve prosthesis and one or more of the following risk factors: AF, prior CVA or TIA, hypertension, diabetes, congestive heart failure, age > 75 yr; (2) VTE within the past 3-12 monon-severe thrombophilia (heterozygous factor V Leiden or prothrombin gene mutation); and (3) Recurrent VTEactive cancer (treated within 6 mo or palliative) | ||
| High-risk | Anticoagulant therapy: (1) Any mitral valve prosthesis; (2) Any caged-ball or tilting disc aortic valve prosthesis; (3) Recent (within 6 mo) CVA or TIA; and (4) Atrial fibrillation with CHA2DS2-VASc score ≥ 2 | Antithrombotic therapy: (1) Drug eluting coronary artery stents within 12 mo of placement; and (2) Bare metal coronary artery stents within 1 mo of placement. | Antithrombotic therapy: (1) Acute coronary syndrome or percutaneous coronary intervention 6 wk–6 mo. |
| Anticoagulant therapy: (1) Non-valvular atrial fibrillation with a CHA2DS2-VASc score > 5; (2) Metallic mitral valve; (3) Prosthetic valve with atrial fibrillation; (4) < 3 mo after venous thromboembolism; and (5) Severe thrombophilia (protein C or protein S deficiency and (6) antiphospholipid syndrome) | |||
| Anticoagulant therapy: (1) Prosthetic metal heart valve in mitral position; (2) Prosthetic heart valve and atrial fibrillation; (3) Atrial fibrillation and mitral stenosis; and (4) < 3 mo after venous thromboembolism | |||
| Very high-risk | Antithrombotic therapy: Acute coronary syndrome or percutaneous coronary intervention < 6 wk | ||
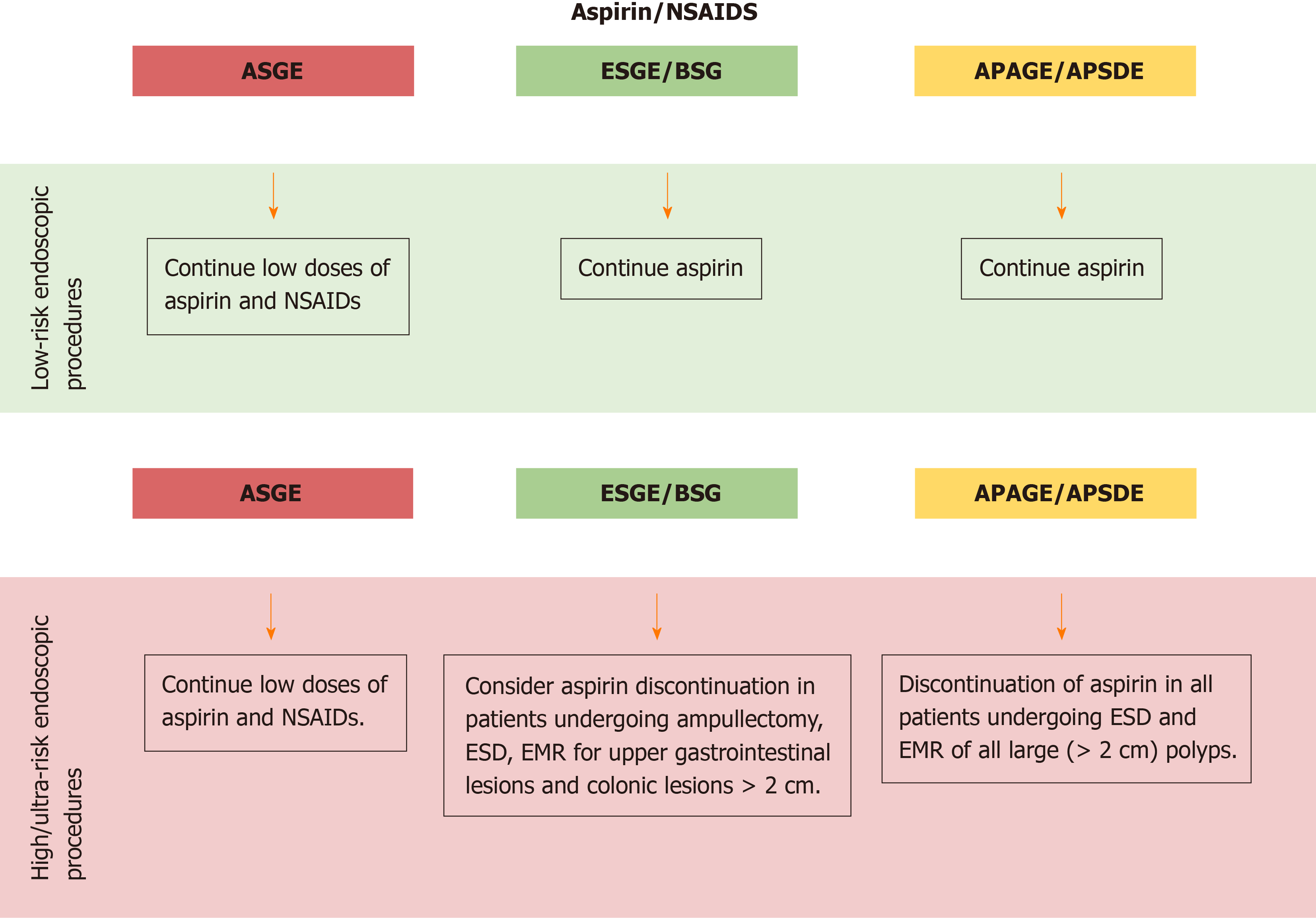
With regards to aspirin, ASGE guidelines judge the use of low doses of aspirin and NSAIDs safe and suggest to continue these drugs in the periendoscopic period[3].
On the other side, ESGE/BSG and APAGE/APSDE guidelines recommend continuing aspirin therapy for almost all endoscopic procedures, with some exceptions.
ESGE/BSG recommend aspirin discontinuation, on an individual basis, in patients undergoing ESD, EMR for upper gastrointestinal lesions and colonic lesions > 2 cm, and ampullectomy[4]. Besides, APAGE/APSDE recommend discontinuation of aspirin in all patients undergoing ESD and EMR of all large (> 2 cm) polyps[5] (Figure 1).
