Published online Feb 16, 2020. doi: 10.4253/wjge.v12.i2.72
Peer-review started: June 23, 2019
First decision: August 2, 2019
Revised: November 25, 2019
Accepted: December 14, 2019
Article in press: December 14, 2019
Published online: February 16, 2020
Processing time: 204 Days and 13.2 Hours
In nonvariceal upper gastrointestinal bleeding (NVUGIB), the optimal volume of adrenaline, the optimal number of hemoclips, and the application of thermal coagulation in determining patient outcomes have not been well studied.
To demonstrate a dose-response relationship between the commonly used endoscopic modalities for the treatment of non-variceal upper gastrointestinal bleeding and various clinical outcomes.
Patients presenting with NVUGIB were retrospectively identified and analyzed. These patients were stratified as follows: (1) > 10 mL of adrenaline injected vs ≤ 10 mL; (2) > 1 hemoclip placed vs ≤ 1 hemoclip; (3) Heater probe used or not; and (4) > 2 treatment modalities used vs ≤ 2. The primary outcomes were rebleeding and the need for repeat endoscopy. The secondary outcomes were the need for surgery, required transfusions, length of hospital stay, death during the same admission period and 30 d mortality. Patients with NVUGIB who required endoscopic therapy were included. Those who did not require endoscopic therapy or were initially treated with surgery or embolization were excluded.
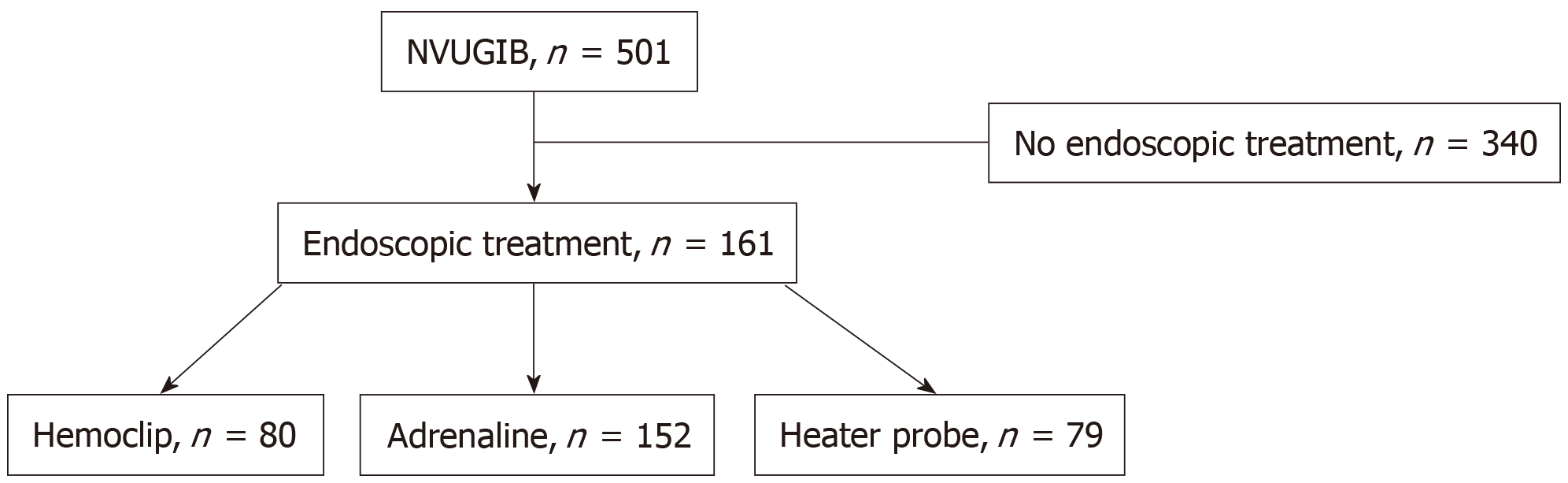
In all, 501 patients with NVUGIB were treated. One hundred sixty-one (32.1%) patients needed endoscopic therapy. The injection of < 10 mL of adrenaline was associated with less rebleeding (P < 0.0001), the need for repeat endoscopy (P = 0.001) and a decreased length of hospital stay (P = 0.026). The use of > 2 treatment modalities were associated with increased rebleeding (P = 0.009) and the need for repeat endoscopy (P = 0.048). The placement of > 1 hemoclip was associated with a decreased length of hospital stay (P = 0.044). The rates of surgery and death were low, and there were no other significant differences between the patient groups.
The more restrictive use of adrenaline and number of endoscopic modalities to treat NVUGIB with the more liberal use of hemoclips was associated with better patient outcomes.
Core tip: This is the first study to our knowledge attempting to demonstrate a dose-response relationship between the commonly used endoscopic modalities for the treatment of non-variceal upper gastrointestinal bleeding and various clinical outcomes. Ours is also the first study to show that a greater number of hemoclips deployed led to a better outcome. It is a real-world study and results were generated from patient care in daily clinical practice.
- Citation: Yip BCH, Sayeed Sajjad H, Wang JX, Anastassiades CP. Endoscopic treatment modalities and outcomes in nonvariceal upper gastrointestinal bleeding. World J Gastrointest Endosc 2020; 12(2): 72-82
- URL: https://www.wjgnet.com/1948-5190/full/v12/i2/72.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i2.72
Nonvariceal bleeding of the upper gastrointestinal tract (NVUGIB) is a common condition that results in significant morbidity and mortality. Mortality rates have not improved over the years despite advances in endoscopic treatment and other aspects of patient care and ranges from 5.6% to 14.3%[1]. Moreover, rebleeding rates are as high as 13%, based on a recent Danish cohort study of 13498 patients[2]. Endoscopic modalities for the treatment of NVUGIB include hemoclip placement, dilute adrenaline injection, thermal coagulation (heat probe, gold probe, bipolar coagulation probe), coagulation grasper forceps, argon plasma coagulation, hemostatic nanopowder spray and over-the-scope clip placement.