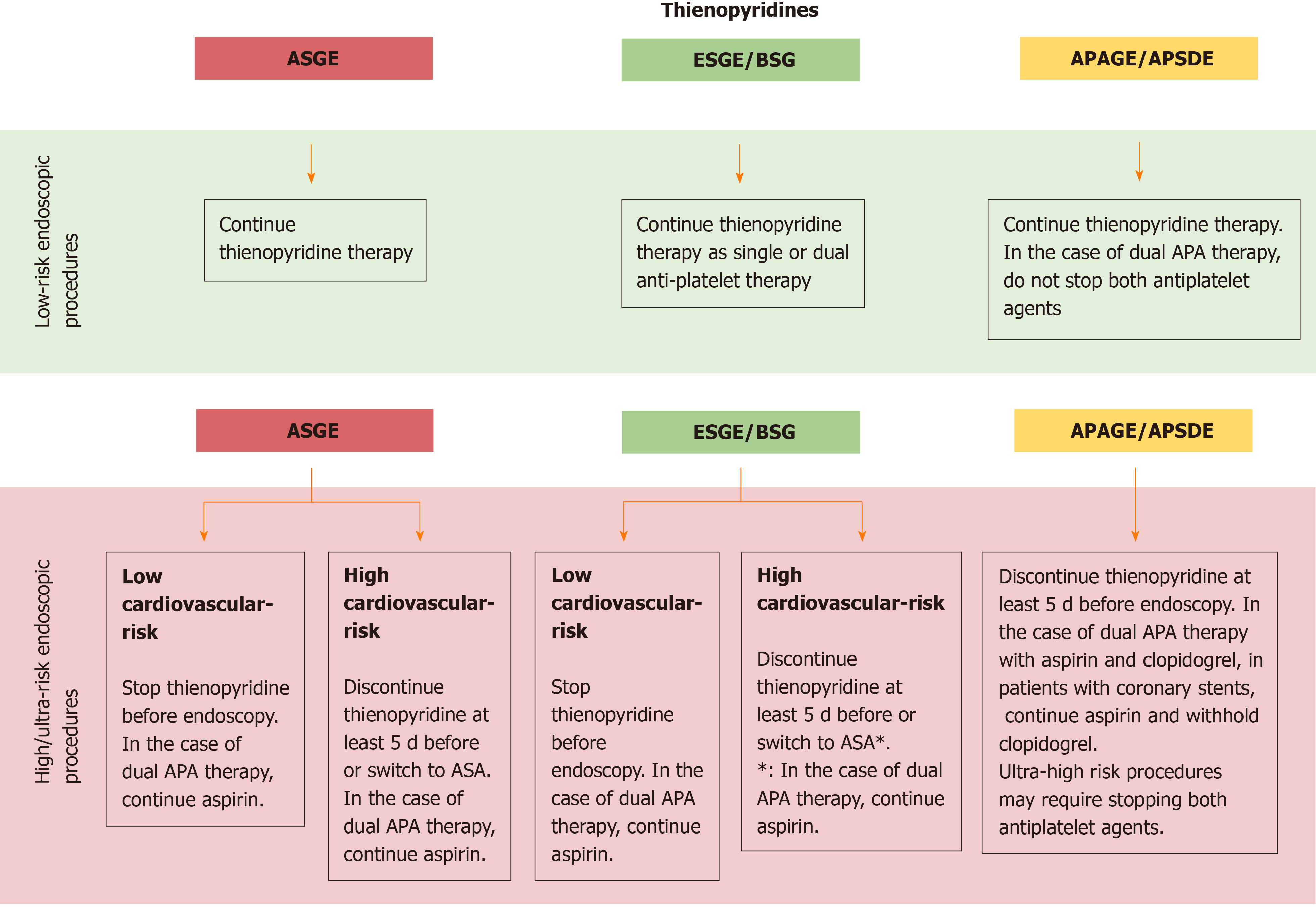
Recommendations on thienopyridines management are provided depending on the estimate procedure risk:
(1) For patients undergoing low-risk endoscopic procedures, all guidelines recommend continuing thienopyridine. Concerning dual antiplatelet therapy, ESGE suggests continuing dual antiplatelet therapy, while APAGE/APSDE advice to don’t stop both antiplatelet agents;
(2) For patients undergoing high-risk endoscopic procedures, ASGE and ESGE/BSG recommend to assess before the cardiovascular risk (CVR, Table 2): If low CVR, stop thienopyridine before endoscopy. In the case of dual APA therapy, both ASGE and ESGE/BSG agree to continue aspirin if already prescribed; if high CVR, ASGE recommends discontinuing thienopyridine at least 5 d before or switch to ASA, while ESGE/BSG suggest considering discontinuation of thienopyridine 5 d before only after 12 mo following insertion of drug-eluting coronary stent or after 1 mo after insertion of a bare metal coronary stent.
APAGE/APSDE recommend discontinuing thienopyridine at least 5 d before endoscopy in all patient undergoing a high-risk endoscopy procedure regardless of the CVR.
With regard to patients with dual APA therapy, all guidelines agree to withhold the thienopyridine and continue aspirin. Moreover, according to APAGE/APSDE ultra-high risk procedures may require stopping both antiplatelet agents (Figure 2).
After the procedure, the suggested management differs according to the guidelines: ASGE suggests to resume thienopyridine after the procedure once hemostasis is achieved; in this setting, a loading dose of thienopyridine should be considered among patients at risk for thrombosis[3]; ESGE/BSG recommend that thienopyridine should be resumed up to 48 h after the procedure depending on the perceived bleeding and thrombotic risks[4]; APAGE/APSDE recommend early resumption of thienopyridine within 5 d after endoscopic hemostasis in patients with drug-eluting coronary stents[5].
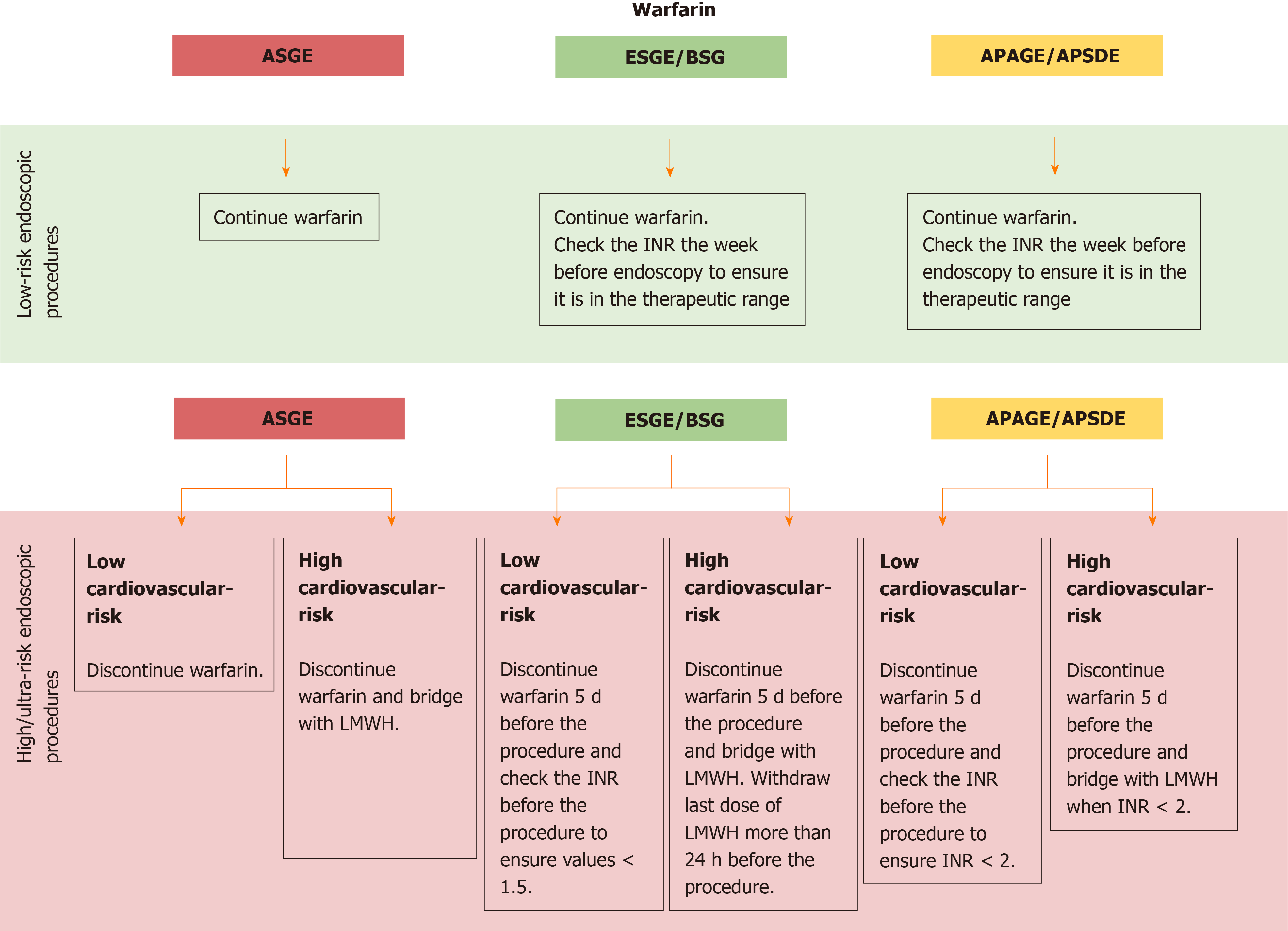
Also for warfarin, recommendations are provided depending on the estimate procedure risk:
(1) For patients undergoing low-risk endoscopic procedures, all guidelines recommend continuing warfarin. In addition, ESGE/BSG and APAGE/APSDE suggest to check the INR the week before endoscopy to ensure it is in the normal range[4,5];
(2) For patients undergoing high or ultra-risk endoscopic procedures, all guidelines recommend to assess before the CVR (Table 2): If low CVR, discontinue warfarin 5 d before the procedure and check the INR before the procedure to ensure values < 1.5 according to ESGE/BSG[4] or less conservative values < 2 according to APAGE/APSDE[5]; if high CVR, discontinue warfarin 5 d before the procedure and administer bridge therapy with LMWH. In addition, ESGE/BSG recommend withdrawing the last dose of LMWH more than 24 h before the procedure[4] (Figure 3).
All guidelines recommend resuming warfarin the same day/evening of the procedure after proper hemostasis has been achieved. Moreover, for patients with high CVR, ESGE/BSG and APAGE/APSDE specify to continue LMWH until therapeutic INR range has been achieved.
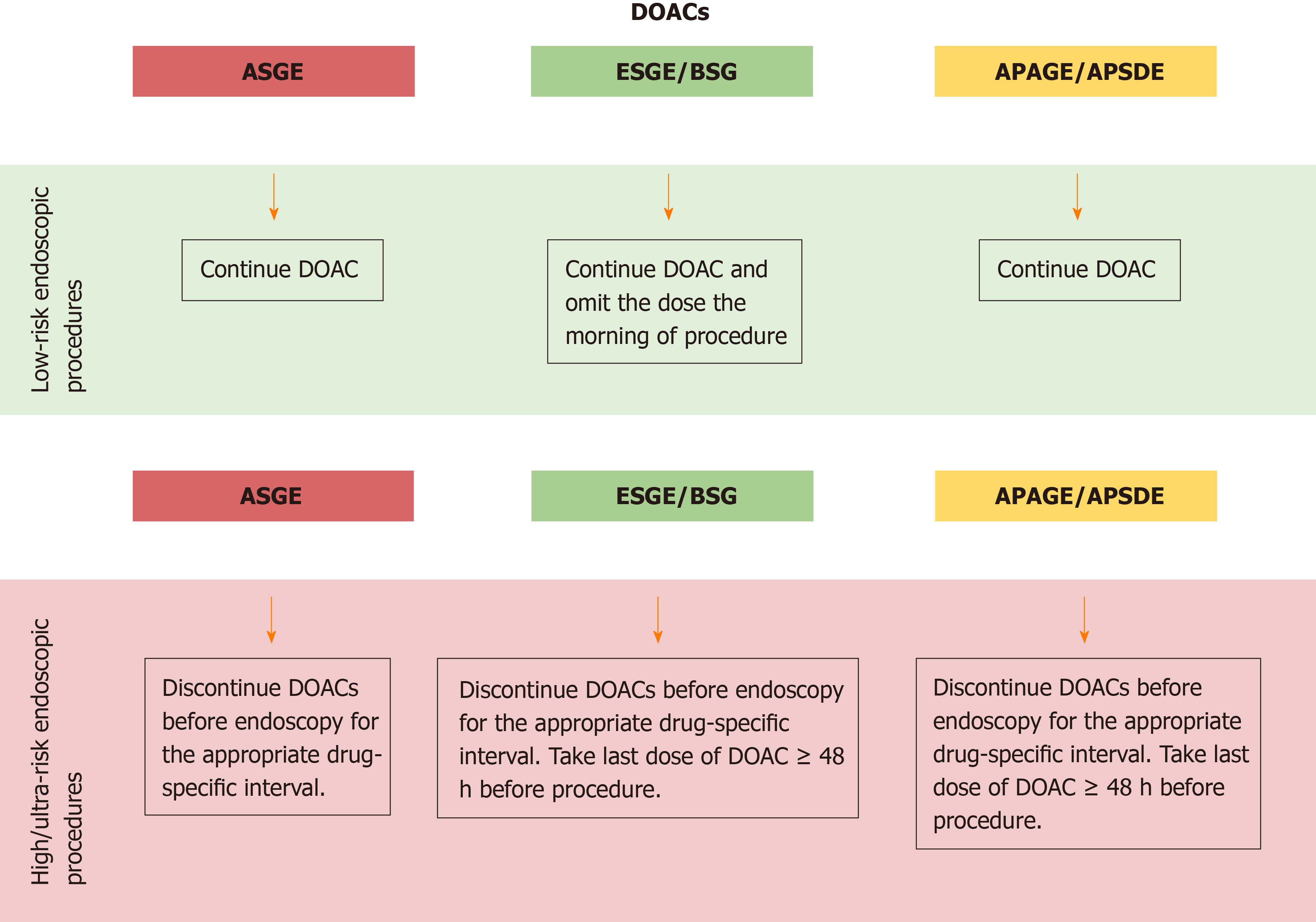
For patients undergoing low-risk endoscopic procedures, ASGE and APAGE/APSDE recommend continuing DOACs in the periendoscopic period, similar to what is suggested for warfarin[3,5]. On the contrary, ESGE/BSG guidelines recommend omitting the DOACs dose the morning of the procedure. Nevertheless, this last statement is reported as a weak recommendation and supported by very low-quality evidence[4].
For patients undergoing high-risk endoscopic procedures, all guidelines recommend discontinuing DOACs before endoscopy for the appropriate drug-specific interval, adjusting for creatinine clearance[3-5]. In this regard, ESGE/BSG and APAGE/APSDE specify to take the last dose of DOACs ≥ 48 h before the procedure[4,5] (Figure 4).
Unlike reintroduction of warfarin, which results in delayed anticoagulation for several days, a therapeutic intensity of anticoagulation is restored within 3 h of taking a therapeutic dose of a DOAC. Because of the high-risk of bleeding associated with the therapeutic intensity of anticoagulation after an invasive procedure, guidelines suggest a delay in reintroducing DOACs after a high-risk procedure. This delay will depend on the bleeding risk associated with the procedure and will usually be 24-48 h[4]. For procedures with a significant risk of delayed bleeding such as EMR or ESD, a longer period of discontinuation may be considered for patients with a relatively low-thrombotic risk.
According to ASGE guidelines, if DOACs cannot be restarted within 24 h after a high-risk procedure because of concern regarding the adequate hemostasis due to their short onset of action, then thromboprophylaxis (e.g., LMWH bridge) should be considered for patients at high-risk for thromboembolism[3].
On the contrary, APAGE/APSDE recommends early resumption of DOACs soon after the procedure after adequate hemostasis has been achieved.
ASGE guidelines recommend consultation with the prescribing specialist before stopping APAs during gastrointestinal bleeding in patients for which the cardiovascular risk overcomes the potential consequences of bleeding, namely patients with: (1) Placed drug eluting intracoronary stents within 1 year, AND (2) Insertion of a bare metal intracoronary stent within 1 mo, or after an acute coronary syndrome within 90 d[3].
The ESGE/BSG guidelines propose an algorithm in which patient management depend from primary or secondary prophylaxis.
The algorithm recommends withholding aspirin until the third day after endoscopic treatment of high-risk stigmata in patients taking APA for secondary prophylaxis[4].
In this regards, two studies have shown that in patients taking low-dose aspirin for secondary cardiovascular prophylaxis, the all-cause mortality was lower if aspirin was continued[106,107].
Besides, APAGE/APSDE guidelines recommend withholding aspirin only in patients with serious or life-threatening bleeding in places where endoscopy is not readily available[5].
With regard to patients taking dual antiplatelet therapy having acute gastrointestinal bleeding, both ESGE/BSG and APAGE/APSDE recommend continuation of aspirin and withholding clopidogrel. In these patients, resumption of therapy preferably within 5 d after endoscopic hemostasis is recommended by APAGE/APSDE guidelines, whereas ESGE/BSG and ASGE suggest consulting with a cardiologist for the management after urgent endoscopic treatment.
The estimated incidence of gastrointestinal bleeding in patients in anticoagulant therapy ranges between 1%-4% per year[108].
The management of patients using anticoagulants with active gastrointestinal bleeding has been the focus of numerous studies for many years.
The ESGE/BSG and ASGE guidelines recommend to stop the therapy and correct the INR in patients taking vitamin K antagonists with signs of severe bleeding, before performing urgent endoscopy[109-111]. Moreover, they also recommend not to delay endoscopy if INR < 2.5. In this regard, Choudari and colleagues described a retrospective series of 52 patients which developed bleeding while taking warfarin. The outcome and the endoscopy efficacy in patients with an INR corrected between 1.5 and 2.5 were good and similar to those recorded in patients not taking anticoagulants. Therefore, even a partial correction of the INR seems to be associated with a good outcome[112].
In a retrospective cohort study of 233 consecutive anticoagulated patients undergoing urgent endoscopy with successful hemostasis, 95% of them had an INR between 1.3 and 2.7. The rate of re-bleeding was 23%, but INR was not a predictor of re-bleeding on multivariable analyzes[113].
Another retrospective study of patients taking warfarin at the moment of admission for gastrointestinal bleeding, reports 55 patients with INR value of 4.0 or greater (supratherapeutic) and 43 with INR in the range 2.0 to 3.9. Patients with supratherapeutic INRs were more likely to have a gastrointestinal pathology supporting the need of endoscopic evaluation, but no differences in the rate of re-bleeding were recorded[114].