It is now common practice for adrenaline injection into the bleeding point to be combined with another modality of endoscopic treatment, such as thermal coagulation or hemoclip placement, to increase the rate of hemostasis and decrease the rate of rebleeding[3-5]. The optimal volume of adrenaline injection to treat these bleeding lesions is not known, although three randomized trials have shown that larger volumes of adrenaline monotherapy (> 13 mL) can reduce the rate of recurrent bleeding[6-8]. However, too large of a volume of adrenaline (> 40 mL) can cause complications, such as ulcer perforation, epigastric pain, and significant elevations in blood pressure[8]. The optimal number of hemoclips to use in the treatment of NVUGIB has not been determined. Furthermore, the utility of using a combination of three or more endoscopic treatment modalities is not known.
Our study aims to investigate the relationship between clinical outcomes in NVUGIB and (1) The volume of adrenaline injected; (2) The number of hemoclips placed; and (3) Combination therapy with more than 2 endoscopic treatment modalities.
This was a retrospective cohort study conducted at our center, which is a 590-bed general hospital in Singapore. Between January 2014 and December 2015, consecutive patients presenting with NVUGIB were identified from our hospital endoscopy reporting module. All patients with NVUGIB who underwent endoscopic treatment were included in the study. Patients with variceal bleeding or patients with NVUGIB who were treated directly with angioembolization or surgery were excluded. This study was approved by our domain-specific research board and local ethics committee. Permission for a patient consent waiver was granted.
Patients with NVUGIB who needed endoscopic hemostasis were further analyzed. Clinical data were retrieved using our hospital electronic medical records. The main endoscopic modalities used included dilute adrenaline injection, hemoclip placement, heater probe application, or a combination of these methods. Dilute adrenaline was prepared with a mixture consisting of 9 mL of 0.9% sodium chloride solution and 1 mL of 1:1000 adrenaline. The types of hemoclip used were the QuickClip2 (Olympus Medical Systems Corp, Japan) and the Resolution clip (Boston Scientific, Natick, MA, United States). The heater probe used was the HeatProbe (Olympus Medical Systems Corp, Japan). Hemostatic powder application (Hemospray, Wilson-Cook, Winston Salem, NC, United States) was used in a few patients. Angioembolization by interventional radiology was used in some patients as a subsequent therapy. The endoscopic procedures were performed by endoscopists with a wide range of expertise, ranging from trainees to senior specialists. All trainees were supervised by accredited endoscopists.
The primary outcomes were the rebleeding rate and need for repeat endoscopy. Rebleeding was defined as one or more signs of ongoing bleeding, including hematemesis, melena, hematochezia, vital sign instability and a significant drop in hemoglobin after initial hemostasis and stabilization of the patient. Repeat endoscopy was defined as upper endoscopy performed within the same hospital admission period. The secondary outcomes included surgical intervention, required transfusions, length of hospital stay (LOS), death during the same admission period and 30 d mortality.
Data were collected for baseline characteristics, such as age, sex, race, presence of comorbid conditions, use of blood thinning agents, bleeding disorders, indication for gastroscopy and whether the patient was admitted to the hospital primarily for NVUGIB or another reason. The endoscopic diagnosis, Forrest classification and number of bleeding lesions were recorded. In terms of the endoscopic treatment modalities, data were collected regarding the volume of adrenaline injected, the number and type of hemoclips used, the heater probe used and all other endoscopic hemostatic treatments employed. For patient outcomes, data regarding rebleeding, repeat endoscopy, required transfusions number of units of packed red blood cells (PRBCs), LOS, angioembolization, surgery, death in the same admission period, and 30 d mortality were captured.
Statistical analysis was carried out using SPSS Statistics, version 22.0 (SPSS, Inc., Chicago, IL, United States). Continuous variables that were approximately normally distributed are expressed as the mean and standard deviation, and those that were skewed are summarized as the median and interquartile range. Categorical variables are summarized by count and frequency.
For binary outcomes (rebleeding, repeat endoscopy and death during the same admission period), the two-sample t-test or Mann-Whitney U test was used to compare continuous variables between these two groups of patients. The chi-squared test or Fisher’s exact test was used to test the association between categorical variables and the outcomes. Logistic regression models were performed for binary outcomes to identify variables significantly associated with the outcomes. Likewise, for continuous outcomes (LOS and number of units of PRBCs transfused), Poisson regression and negative binomial regression models were used to identify these variables. The degree of association was reflected by the odds ratio and 95% confidence interval. A two-tailed P value of less than 0.05 was considered statistically significant.
From January 2014 to December 2015, 501 patients were treated for NVUGIB at our hospital endoscopy center. Of these patients, 161 (32.1%) received endoscopic therapy (Figure 1). The baseline patient characteristics are shown in Table 1. Of the 161 patients, the mean age was 64.0, and there were more males (69.6%) than females (30.4%). The majority of patients (76.5%) were Chinese, 14.3% were Malay, 4.3% were Indian, and 5% were of other races. These figures are reflective of the current demographics of Singapore[9]. More than two-thirds of the patients (68.9%) had an ASA score of 3, 16.8% had an ASA score of 1, and 14.3% had an ASA score of 2. None had ASA scores of > 3. Close to 30% of the patients were taking blood thinning agents, of which aspirin, clopidogrel and warfarin were the most common. A small number (5%) had underlying bleeding dyscrasia, of which thrombocytopenia and coagulopathy were the most common. The majority of patients (73.3%) had upper gastrointestinal bleeding as the admitting diagnosis, while the rest (26.7%) developed bleeding in the hospital after admission for other reasons. Of the study patients who underwent endoscopy, ninety-eight patients had duodenal ulcers, sixty had gastric ulcers, and fourteen had other bleeding lesions, including arteriovenous malformations, Dieulafoy lesions, Mallory Weiss tears and tumor bleeds. Most of the patients had a single bleeding lesion (88.2%). Of a total of 172 lesions, fourteen were Forrest 1a, fifty-four were Forrest 1b, thirty-nine were Forrest 2a, twenty-one were Forrest 2b, sixteen were Forrest 2c, seventeen were Forrest 3, and eleven were nonpeptic ulcer bleeding lesions. The male sex was significantly associated with rebleeding and repeat endoscopy, as well as higher blood transfusion requirements. Having bleeding dyscrasia was significantly associated with an increased need for transfusion.