A large systematic review of 1869 patients with nonvariceal upper GI bleeding, showed that INR value at the initial presentation of the event did not predict the risk of re-bleeding. Rather, the finding of INR > 1.5 during non-variceal digestive hemorrhage was associated with an increase in mortality after correction in patients with specific comorbidities. Hence, in this study, the INR value was found to be useful in the risk stratification than as a predictor of re-bleeding[115]. Furthermore, in a retrospective case-control study, Irwin and colleagues have highlighted that patients with supratherapeutic INR at the time of gastrointestinal bleeding had a lower mortality rate 30 d after the event compared to patients not taking warfarin[116].
This evidence suggests that the strategy aimed to normalize the INR in all patients delaying the timing of endoscopy could not be so useful in the clinical practice. Hence, as suggested by ASGE and ESGE/BSG practice guidelines, the endoscopic treatment can be considered effective and relatively safe with INR values < 2.5[3,4].
In the management of a patient with active gastrointestinal bleeding, the decision to correct the coagulopathy it’s often difficult because of the risk of thromboembolic consequences.
The thrombotic risk after transient withdrawal of anticoagulant therapy in acute gastrointestinal bleeding settings was explored by 2 small studies conducted on 27 and 28 patients. The withdrawal time was variable between 5 and 14 d, in one of the two studies it was also administered vitamin K or frozen plasma was given to 7 patients to reverse anticoagulation. Both studies showed a low-risk of thrombotic complications[117,118].
All the guidelines (ASGE, ESGE/BSG and APAGE/APSDE) agree on the urgent anticoagulation reversal in all patients presenting with life-threatening gastrointestinal bleeding, regardless of therapeutic or supra-therapeutic INR elevations[3-5,119].
Specifically, ESGE/BSG recommend estimating the cardiovascular risk of the patient by consulting with a cardiologist before starting INR correction[4].
For patients without signs of active bleeding and hemodynamically stable, the usefulness of INR correction should be evaluated on a case by case basis.
For an urgent correction of coagulopathy in patients taking warfarin, ASGE and ESGE/BSG guidelines suggest the administration of prothrombin complex concentrates (PCC) or fresh frozen plasma (FFP)[3,4].
Moreover, ESGE/BSG highlight that it is preferable to use PCC in combination with intravenous vitamin K at the dosage of 5-10 mg K to prevent “rebound coagulopathy” limiting the use of FFP when PCC is not available[4].
Other guidelines, such as those of the APAGE/APSDE recommend only the combination of PCC with concomitant low-dose vitamin K (< 5 mg instead of 5-10 mg) administration[5]. With regard to the latter, the decision to prefer low-dose vitamin K is based on the evidence from four randomized clinical trials that the optimal dose of vitamin K to achieve a normalization of the INR value ranges between 1 and 2.5 mg[120-123].
Currently, there are no randomized clinical trials comparing prothrombin PCC and FFP for warfarin reversal in acute GI bleeding.
In 2014 Karaca et al[124] performed a prospective, non-randomized, comparative study of 40 patients taking warfarin with upper gastrointestinal haemorrhage and INR > 2.1. Patients received either PCC or FFP, and INR levels were reversed more quickly in PCC group at the second and sixth h (second h INR: 1.53 vs 4.50, P < 0.01, sixth h INR: 1.52 vs 2.41, P < 0.01). At the time of endoscopy, no patient in the PCC group had active bleeding compared with 7 in the FFP group (0% vs 35 %, P < 0.01).
Concerning the thrombotic risk, a meta-analysis of 2011 showed a similar risk for the two agents (~ 1%)[125].
In addition to a faster onset of action, in the clinical practice, PCC have other advantages, as no need for ABO matching, less risk for volume overload because of smaller transfusion volume and minimal risk of infectious transmission. On the contrary, a disadvantage is represented by the higher cost[126].
With regard to the resumption of warfarin after the bleeding event, APAGE-APSDE guidelines recommend resuming warfarin by day 3 after adequate hemostasis is achieved and to consider bridge with LMWH in patients with high thrombotic risk[5]. On the other side, ESGE/BSG suggest resuming warfarin between 7 and 15 d following the bleeding event if hemostasis is successfully achieved[4].
The ESGE/BSG guidelines recommend the use of platelet transfusion or desmopressin for more severe bleeding, although, there is no clear evidence to support this approach[4]. As evidenced by recent data, platelet transfusion is associated with an increase in mortality[127].
The most recent endoscopic APAGE/APSDE guidelines, do not recommend making specific assays to estimate the anticoagulant activity of DOACs in acute bleeding[5]. Prothrombin time and activated partial thromboplastin time could be inaccurate in DOACs activity evaluation and currently specific assays are not routinely available.
With the exception of patients with reduced renal clearance, generally, the best strategy in patients taking DOACs is their withdrawal due to their short half-lives.
Moreover, it should also be considered that, to date, antidotes and direct inhibitor of DOACs are available. In a multicenter open-label study of 503 patients, idarucizumab proved to be safe and effective in neutralizing the anticoagulant effect of dabigatran in emergency situations[15].
Another study assessed the effectiveness of andexanet alfa, a modified recombinant inactive form of human factor Xa, for bleeding associated with Factor Xa Inhibitors such as rivaroxaban, apixaban and edoxaban. The study showed that treatment with andexanet in patients with major acute bleeding associated with the use of a factor Xa inhibitor, markedly reduced anti-factor Xa activity, with an excellent or good hemostatic efficacy in 82% of patients[16].
After index bleeding episode, APAGE/APSDE recommend resuming DOACs by day 3 after hemostasis is successfully achieved, without heparin bridging[5].
On the contrary, ASGE and ESGE/BSG guidelines do not provide a specific recommendation on the resumption of DOACs[3,4].
Currently, there is sufficient evidence from the literature to assess the basal risk of bleeding associated with main endoscopic procedures in patients who do not take antithrombotic agents.
On the contrary, there are not sufficient data allowing to evaluate as this risk increases in patients taking anticoagulants, especially in some specific procedures (e.g. endoscopic dilatation, stenting, esophageal variceal ligation, EUS-FNA etc). Nevertheless, the high-risk associated with these procedures makes further studies unlikely to be carried out in the future.
To date, current international practice guidelines provide specific recommendations for the management of anticoagulants in endoscopy, with a good agreement and some major differences that have already been discussed (Figures 1-4).
Some of these differences may be explained by a different proposed approach, different geographical area of reference and different year of publication.
Nevertheless, even with some discrepancy, guidelines only represent the guide for a common policy, over which a tailored approach should always be applied. On this line, the assessment of the individual risk-benefit ratio is essential.
As a matter of fact, the interruption of anticoagulants is undoubtful effective in reducing the risk of bleeding during endoscopic procedures. Despite this, the thromboembolic risk that follows the therapy suspension should not be underestimated. For this reason, the individual decision should be taken on a case by case basis taking into account every single factor.
In addition, since several endoscopic examinations are commonly requested by the general practitioners or by other consultants as “open access”, a preliminary gastroenterological visit with the patient is essential to better assess the procedure risk and the proper schedule for withdrawal of anticoagulants, as well as written information on their resumption, should be provided at discharge.
Moreover, when necessary, consultation with the cardiologist or the hematologist has primary importance for a tailored approach on the single patient.
In the future, further studies are needed to clarify better the grey areas and topics on which the guidelines still present some controversial points. In the meantime, we suggest to comply with the international guidelines strictly and to discuss difficult decisions within multidisciplinary boards.
| 1. | Baker E, Roberts AP, Wilde K, Walton H, Suri S, Rull G, Webb A. Development of a core drug list towards improving prescribing education and reducing errors in the UK. Br J Clin Pharmacol. 2011;71:190-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Hansen ML, Sørensen R, Clausen MT, Fog-Petersen ML, Raunsø J, Gadsbøll N, Gislason GH, Folke F, Andersen SS, Schramm TK, Abildstrøm SZ, Poulsen HE, Køber L, Torp-Pedersen C. Risk of bleeding with single, dual, or triple therapy with warfarin, aspirin, and clopidogrel in patients with atrial fibrillation. Arch Intern Med. 2010;170:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 650] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 3. | ASGE Standards of Practice Committee, Roberts Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, Hwang JH, Khashab MA, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Shergill AK, Wang A, Cash BD, DeWitt JM. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 483] [Article Influence: 48.3] [Reference Citation Analysis (2)] |
| 4. | Veitch AM, Vanbiervliet G, Gershlick AH, Boustiere C, Baglin TP, Smith LA, Radaelli F, Knight E, Gralnek IM, Hassan C, Dumonceau JM. Endoscopy in patients on antiplatelet or anticoagulant therapy, including direct oral anticoagulants: British Society of Gastroenterology (BSG) and European Society of Gastrointestinal Endoscopy (ESGE) guidelines. Gut. 2016;65:374-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 5. | Chan FKL, Goh KL, Reddy N, Fujimoto K, Ho KY, Hokimoto S, Jeong YH, Kitazono T, Lee HS, Mahachai V, Tsoi KKF, Wu MS, Yan BP, Sugano K. Management of patients on antithrombotic agents undergoing emergency and elective endoscopy: joint Asian Pacific Association of Gastroenterology (APAGE) and Asian Pacific Society for Digestive Endoscopy (APSDE) practice guidelines. Gut. 2018;67:405-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 6. | Patrono C, Loscalzo J, Schafer AI. Pharmacology of antiplatelet agents. Thrombosis and Hemorrhage. Baltimore: William & Wilkins; 1998; 1181-1192. |
| 7. | Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, Steg PG, Ferrières J, Danchin N, Becquemont L; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1280] [Cited by in RCA: 1231] [Article Influence: 72.4] [Reference Citation Analysis (0)] |
| 8. | Wiviott SD, Trenk D, Frelinger AL, O'Donoghue M, Neumann FJ, Michelson AD, Angiolillo DJ, Hod H, Montalescot G, Miller DL, Jakubowski JA, Cairns R, Murphy SA, McCabe CH, Antman EM, Braunwald E; PRINCIPLE-TIMI 44 Investigators. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation. 2007;116:2923-2932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 664] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 9. | Gurbel PA, Bliden KP, Butler K, Tantry US, Gesheff T, Wei C, Teng R, Antonino MJ, Patil SB, Karunakaran A, Kereiakes DJ, Parris C, Purdy D, Wilson V, Ledley GS, Storey RF. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577-2585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 917] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 10. | Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabaté M, Senior R, Taggart DP, van der Wall EE, Vrints CJ; ESC Committee for Practice Guidelines, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Document Reviewers, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Rydén L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949-3003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2772] [Cited by in RCA: 3032] [Article Influence: 233.2] [Reference Citation Analysis (1)] |
| 11. | Jardine MJ, Ninomiya T, Perkovic V, Cass A, Turnbull F, Gallagher MP, Zoungas S, Lambers Heerspink HJ, Chalmers J, Zanchetti A. Aspirin is beneficial in hypertensive patients with chronic kidney disease: a post-hoc subgroup analysis of a randomized controlled trial. J Am Coll Cardiol. 2010;56:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 179] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 12. | Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, Sandercock PA, Fox KA, Lowe GD, Murray GD; Aspirin for Asymptomatic Atherosclerosis Trialists. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 491] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 13. | Schulman S, Crowther MA. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood. 2012;119:3016-3023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3181] [Cited by in RCA: 3780] [Article Influence: 315.0] [Reference Citation Analysis (1)] |
| 15. | Abraham NS, Singh S, Alexander GC, Heien H, Haas LR, Crown W, Shah ND. Comparative risk of gastrointestinal bleeding with dabigatran, rivaroxaban, and warfarin: population based cohort study. BMJ. 2015;350:h1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (1)] |
| 16. | Abraham NS, Noseworthy PA, Yao X, Sangaralingham LR, Shah ND. Gastrointestinal Safety of Direct Oral Anticoagulants: A Large Population-Based Study. Gastroenterology. 2017;152:1014-1022.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 169] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 17. | Lahaye S, Regpala S, Lacombe S, Sharma M, Gibbens S, Ball D, Francis K. Evaluation of patients' attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost. 2014;111:465-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Pollack CV, Reilly PA, Eikelboom J, Glund S, Verhamme P, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kamphuisen PW, Kreuzer J, Levy JH, Sellke FW, Stangier J, Steiner T, Wang B, Kam CW, Weitz JI. Idarucizumab for Dabigatran Reversal. N Engl J Med. 2015;373:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1176] [Cited by in RCA: 1103] [Article Influence: 100.3] [Reference Citation Analysis (0)] |
| 19. | Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, Yue P, Bronson MD, Lu G, Conley PB, Verhamme P, Schmidt J, Middeldorp S, Cohen AT, Beyer-Westendorf J, Albaladejo P, Lopez-Sendon J, Demchuk AM, Pallin DJ, Concha M, Goodman S, Leeds J, Souza S, Siegal DM, Zotova E, Meeks B, Ahmad S, Nakamya J, Milling TJ; ANNEXA-4 Investigators. Full Study Report of Andexanet Alfa for Bleeding Associated with Factor Xa Inhibitors. N Engl J Med. 2019;380:1326-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 688] [Article Influence: 98.3] [Reference Citation Analysis (0)] |
| 20. | Macrae FA, Tan KG, Williams CB. Towards safer colonoscopy: a report on the complications of 5000 diagnostic or therapeutic colonoscopies. Gut. 1983;24:376-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 316] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Vu CK, Korman MG, Bejer I, Davis S. Gastrointestinal bleeding after cold biopsy. Am J Gastroenterol. 1998;93:1141-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Whitson MJ, Dikman AE, von Althann C, Sanyal S, Desai JC, Bamji ND, Kornacki S, Harpaz N, Bodian CA, Cohen LB, Miller KM, Aisenberg J. Is gastroduodenal biopsy safe in patients receiving aspirin and clopidogrel?: a prospective, randomized study involving 630 biopsies. J Clin Gastroenterol. 2011;45:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Ono S, Fujishiro M, Kodashima S, Takahashi Y, Minatsuki C, Mikami-Matsuda R, Asada-Hirayama I, Konno-Shimizu M, Tsuji Y, Mochizuki S, Niimi K, Yamamichi N, Kaneko M, Yatomi Y, Koike K. Evaluation of safety of endoscopic biopsy without cessation of antithrombotic agents in Japan. J Gastroenterol. 2012;47:770-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Gerson LB, Tokar J, Chiorean M, Lo S, Decker GA, Cave D, Bouhaidar D, Mishkin D, Dye C, Haluszka O, Leighton JA, Zfass A, Semrad C. Complications associated with double balloon enteroscopy at nine US centers. Clin Gastroenterol Hepatol. 2009;7:1177-1182, 1182.e1-1182.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Gavin DR, Valori RM, Anderson JT, Donnelly MT, Williams JG, Swarbrick ET. The national colonoscopy audit: a nationwide assessment of the quality and safety of colonoscopy in the UK. Gut. 2013;62:242-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (1)] |