| Patient characteristic | Number (n = 161) |
| Mean age (yr) ± SD | 64.0 ± 14.7 |
| Gender | |
| Male | 112 (69.6) |
| Female | 49 (30.4) |
| Race | |
| Chinese | 123 (76.4) |
| Malay | 23 (14.3) |
| Indian | 7 (4.3) |
| Others | 8 (5.0) |
| ASA score | |
| 1 | 27 (16.8) |
| 2 | 23 (14.3) |
| 3 | 111 (68.9) |
| 4 | 0 |
| 5 | 0 |
| Blood thinning agent | |
| Taking | 48 (29.8) |
| Not taking | 113 (70.2) |
| Bleeding dyscrasia | |
| Present | 8 (5.0) |
| Absent | 152 (94.4) |
| Bleeding point | |
| Single | 142 (88.2) |
| > 1 | 19 (11.8) |
| Admitted for bleeding initially | |
| Yes | 118 (73.3) |
| No | 43 (26.7) |
The baseline treatment characteristics are shown in Table 2. Hemoclips were used in almost half (46%) of the patients. Of the patients who were treated with hemoclips, 50% received one hemoclip, 64.9% received two hemoclips, and 5.4% received more than two hemoclips. Dilute adrenaline injection was used in the majority (94.4%) of patients, with close to two-thirds of