| 26. | Wexner SD, Garbus JE, Singh JJ; SAGES Colonoscopy Study Outcomes Group. A prospective analysis of 13,580 colonoscopies. Reevaluation of credentialing guidelines. Surg Endosc. 2001;15:251-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 27. | Bowles CJ, Leicester R, Romaya C, Swarbrick E, Williams CB, Epstein O. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 421] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 28. | Sieg A, Hachmoeller-Eisenbach U, Eisenbach T. Prospective evaluation of complications in outpatient GI endoscopy: a survey among German gastroenterologists. Gastrointest Endosc. 2001;53:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 211] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Rutter MD, Nickerson C, Rees CJ, Patnick J, Blanks RG. Risk factors for adverse events related to polypectomy in the English Bowel Cancer Screening Programme. Endoscopy. 2014;46:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 30. | Kim HS, Kim TI, Kim WH, Kim YH, Kim HJ, Yang SK, Myung SJ, Byeon JS, Lee MS, Chung IK, Jung SA, Jeen YT, Choi JH, Choi KY, Choi H, Han DS, Song JS. Risk factors for immediate postpolypectomy bleeding of the colon: a multicenter study. Am J Gastroenterol. 2006;101:1333-1341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 198] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 31. | Sawhney MS, Salfiti N, Nelson DB, Lederle FA, Bond JH. Risk factors for severe delayed postpolypectomy bleeding. Endoscopy. 2008;40:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 32. | Shiffman ML, Farrel MT, Yee YS. Risk of bleeding after endoscopic biopsy or polypectomy in patients taking aspirin or other NSAIDS. Gastrointest Endosc. 1994;40:458-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 124] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Hui AJ, Wong RM, Ching JY, Hung LC, Chung SC, Sung JJ. Risk of colonoscopic polypectomy bleeding with anticoagulants and antiplatelet agents: analysis of 1657 cases. Gastrointest Endosc. 2004;59:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 34. | Repici A, Hassan C, Vitetta E, Ferrara E, Manes G, Gullotti G, Princiotta A, Dulbecco P, Gaffuri N, Bettoni E, Pagano N, Rando G, Strangio G, Carlino A, Romeo F, de Paula Pessoa Ferreira D, Zullo A, Ridola L, Malesci A. Safety of cold polypectomy for <10mm polyps at colonoscopy: a prospective multicenter study. Endoscopy. 2012;44:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 188] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 35. | Feagins LA, Uddin FS, Davila RE, Harford WV, Spechler SJ. The rate of post-polypectomy bleeding for patients on uninterrupted clopidogrel therapy during elective colonoscopy is acceptably low. Dig Dis Sci. 2011;56:2631-2638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Gandhi S, Narula N, Mosleh W, Marshall JK, Farkouh M. Meta-analysis: colonoscopic post-polypectomy bleeding in patients on continued clopidogrel therapy. Aliment Pharmacol Ther. 2013;37:947-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Friedland S, Soetikno R. Colonoscopy with polypectomy in anticoagulated patients. Gastrointest Endosc. 2006;64:98-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Friedland S, Sedehi D, Soetikno R. Colonoscopic polypectomy in anticoagulated patients. World J Gastroenterol. 2009;15:1973-1976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Horiuchi A, Nakayama Y, Kajiyama M, Tanaka N, Sano K, Graham DY. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc. 2014;79:417-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 40. | Li HK, Chen FC, Rea RF, Asirvatham SJ, Powell BD, Friedman PA, Shen WK, Brady PA, Bradley DJ, Lee HC, Hodge DO, Slusser JP, Hayes DL, Cha YM. No increased bleeding events with continuation of oral anticoagulation therapy for patients undergoing cardiac device procedure. Pacing Clin Electrophysiol. 2011;34:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Douketis JD, Spyropoulos AC, Kaatz S, Becker RC, Caprini JA, Dunn AS, Garcia DA, Jacobson A, Jaffer AK, Kong DF, Schulman S, Turpie AG, Hasselblad V, Ortel TL; BRIDGE Investigators. Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation. N Engl J Med. 2015;373:823-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 774] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 42. | Yanagisawa N, Nagata N, Watanabe K, Iida T, Hamada M, Kobayashi S, Shimbo T, Akiyama J, Uemura N. Post-polypectomy bleeding and thromboembolism risks associated with warfarin vs direct oral anticoagulants. World J Gastroenterol. 2018;24:1540-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 43. | Yu JX, Oliver M, Lin J, Chang M, Limketkai BN, Soetikno R, Bhattacharya J, Kaltenbach T. Patients Prescribed Direct-Acting Oral Anticoagulants Have Low Risk of Postpolypectomy Complications. Clin Gastroenterol Hepatol. 2019;17:2000-2007.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Kodama M, Kakegawa T. Treatment of superficial cancer of the esophagus: a summary of responses to a questionnaire on superficial cancer of the esophagus in Japan. Surgery. 1998;123:432-439. [PubMed] |
| 45. | Katada C, Muto M, Momma K, Arima M, Tajiri H, Kanamaru C, Ooyanagi H, Endo H, Michida T, Hasuike N, Oda I, Fujii T, Saito D. Clinical outcome after endoscopic mucosal resection for esophageal squamous cell carcinoma invading the muscularis mucosae--a multicenter retrospective cohort study. Endoscopy. 2007;39:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 131] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 46. | Ciocirlan M, Lapalus MG, Hervieu V, Souquet JC, Napoléon B, Scoazec JY, Lefort C, Saurin JC, Ponchon T. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy. 2007;39:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 47. | Nonaka S, Oda I, Tada K, Mori G, Sato Y, Abe S, Suzuki H, Yoshinaga S, Nakajima T, Matsuda T, Taniguchi H, Saito Y, Maetani I. Clinical outcome of endoscopic resection for nonampullary duodenal tumors. Endoscopy. 2015;47:129-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Qumseya BJ, Wolfsen C, Wang Y, Othman M, Raimondo M, Bouras E, Wolfsen H, Wallace MB, Woodward T. Factors associated with increased bleeding post-endoscopic mucosal resection. J Dig Dis. 2013;14:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Heresbach D, Kornhauser R, Seyrig JA, Coumaros D, Claviere C, Bury A, Cottereau J, Canard JM, Chaussade S, Baudet A, Casteur A, Duval O, Ponchon T; OMEGA group. A national survey of endoscopic mucosal resection for superficial gastrointestinal neoplasia. Endoscopy. 2010;42:806-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Albéniz E, Gimeno-García AZ, Fraile M, Ibáñez B, Guarner-Argente C, Alonso-Aguirre P, Álvarez MA, Gargallo CJ, Pellisé M, Ramos Zabala F, Herreros de Tejada A, Nogales Ó, Martínez-Ares D, Múgica F, de la Peña J, Espinós J, Huerta A, Álvarez A, Gonzalez-Santiago JM, Navajas F, Martínez-Cara JG, Redondo-Cerezo E, Merlo Mas J, Sábado F, Rivero L, Saperas E, Soto S, Rodríguez-Sánchez J, López-Roses L, Rodríguez-Téllez M, Rullán Iriarte M, Elosua González A, Pardeiro R, Valdivielso Cortázar E, Concepción-Martín M, Huelin Álvarez P, Colán Hernández J, Cobian J, Santiago J, Jiménez A, Remedios D, López-Viedma B, García O, Martínez-Alcalá F, Pérez-Roldán F, Carbó J, Enguita M. Clinical validation of risk scoring systems to predict risk of delayed bleeding after EMR of large colorectal lesions. Gastrointest Endosc. 2020;91:868-878.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 51. | Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest Endosc. 2013;77:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 52. | Feagins LA, Nguyen AD, Iqbal R, Spechler SJ. The prophylactic placement of hemoclips to prevent delayed post-polypectomy bleeding: an unnecessary practice? A case control study. Dig Dis Sci. 2014;59:823-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Shioji K, Suzuki Y, Kobayashi M, Nakamura A, Azumaya M, Takeuchi M, Baba Y, Honma T, Narisawa R. Prophylactic clip application does not decrease delayed bleeding after colonoscopic polypectomy. Gastrointest Endosc. 2003;57:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 188] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Metz AJ, Bourke MJ, Moss A, Williams SJ, Swan MP, Byth K. Factors that predict bleeding following endoscopic mucosal resection of large colonic lesions. Endoscopy. 2011;43:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 55. | Namasivayam V, Prasad GA, Lutzke LS, Dunagan KT, Borkenhagen LS, Okoro NI, Tomizawa Y, Buttar NS, Michel WL, Wang KK. The risk of endoscopic mucosal resection in the setting of clopidogrel use. ISRN Gastroenterol. 2014;2014:494157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 288] [Article Influence: 16.9] [Reference Citation Analysis (2)] |