patients (65.8%) receiving ≤ 10 mL and the rest (34.2%) receiving > 10 mL of dilute adrenaline. A heater probe was applied in 49.1% of the patients. Most patients (81.5%) received at least two different types of endoscopic treatment.
| Treatment characteristic | Number (n = 161) |
| Number of hemoclips used | |
| 0 | 87 (54.0) |
| 1 | 22 (13.7) |
| 2 | 48 (29.8) |
| 3 | 2 (1.2) |
| 4 | 1 (0.6) |
| Volume of adrenaline used (mL) | |
| 0 | 9 (5.6) |
| ≤ 10 | 100 (62.1) |
| > 10 | 52 (32.3) |
| Heater probe use | |
| Yes | 79 (49.1) |
| No | 82 (50.1) |
| Number of treatment modalities | |
| ≤ 2 | 127 (78.9) |
| > 2 | 34 (21.1) |
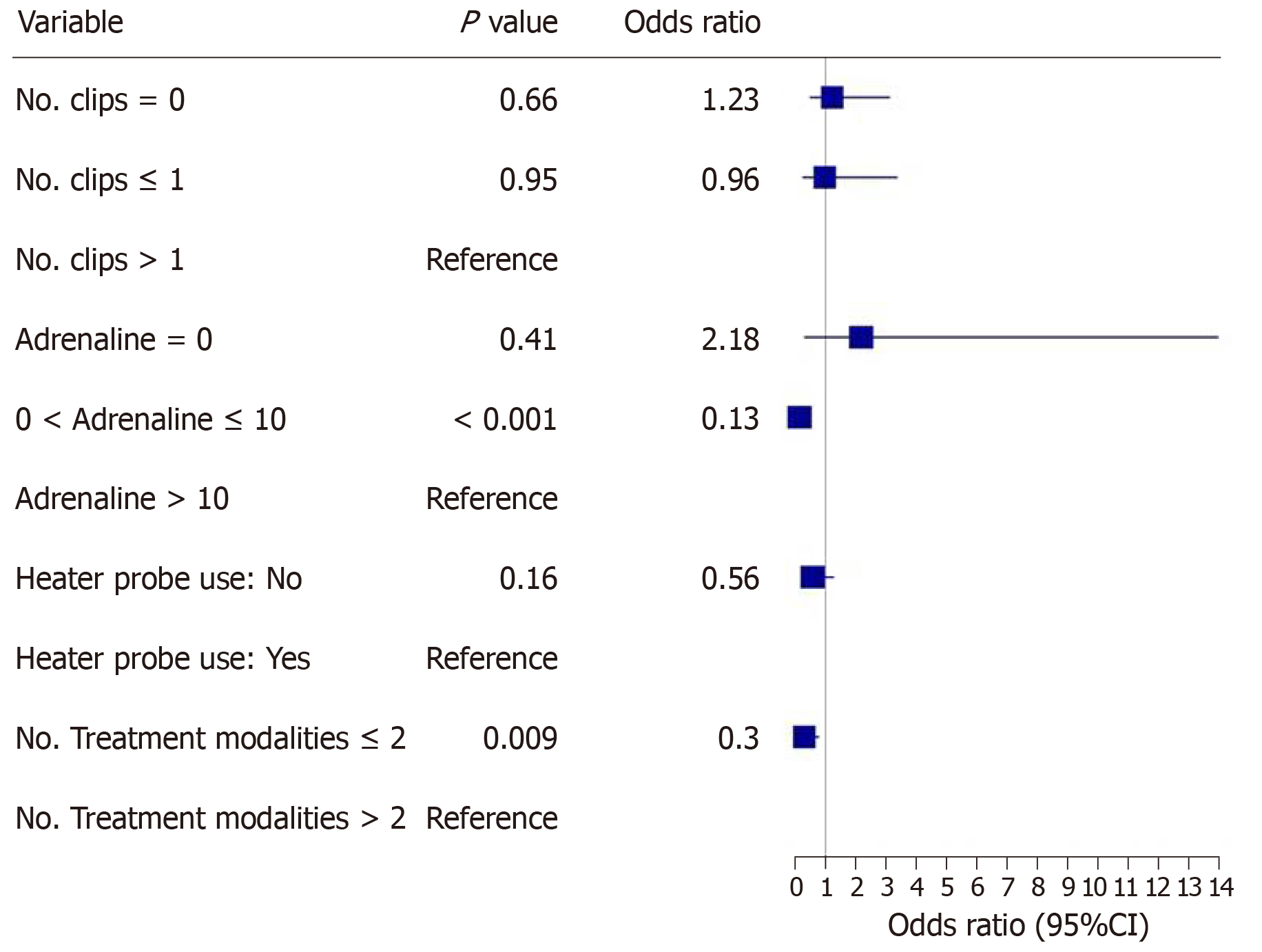
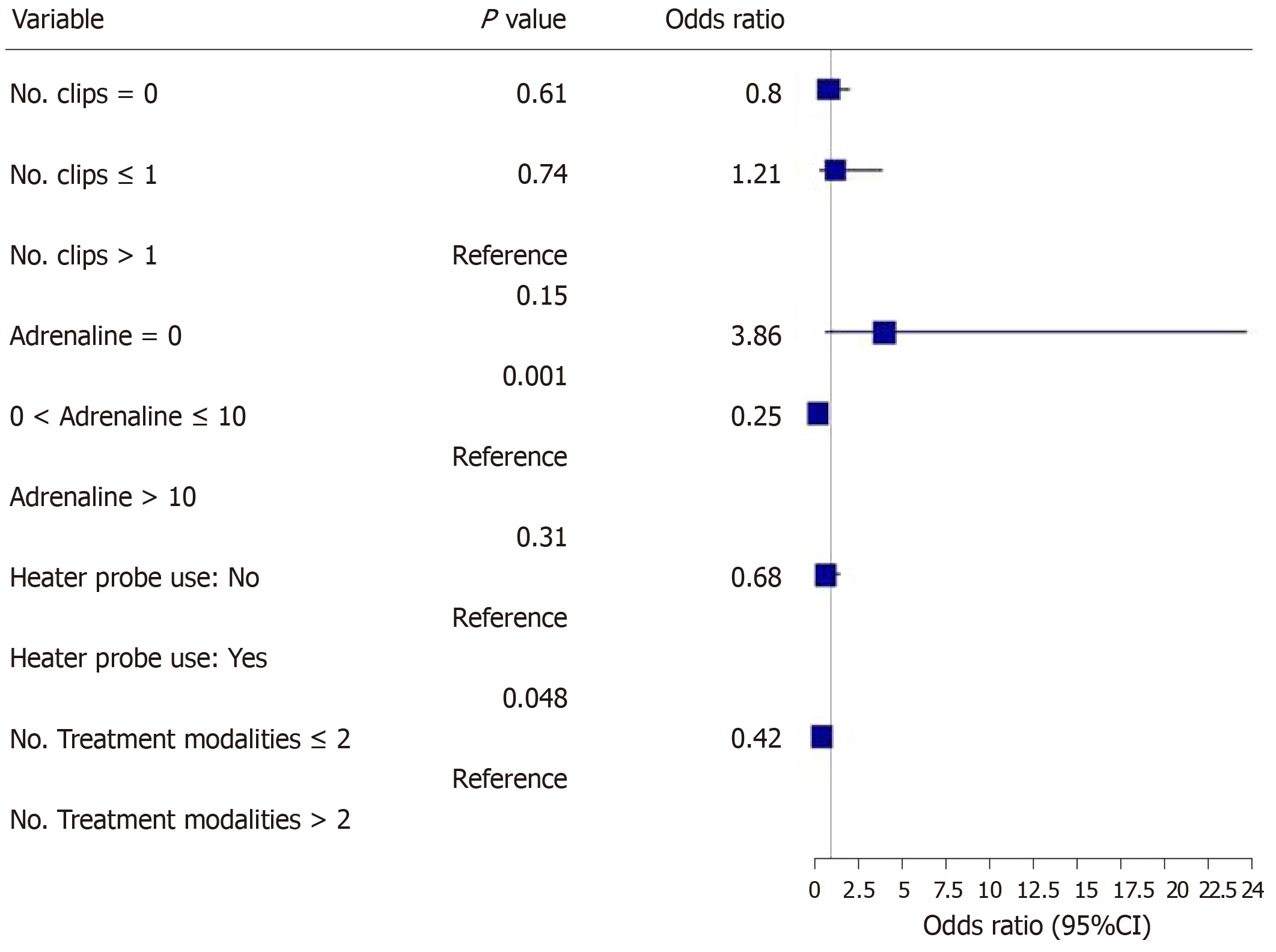
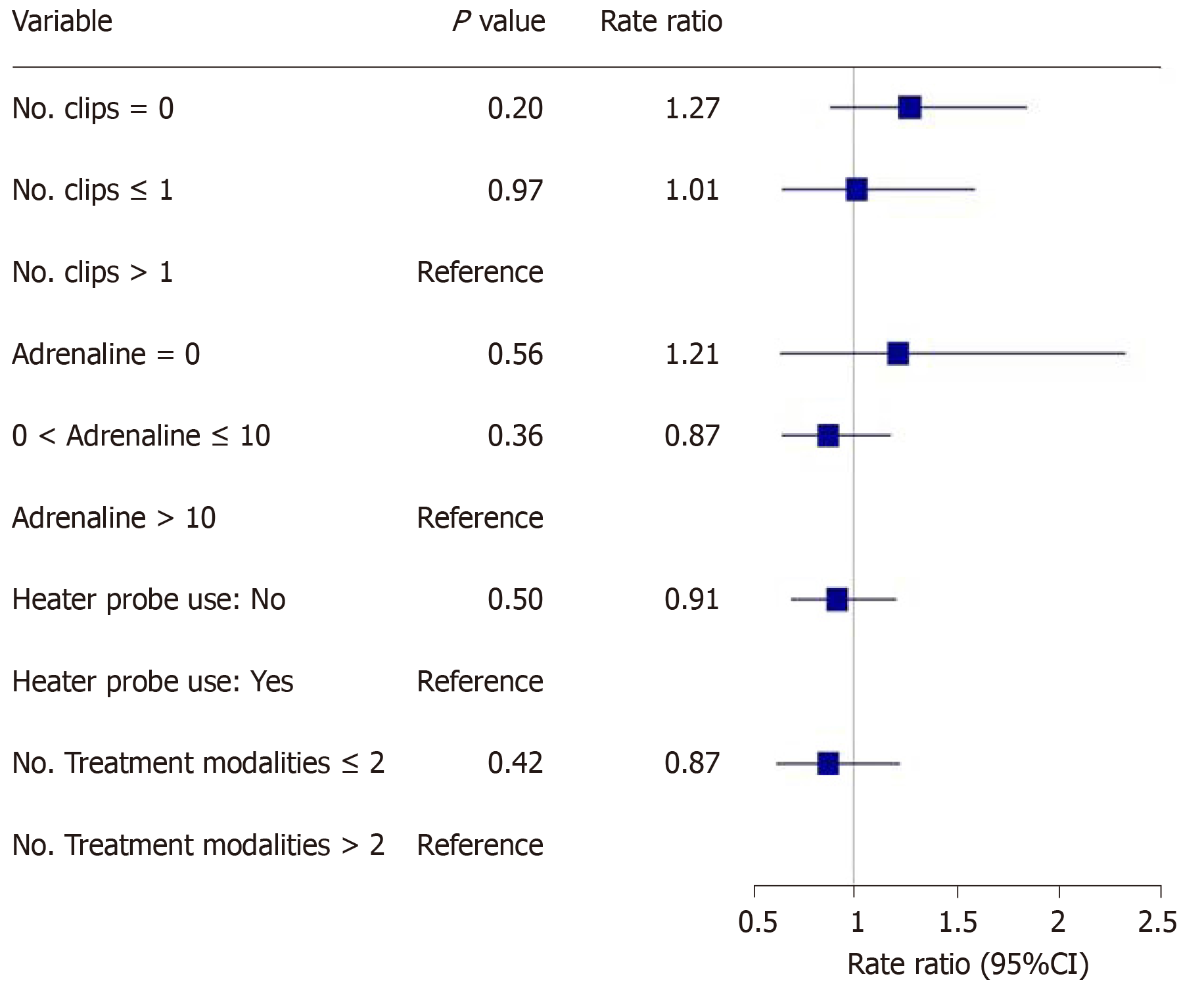
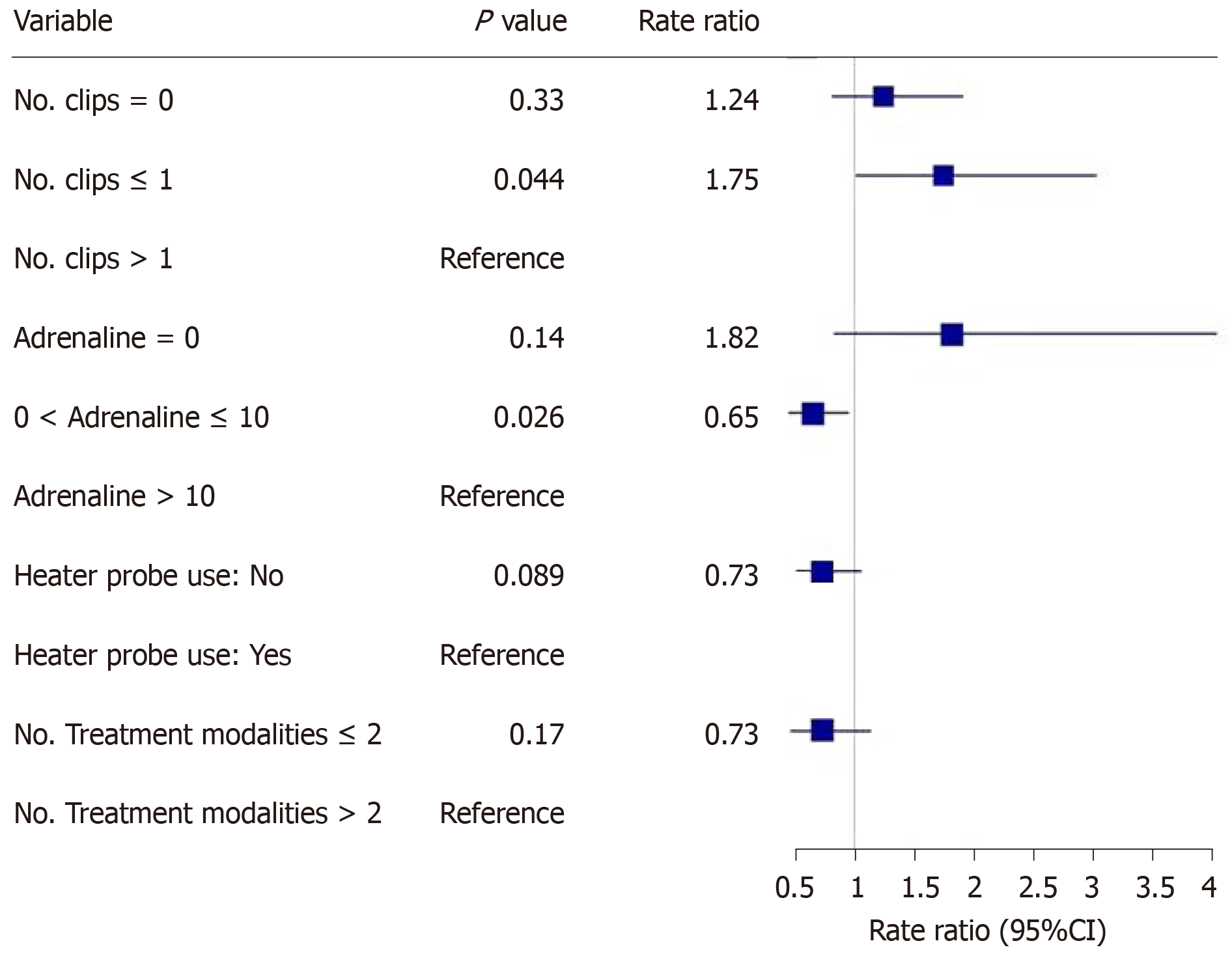
The main outcomes are shown in Figures 2-5. An injected volume of adrenaline of less than 10 mL was associated with significantly less rebleeding (P < 0.0001), a lower frequency of repeat endoscopy (P = 0.001), and a decreased LOS (P = 0.026). A combination treatment of more than two modalities was associated with significantly more rebleeding (P = 0.009) and an increased need for repeat endoscopy (P = 0.048). The placement of more than one hemoclip was associated with a significant decrease in the LOS (P = 0.044). No significant association was shown with the use of the heater probe. Twenty-six percent of the patients experienced rebleeding, and 29% underwent repeat endoscopy. One patient required surgery for a Forrest 1a duodenal ulcer immediately after endoscopy. The patients received a median of two units of PRBCs. The median LOS was six days. Ten patients (6.2%) died during the same admission period. This number was too small for any meaningful statistical analysis. Four patients died from pneumonia, two from myocardial infarction, two from NVUGIB, one from sepsis and one from intracranial hemorrhage. There were no cases of 30 d mortality in our cohort (Table 3).