| 57. | Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci. 2014;59:1862-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 58. | Repici A, Hassan C, De Paula Pessoa D, Pagano N, Arezzo A, Zullo A, Lorenzetti R, Marmo R. Efficacy and safety of endoscopic submucosal dissection for colorectal neoplasia: a systematic review. Endoscopy. 2012;44:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 59. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A, Taguri M, Morita S, Maeda S, Tanaka K. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Lim JH, Kim SG, Kim JW, Choi YJ, Kwon J, Kim JY, Lee YB, Choi J, Im JP, Kim JS, Jung HC, Song IS. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc. 2012;75:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 61. | Miyahara K, Iwakiri R, Shimoda R, Sakata Y, Fujise T, Shiraishi R, Yamaguchi K, Watanabe A, Yamaguchi D, Higuchi T, Tominaga N, Ogata S, Tsuruoka N, Noda T, Hidaka H, Mannen K, Endo H, Yamanouchi K, Yamazato T, Sakata H, Fujimoto K. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion. 2012;86:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 62. | Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, Morikawa T, Murakami T, Tomoda J. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 63. | Toyonaga T, Man-i M, East JE, Nishino E, Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T, Hirooka T, Fujita T, Inokuchi H, Azuma T. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000-1008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 64. | Kim TS, Kim B, Min BH, Min YW, Lee H, Lee JH, Rhee PL, Kim JJ, Kushima R, Kim KM. Outcomes of endoscopic submucosal dissection for intestinal-type adenocarcinoma with anastomosing glands of the stomach. J Gastroenterol Hepatol. 2020;35:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 65. | Nam HS, Choi CW, Kim SJ, Kim HW, Kang DH, Park SB, Ryu DG. Risk factors for delayed bleeding by onset time after endoscopic submucosal dissection for gastric neoplasm. Sci Rep. 2019;9:2674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 66. | Cho SJ, Choi IJ, Kim CG, Lee JY, Nam BH, Kwak MH, Kim HJ, Ryu KW, Lee JH, Kim YW. Aspirin use and bleeding risk after endoscopic submucosal dissection in patients with gastric neoplasms. Endoscopy. 2012;44:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 67. | Koh R, Hirasawa K, Yahara S, Oka H, Sugimori K, Morimoto M, Numata K, Kokawa A, Sasaki T, Nozawa A, Taguri M, Morita S, Maeda S, Tanaka K. Antithrombotic drugs are risk factors for delayed postoperative bleeding after endoscopic submucosal dissection for gastric neoplasms. Gastrointest Endosc. 2013;78:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Takeuchi T, Ota K, Harada S, Edogawa S, Kojima Y, Tokioka S, Umegaki E, Higuchi K. The postoperative bleeding rate and its risk factors in patients on antithrombotic therapy who undergo gastric endoscopic submucosal dissection. BMC Gastroenterol. 2013;13:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 69. | Nakamura M, Nishikawa J, Hamabe K, Nishimura J, Satake M, Goto A, Kiyotoki S, Saito M, Fukagawa Y, Shirai Y, Okamoto T, Sakaida I. Risk factors for delayed bleeding from endoscopic submucosal dissection of gastric neoplasms. Scand J Gastroenterol. 2012;47:1108-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 70. | Lim JH, Kim SG, Kim JW, Choi YJ, Kwon J, Kim JY, Lee YB, Choi J, Im JP, Kim JS, Jung HC, Song IS. Do antiplatelets increase the risk of bleeding after endoscopic submucosal dissection of gastric neoplasms? Gastrointest Endosc. 2012;75:719-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Suzuki S, Chino A, Kishihara T, Uragami N, Tamegai Y, Suganuma T, Fujisaki J, Matsuura M, Itoi T, Gotoda T, Igarashi M, Moriyasu F. Risk factors for bleeding after endoscopic submucosal dissection of colorectal neoplasms. World J Gastroenterol. 2014;20:1839-1845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 72. | Terasaki M, Tanaka S, Shigita K, Asayama N, Nishiyama S, Hayashi N, Nakadoi K, Oka S, Chayama K. Risk factors for delayed bleeding after endoscopic submucosal dissection for colorectal neoplasms. Int J Colorectal Dis. 2014;29:877-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 73. | Williams VA, Watson TJ, Zhovtis S, Gellersen O, Raymond D, Jones C, Peters JH. Endoscopic and symptomatic assessment of anastomotic strictures following esophagectomy and cervical esophagogastrostomy. Surg Endosc. 2008;22:1470-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 74. | Raymondi R, Pereira-Lima JC, Valves A, Morales GF, Marques D, Lopes CV, Marroni CA. Endoscopic dilation of benign esophageal strictures without fluoroscopy: experience of 2750 procedures. Hepatogastroenterology. 2008;55:1342-1348. [PubMed] |
| 75. | Metman EH, Lagasse JP, d'Alteroche L, Picon L, Scotto B, Barbieux JP. Risk factors for immediate complications after progressive pneumatic dilation for achalasia. Am J Gastroenterol. 1999;94:1179-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 76. | Polese L, Angriman I, Bonello E, Erroi F, Scarpa M, Frego M, D'Amico DF, Norberto L. Endoscopic dilation of benign esophageal strictures in a surgical unit: a report on 95 cases. Surg Laparosc Endosc Percutan Tech. 2007;17:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Pereira-Lima JC, Ramires RP, Zamin I, Cassal AP, Marroni CA, Mattos AA. Endoscopic dilation of benign esophageal strictures: report on 1043 procedures. Am J Gastroenterol. 1999;94:1497-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 136] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 78. | Dear KL, Hunter JO. Colonoscopic hydrostatic balloon dilatation of Crohn's strictures. J Clin Gastroenterol. 2001;33:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Thomas-Gibson S, Brooker JC, Hayward CM, Shah SG, Williams CB, Saunders BP. Colonoscopic balloon dilation of Crohn's strictures: a review of long-term outcomes. Eur J Gastroenterol Hepatol. 2003;15:485-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 80. | Sabaté JM, Villarejo J, Bouhnik Y, Allez M, Gornet JM, Vahedi K, Modigliani R, Lémann M. Hydrostatic balloon dilatation of Crohn's strictures. Aliment Pharmacol Ther. 2003;18:409-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 81. | Suchan KL, Muldner A, Manegold BC. Endoscopic treatment of postoperative colorectal anastomotic strictures. Surg Endosc. 2003;17:1110-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Nomura E, Takagi S, Kikuchi T, Negoro K, Takahashi S, Kinouchi Y, Hiwatashi N, Shimosegawa T. Efficacy and safety of endoscopic balloon dilation for Crohn's strictures. Dis Colon Rectum. 2006;49:S59-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 83. | Ajlouni Y, Iser JH, Gibson PR. Endoscopic balloon dilatation of intestinal strictures in Crohn's disease: safe alternative to surgery. J Gastroenterol Hepatol. 2007;22:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 84. | Araujo SE, Costa AF. Efficacy and safety of endoscopic balloon dilation of benign anastomotic strictures after oncologic anterior rectal resection: report on 24 cases. Surg Laparosc Endosc Percutan Tech. 2008;18:565-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Neale JC, Goulden JW, Allan SG, Dixon PD, Isaacs RJ. Esophageal stents in malignant dysphagia: a two-edged sword? J Palliat Care. 2004;20:28-31. [PubMed] |
| 86. | Wang MQ, Sze DY, Wang ZP, Wang ZQ, Gao YA, Dake MD. Delayed complications after esophageal stent placement for treatment of malignant esophageal obstructions and esophagorespiratory fistulas. J Vasc Interv Radiol. 2001;12:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 87. | Dormann A, Meisner S, Verin N, Wenk Lang A. Self-expanding metal stents for gastroduodenal malignancies: systematic review of their clinical effectiveness. Endoscopy. 2004;36:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 290] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 88. | Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 421] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 89. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 413] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 90. | Anderloni A, Di Leo M, Barzaghi F, Semeraro R, Meucci G, Marino R, Amato L, Frigerio M, Saladino V, Toldi A, Manfredi G, Redaelli A, Feliziani M, De Roberto G, Boni F, Scacchi G, Mosca D, Devani M, Arena M, Massidda M, Zanoni P, Ciscato C, Casini V, Beretta P, Forti E, Salerno R, Caramia V, Bianchetti M, Tomba C, Evangelista A, Repici A, Soncini M, Maconi G, Manes G, Gullotta R. Complications and early mortality in percutaneous endoscopic gastrostomy placement in lombardy: A multicenter prospective cohort study. Dig Liver Dis. 2019;51:1380-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Richter JA, Patrie JT, Richter RP, Henry ZH, Pop GH, Regan KA, Peura DA, Sawyer RG, Northup PG, Wang AY. Bleeding after percutaneous endoscopic gastrostomy is linked to serotonin reuptake inhibitors, not aspirin or clopidogrel. Gastrointest Endosc. 2011;74:22-34.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 92. | Vanbiervliet G, Giudicelli-Bornard S, Piche T, Berthier F, Gelsi E, Filippi J, Anty R, Arab K, Huet PM, Hebuterne X, Tran A. Predictive factors of bleeding related to post-banding ulcer following endoscopic variceal ligation in cirrhotic patients: a case-control study. Aliment Pharmacol Ther. 2010;32:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 93. | Freeman ML, Nelson DB, Sherman S, Haber GB, Herman ME, Dorsher PJ, Moore JP, Fennerty MB, Ryan ME, Shaw MJ, Lande JD, Pheley AM. Complications of endoscopic biliary sphincterotomy. N Engl J Med. 1996;335:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1716] [Cited by in RCA: 1703] [Article Influence: 56.8] [Reference Citation Analysis (2)] |
| 94. | Cui PJ, Yao J, Zhao YJ, Han HZ, Yang J. Biliary stenting with or without sphincterotomy for malignant biliary obstruction: a meta-analysis. World J Gastroenterol. 2014;20:14033-14039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 95. | Verma D, Kapadia A, Adler DG. Pure versus mixed electrosurgical current for endoscopic biliary sphincterotomy: a meta-analysis of adverse outcomes. Gastrointest Endosc. 2007;66:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 96. | Zhao HC, He L, Zhou DC, Geng XP, Pan FM. Meta-analysis comparison of endoscopic papillary balloon dilatation and endoscopic sphincteropapillotomy. World J Gastroenterol. 2013;19:3883-3891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 97. | Lee MG, Kim J, Lee SH, Lee BS, Lee SJ, Lee YS, Cha BH, Hwang JH, Ryu JK, Kim YT. Effect of sustained use of platelet aggregation inhibitors on post-endoscopic sphincterotomy bleeding. Dig Endosc. 2014;26:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Onal IK, Parlak E, Akdogan M, Yesil Y, Kuran SO, Kurt M, Disibeyaz S, Ozturk E, Odemis B. Do aspirin and nonsteroidal anti-inflammatory drugs increase the risk of post-sphincterotomy hemorrhage--a case-control study. Clin Res Hepatol Gastroenterol. 2013;37:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 99. | Patai Á, Solymosi N, Patai AV. Does rectal indomethacin given for prevention of post-ERCP pancreatitis increase bleeding after biliary endoscopic sphincterotomy or cardiovascular mortality?: Post hoc analysis using prospective clinical trial data. Medicine (Baltimore). 2014;93:e159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 100. | Hussain N, Alsulaiman R, Burtin P, Toubouti Y, Rahme E, Boivin JF, Barkun AN. The safety of endoscopic sphincterotomy in patients receiving antiplatelet agents: a case-control study. Aliment Pharmacol Ther. 2007;25:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 101. | Hui CK, Lai KC, Yuen MF, Wong WM, Lam SK, Lai CL. Does withholding aspirin for one week reduce the risk of post-sphincterotomy bleeding? Aliment Pharmacol Ther. 2002;16:929-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Wang KX, Ben QW, Jin ZD, Du YQ, Zou DW, Liao Z, Li ZS. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 302] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 103. | Kien-Fong Vu C, Chang F, Doig L, Meenan J. A prospective control study of the safety and cellular yield of EUS-guided FNA or Trucut biopsy in patients taking aspirin, nonsteroidal anti-inflammatory drugs, or prophylactic low molecular weight heparin. Gastrointest Endosc. 2006;63:808-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Familiari P, Gigante G, Marchese M, Boskoski I, Tringali A, Perri V, Costamagna G. Peroral Endoscopic Myotomy for Esophageal Achalasia: Outcomes of the First 100 Patients With Short-term Follow-up. Ann Surg. 2016;263:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 105. | Akintoye E, Kumar N, Obaitan I, Alayo QA, Thompson CC. Peroral endoscopic myotomy: a meta-analysis. Endoscopy. 2016;48:1059-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 106. | Sung JJ, Lau JY, Ching JY, Wu JC, Lee YT, Chiu PW, Leung VK, Wong VW, Chan FK. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med. 2010;152:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 283] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 107. | Derogar M, Sandblom G, Lundell L, Orsini N, Bottai M, Lu Y, Sadr-Azodi O. Discontinuation of low-dose aspirin therapy after peptic ulcer bleeding increases risk of death and acute cardiovascular events. Clin Gastroenterol Hepatol. 2013;11:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 108. | Guerrouij M, Uppal CS, Alklabi A, Douketis JD. The clinical impact of bleeding during oral anticoagulant therapy: assessment of morbidity, mortality and post-bleed anticoagulant management. J Thromb Thrombolysis. 2011;31:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 109. | Holbrook A, Schulman S, Witt DM, Vandvik PO, Fish J, Kovacs MJ, Svensson PJ, Veenstra DL, Crowther M, Guyatt GH. Evidence-based management of anticoagulant therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e152S-e184S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 921] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 110. | Tran HA, Chunilal SD, Harper PL, Tran H, Wood EM, Gallus AS; Australasian Society of Thrombosis and Hemostasis (ASTH). An update of consensus guidelines for warfarin reversal. Med J Aust. 2013;198:198-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 111. | Patriquin C, Crowther M. Treatment of warfarin-associated coagulopathy with vitamin K. Expert Rev Hematol. 2011;4:657-65; quiz 666-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 112. | Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal haemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut. 1994;35:464-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 113. | Wolf AT, Wasan SK, Saltzman JR. Impact of anticoagulation on rebleeding following endoscopic therapy for nonvariceal upper gastrointestinal hemorrhage. Am J Gastroenterol. 2007;102:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Rubin TA, Murdoch M, Nelson DB. Acute GI bleeding in the setting of supratherapeutic international normalized ratio in patients taking warfarin: endoscopic diagnosis, clinical management, and outcomes. Gastrointest Endosc. 2003;58:369-373. [PubMed] |
| 115. | Shingina A, Barkun AN, Razzaghi A, Martel M, Bardou M, Gralnek I; RUGBE Investigators. Systematic review: the presenting international normalised ratio (INR) as a predictor of outcome in patients with upper nonvariceal gastrointestinal bleeding. Aliment Pharmacol Ther. 2011;33:1010-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 116. | Irwin J, Ferguson R, Weilert F, Smith A. Supratherapeutic anticoagulation at presentation is associated with reduced mortality in nonvariceal upper gastrointestinal hemorrhage. Endosc Int Open. 2014;2:E148-E152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 117. | Kuwada SK, Balm R, Gostout CJ. The risk of withdrawing chronic anticoagulation because of acute GI bleeding. Am J Gastroenterol. 1996;91:1116-1119. [PubMed] |
| 118. | Ananthasubramaniam K, Beattie JN, Rosman HS, Jayam V, Borzak S. How safely and for how long can warfarin therapy be withheld in prosthetic heart valve patients hospitalized with a major hemorrhage? Chest. 2001;119:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 119. | Patriquin C, Crowther M. Treatment of warfarin-associated coagulopathy with vitamin K. Expert Rev Hematol. 2011;4:657-65; quiz 666-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 120. | Patel RJ, Witt DM, Saseen JJ, Tillman DJ, Wilkinson DS. Randomized, placebo-controlled trial of oral phytonadione for excessive anticoagulation. Pharmacotherapy. 2000;20:1159-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 121. | Crowther MA, Julian J, McCarty D, Douketis J, Kovacs M, Biagoni L, Schnurr T, McGinnis J, Gent M, Hirsh J, Ginsberg J. Treatment of warfarin-associated coagulopathy with oral vitamin K: a randomised controlled trial. Lancet. 2000;356:1551-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 122. | Crowther MA, Douketis JD, Schnurr T, Steidl L, Mera V, Ultori C, Venco A, Ageno W. Oral vitamin K lowers the international normalized ratio more rapidly than subcutaneous vitamin K in the treatment of warfarin-associated coagulopathy. A randomized, controlled trial. Ann Intern Med. 2002;137:251-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 123. | Lubetsky A, Yonath H, Olchovsky D, Loebstein R, Halkin H, Ezra D. Comparison of oral vs intravenous phytonadione (vitamin K1) in patients with excessive anticoagulation: a prospective randomized controlled study. Arch Intern Med. 2003;163:2469-2473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 143] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 124. | Karaca MA, Erbil B, Ozmen MM. Use and effectiveness of prothrombin complex concentrates vs fresh frozen plasma in gastrointestinal hemorrhage due to warfarin usage in the ED. Am J Emerg Med. 2014;32:660-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 125. | Dentali F, Marchesi C, Giorgi Pierfranceschi M, Crowther M, Garcia D, Hylek E, Witt DM, Clark NP, Squizzato A, Imberti D, Ageno W. Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta-analysis. Thromb Haemost. 2011;106:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 126. | Hickey M, Gatien M, Taljaard M, Aujnarain A, Giulivi A, Perry JJ. Outcomes of urgent warfarin reversal with frozen plasma versus prothrombin complex concentrate in the emergency department. Circulation. 2013;128:360-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 127. | Zakko L, Rustagi T, Douglas M, Laine L. No Benefit From Platelet Transfusion for Gastrointestinal Bleeding in Patients Taking Antiplatelet Agents. Clin Gastroenterol Hepatol. 2017;15:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hu B, Huang LY, Sporea I S-Editor: Zhang H L-Editor: A E-Editor: Liu MY