| Outcomes | Number (n = 161) |
| Rebleeding | |
| Yes | 42 (26.1) |
| No | 119 (73.9) |
| Need for repeat endoscopy | |
| Yes | 47 (29.2) |
| No | 114 (70.8) |
| Need for surgery | |
| Yes | 1 (0.6) |
| No | 160 (99.4) |
| Transfusion requirement | |
| -Number of units of PRBC’s (Median/IQR) | 2/0-3 |
| Length of hospital stay | |
| -Number of days (Median/IQR) | 6/4-13 |
| Death during same admission | |
| Yes | 10 (6.2) |
| No | 151 (93.8) |
| 30-d mortality | |
| Yes | 0 |
| No | 151 (93.8) |
Our study revealed that the more restrictive use of adrenaline (< 10 mL) was associated with significantly less rebleeding, a reduced need for repeat endoscopy and a decreased LOS. Similarly, the more conservative use of endoscopic modalities was associated with significantly less rebleeding and a reduced need for repeat endoscopy. The use of more hemoclips was associated with a decreased LOS.
Less than one-third of our cohort of NVUGIB patients underwent endoscopic therapy. A study by Matthewson et al[10] in the 1980s revealed that for patients presenting with bleeding due to peptic ulcer disease, stigmata of recent hemorrhage was present in 79%. Although this group might comprise some patients with Forrest IIc lesions that would not require treatment (not specified in the paper), the number that would require endoscopic therapy is still likely to be much higher than that in our cohort. An important reason for this could be improved care for NVUGIB over the years. One notable example would be the introduction of proton pump inhibitor use as an adjunct therapy for upper GI bleeding, which can downgrade the bleeding stigma and thus reduce the need for endoscopic therapy. This may not have been the case before 2007[11].
In our cohort, the rebleeding rate was 26%. This was significantly higher than the rate reported in a recent systematic review studying the timing of rebleeding in patients at a high risk for this problem after successful hemostasis for peptic ulcer disease[12]. The rebleeding rate in this study ranged from 11.5% to 14.4%. This may reflect differences in the patient cohorts. In this systematic review, the demographics were different, with a mix of Asian and Caucasian patients, while our study population was predominantly Asian. Furthermore, patients in 50% of the studies included in the systematic review had lower rates of comorbid diseases compared to the patients in our study. Finally, the different rebleeding rate may reflect differences in the skill level of the endoscopist. Our study reflects real-life endoscopic practice. The endoscopists who performed the procedures in patients included in the study had a wide range of experience. This may be relevant, as the endoscopic identification of lesions that are amenable to clipping and proficiency in hemoclip application are key determinants of successful outcomes[13].
In our study, it was found that a lower volume of injected adrenaline (< 10 mL) was associated with better clinical outcomes, i.e., a lower rebleeding rate and a lower frequency of repeat endoscopy. At least three randomized controlled trials had previously shown that larger volumes of adrenaline result in better outcomes, mainly in terms of reduced recurrent bleeding rates[6-8]. However, these are older studies, and all of the patients were treated with adrenaline monotherapy. In contrast, almost all of the patients in our study who received an adrenaline injection also received another form of endoscopic therapy. This is consistent with the relatively new standards of endoscopic practice and in line with the newer guidelines for the management of NVUGIB[3-4]. It is probable that the additional use of another endoscopic modality in combination with adrenaline, such as endoscopic clip placement, contributes to successful hemostasis and thus reduces the volume of adrenaline that needs to be injected. However, due to the retrospective nature of this study, other unknown variables may be present. For example, the patients who achieved better outcomes may have had smaller or few bleeding vessels for each bleeding lesion. Hence, the minimum volume of adrenaline should be used to achieve initial hemostasis. Thereafter, a second endoscopic treatment modality should be used.
Another finding in our study was that the use of more than 2 endoscopic modalities was associated with poorer outcomes (i.e., higher rebleeding and repeat endoscopy rates) than the use of ≤ 2 endoscopic modalities. This appears counterintuitive, especially since current guidelines for the management of NVUGIB[3,4] advocate combination therapy for endoscopic hemostasis. However, the poor outcomes associated with the use of 3 or more endoscopic modalities may be related to variables not captured in this retrospective study. For example, torrential or difficult-to-control bleeding may have prompted endoscopists in desperation to try more treatment modalities that ultimately did not prevent rebleeding or repeat endoscopy. There are currently no studies available that have shown that the use of 3 or more endoscopic modalities leads to poorer clinical outcomes compared with ≤ 2 modalities. This may be further evaluated in prospective trials.
In our study, we found that the use of more than one hemoclip was associated with a significant decrease in the LOS. Several studies have shown that hemoclip placement may be superior to other forms of endoscopic therapy or noninferior to combination endoscopic therapy. In a study by Hepworth et al[14], mechanical methods, such as hemoclip placement and thermal therapy, were more effective for achieving hemostasis in bleeding mesenteric vessels in dogs than injection sclerotherapy. In a study by Ljubicic et al[15], hemoclips were found to be superior to both small and large volumes of injected adrenaline in the prevention of recurrent bleeding in patients with peptic ulcers. A randomized trial conducted by Saltzman et al[16] in 47 patients with NVUGIB found that hemoclip placement was not inferior to combination treatment with adrenaline injection and bipolar electrocautery in terms of efficiency, efficacy or complications. Randomized controlled trials and meta-analyses have shown comparable efficacy between clipping and conventional contact thermal therapy for definitive hemostasis in NVUGIB[13]. Furthermore, clipping is considered safer than thermal coagulation techniques owing to the lower risk of perforation. While it is difficult to make a causative association in this retrospective study, our findings suggest that the more liberal use of mechanical hemostasis with endoscopic clips may have been beneficial in our patient population. Hence, any number of hemoclips should be used to definitively control the bleeding.
With regard to the other outcomes, the median number of two units of PRBCs transfused per patient was lower than in a Danish cohort of 5107 patients, which reported a median PRBC requirement of four units per patient[17]. The mean age in the Danish cohort was greater, at 74 years, than the median age in our cohort, which was 64 years. Older patients are generally frailer, and their comorbidities may necessitate a more liberal transfusion strategy.
Our cohort had a median LOS of 6 d. This was longer than the LOS in several other cohorts of patients. An American cohort of 1929 patients with upper bleeding gastrointestinal tract (variceal and nonvariceal) had a median LOS of 4 d[18]. A second American cohort of more than 19000 patients with NVUGIB had a mean LOS of 3 to 4 d[19]. An Australian cohort of 507 patients with peptic ulcer bleeding had a median LOS of 4 to 5 d[20]. This may be explained by differences among the patient cohorts. There was no mention of associated comorbid medical conditions in the two American cohorts, and there were fewer comorbidities overall in the Australian cohort than in our cohort, which may have led to a decreased LOS. Furthermore, the LOS may reflect differences in healthcare settings or even cultural differences and expectations. In Singapore, healthcare is still largely hospital-based, and there may be infrastructure limitations that may delay the transition to outpatient care compared to Western healthcare systems. The mortality rate in our cohort was 6.2%. This figure is within the reported mortality range (3% to 14%) for patients with NVUGIB in studies conducted from 2000 to 2010[1].
Our study has several strengths. First, this is the first study to demonstrate a dose-response relationship between the commonly used endoscopic modalities for the treatment of NVUGIB and various clinical outcomes. Ours is also the first study to show that the use of a greater number of hemoclips led to a better outcome. Second, this is a real-world study, and the results were generated from patient care in daily clinical practice. The weaknesses of the study are as follows. First, our study is prone to biases inherent to retrospective cohort studies, such as selection bias and information bias. We are only able to determine whether there is an association between the variables, and the determination of direct cause and effect is not possible. Second, there may be unknown confounders that are impossible to control. We have attempted to list all of the patient variables that can potentially affect the outcomes and have controlled for these variables using multivariate analysis. Third, there is missing clinical information due to the retrospective nature of the study, although this deficiency is minimal.
In conclusion, our study of a cohort of patients undergoing endoscopic therapy for NVUGIB with high-risk stigmata shows that a relatively lower adrenaline injection volume and a combination of up to 2 endoscopic modalities to treat NVUGIB were associated with lower rebleeding and repeat endoscopy rates and a decreased LOS. The more liberal use of endoscopic clips was associated with a decreased LOS. To the best of our knowledge, this is the first study that has attempted to demonstrate a dose-dependent relationship between various endoscopic treatment modalities and patient outcomes in NVUGIB. We propose that the minimum volume of adrenaline be used for initial hemostasis along with any number of hemoclips for complete hemostasis. A total of 2 endoscopic modalities should be used.
Nonvariceal upper gastrointestinal bleeding (NVUGIB) is a common condition that results in significant morbidity and mortality. Mortality rates have not improved over the years. There are currently several endoscopic modalities for the treatment of this condition.
However, the dosage or amount of treatment to be used for each modality is not well studied. Moreover, it is not known whether a combination of three or more modalities combined is associated with better outcomes.
Our study aims to investigate whether various clinical outcomes in NVUGIB are influenced by the volume of adrenaline injected, the number of hemoclips placed and the number of treatment modalities used.
A retrospective cohort study conducted in a single large district general hospital. All patients admitted for NVUGIB and needing endoscopic treatment over a two-year period were analyzed. The various endoscopic treatment modalities were compared against several outcomes including rebleeding, repeat endoscopy rates, surgical intervention, transfusion requirements, length of hospital stay, death during the same admission and 30 d mortality.
Close to one third of our patients needed endoscopic therapy. < 10 mL adrenaline injected was associated with less re-bleeding (P < 0.0001), need for repeat endoscopy (P = 0.001) and decreased length of hospital stay (P = 0.026). > 2 treatment modalities used was associated with more re-bleeding (P = 0.009) and need for repeat endoscopy (P = 0.048). > 1 hemoclip placed was associated with decreased length of hospital stay (P = 0.044).
Our study is the first to show that more hemoclips placed was associated with a decreased length of stay. Also, we report novel findings that a reduced volume of adrenaline injected and a reduced number of endoscopic treatment modalities used was associated with better outcomes. More hemoclips used being associated with a better outcome is intuitive. However, previous studies have shown that larger volumes of adrenaline used led to better outcomes. These studies were conducted with adrenaline as the only treatment modality. This is not in line with the current management guidelines of NVUGIB which states that adrenaline use needs to be combined with another modality. Most of our patients who received adrenaline also received at least another treatment modality, this may be one reason why the volume of adrenaline required to arrest the bleeding may be smaller in our study. There are no previous studies that have shown that > 2 treatment modalities led to poorer outcomes. These findings are counter-intuitive but may be due to certain variables not captured in this retrospective study causing poorer outcomes. A prospective study with a larger sample size is needed to compare the various dosages and amounts of treatment used to manage this common condition.
In the endoscopic management of NVUGIB, more may not be merrier for all treatment modalities. A prospective trial is needed to confirm this.
| 1. | Holster IL, Kuipers EJ. Management of acute nonvariceal upper gastrointestinal bleeding: current policies and future perspectives. World J Gastroenterol. 2012;18:1202-1207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 2. | Rosenstock SJ, Møller MH, Larsson H, Johnsen SP, Madsen AH, Bendix J, Adamsen S, Jensen AG, Zimmermann-Nielsen E, Nielsen AS, Kallehave F, Oxholm D, Skarbye M, Jølving LR, Jørgensen HS, Schaffalitzky de Muckadell OB, Thomsen RW. Improving quality of care in peptic ulcer bleeding: nationwide cohort study of 13,498 consecutive patients in the Danish Clinical Register of Emergency Surgery. Am J Gastroenterol. 2013;108:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P; International Consensus Upper Gastrointestinal Bleeding Conference Group. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 840] [Cited by in RCA: 715] [Article Influence: 44.7] [Reference Citation Analysis (1)] |
| 4. | Hwang JH, Fisher DA, Ben-Menachem T, Chandrasekhara V, Chathadi K, Decker GA, Early DS, Evans JA, Fanelli RD, Foley K, Fukami N, Jain R, Jue TL, Khan KM, Lightdale J, Malpas PM, Maple JT, Pasha S, Saltzman J, Sharaf R, Shergill AK, Dominitz JA, Cash BD; Standards of Practice Committee of the American Society for Gastrointestinal Endoscopy. The role of endoscopy in the management of acute non-variceal upper GI bleeding. Gastrointest Endosc. 2012;75:1132-1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 223] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R, Racz I, Arezzo A, Hoffmann RT, Lesur G, de Franchis R, Aabakken L, Veitch A, Radaelli F, Salgueiro P, Cardoso R, Maia L, Zullo A, Cipolletta L, Hassan C. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:a1-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 517] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 6. | Lin HJ, Hsieh YH, Tseng GY, Perng CL, Chang FY, Lee SD. A prospective, randomized trial of large- versus small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc. 2002;55:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Park CH, Lee SJ, Park JH, Park JH, Lee WS, Joo YE, Kim HS, Choi SK, Rew JS, Kim SJ. Optimal injection volume of epinephrine for endoscopic prevention of recurrent peptic ulcer bleeding. Gastrointest Endosc. 2004;60:875-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Liou TC, Lin SC, Wang HY, Chang WH. Optimal injection volume of epinephrine for endoscopic treatment of peptic ulcer bleeding. World J Gastroenterol. 2006;12:3108-3113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Information paper on economic statistics. Available from: https://www.singstat.gov.sg/-/media/files/publications/economy/ip-e40ulc.pdf. |
| 10. | Matthewson K, Pugh S, Northfield TC. Which peptic ulcer patients bleed? Gut. 1988;29:70-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Lau JY, Leung WK, Wu JC, Chan FK, Wong VW, Chiu PW, Lee VW, Lee KK, Cheung FK, Siu P, Ng EK, Sung JJ. Omeprazole before endoscopy in patients with gastrointestinal bleeding. N Engl J Med. 2007;356:1631-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 196] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | El Ouali S, Barkun A, Martel M, Maggio D. Timing of rebleeding in high-risk peptic ulcer bleeding after successful hemostasis: a systematic review. Can J Gastroenterol Hepatol. 2014;28:543-548. [PubMed] |
| 13. | Anastassiades CP, Baron TH, Wong Kee Song LM. Endoscopic clipping for the management of gastrointestinal bleeding. Nat Clin Pract Gastroenterol Hepatol. 2008;5:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Hepworth CC, Kadirkamanathan SS, Gong F, Swain CP. A randomised controlled comparison of injection, thermal, and mechanical endoscopic methods of haemostasis on mesenteric vessels. Gut. 1998;42:462-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Ljubicic N, Budimir I, Biscanin A, Nikolic M, Supanc V, Hrabar D, Pavic T. Endoclips vs large or small-volume epinephrine in peptic ulcer recurrent bleeding. World J Gastroenterol. 2012;18:2219-2224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Saltzman JR, Strate LL, Di Sena V, Huang C, Merrifield B, Ookubo R, Carr-Locke DL. Prospective trial of endoscopic clips versus combination therapy in upper GI bleeding (PROTECCT--UGI bleeding). Am J Gastroenterol. 2005;100:1503-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Fabricius R, Svenningsen P, Hillingsø J, Svendsen LB, Sillesen M. Effect of Transfusion Strategy in Acute Non-variceal Upper Gastrointestinal Bleeding: A Nationwide Study of 5861 Hospital Admissions in Denmark. World J Surg. 2016;40:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Kim JJ, Sheibani S, Park S, Buxbaum J, Laine L. Causes of bleeding and outcomes in patients hospitalized with upper gastrointestinal bleeding. J Clin Gastroenterol. 2014;48:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Adam V, Barkun AN. Estimates of costs of hospital stay for variceal and nonvariceal upper gastrointestinal bleeding in the United States. Value Health. 2008;11:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Boyapati R, Ong SY, Ye B, Kruavit A, Lee N, Vaughan R, Nandurkar S, Gibson P, Garg M. One fifth of hospitalizations for peptic ulcer-related bleeding are potentially preventable. World J Gastroenterol. 2014;20:10504-10511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Singapore
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tandon RK, Wang YP S-Editor: Zhang L L-Editor: A E-Editor: Qi LL