Published online Jan 16, 2019. doi: 10.4253/wjge.v11.i1.54
Peer-review started: November 20, 2018
First decision: December 9, 2018
Revised: December 27, 2018
Accepted: January 8, 2019
Article in press: January 8, 2019
Published online: January 16, 2019
Processing time: 57 Days and 19 Hours
Enteropathy associated T-cell lymphoma (EATL) is a rare form of peripheral T-cell lymphoma and makes up less than 5% of gastrointestinal lymphomas. EATL can be divided into type 1 which is associated with celiac disease, and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), formally type 2, which is not associated with celiac disease.
We present a 60-year-old African American female, without celiac disease, who presented with abdominal pain, diarrhea, and 30 lb. weight loss over a 3 month period. She was subsequently diagnosed with EATL throughout her entire gastrointestinal tract. She is currently undergoing chemotherapy with EOCH (Etoposide, Oncovin, Cyclophosphamide, and Hydroxydaunorubicin). EATL is most common in the Asian and Hispanic population yet the incidence in African Americans is uncertain and emphasizes the rarity of this case. A literature review was included to further emphasize similarities and differences between our case and previously reported cases of MEITL.
The patient was diagnosed with EATL, immunochemical testing was not conclusive for MEITL however was suggestive of the disease.
Core Tip: The purpose of this case is to highlight an unusual presentation and demographic of monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL). A comprehensive literature review of MEITL is included in the case to further emphasize similarities and differences between our case and previously reported cases of MEITL.
- Citation: Fisher A, Yousif E, Piper M. Truth lies below: A case report and literature review of typical appearing polyps yet with an atypical diagnosis. World J Gastrointest Endosc 2019; 11(1): 54-60
- URL: https://www.wjgnet.com/1948-5190/full/v11/i1/54.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i1.54
Peripheral T-cell lymphoma (PTCL) is a small subset of aggressive non-Hodgkin lymphomas (NHL)[1]. One of the rarer entities of PTCL is enteropathy associated T-cell lymphoma (EATL). In general, EATL is diagnosed upon immunophenotype of small intestine biopsy and is only associated with approximately 5% of gastrointestinal (GI) lymphomas and less than 1% of NHL[2]. EATL is most commonly found in adult populations with a high incidence of celiac disease[3]. However, EATL can be divided into type 1 disease, which is associated with celiac disease, and monomorphic epitheliotropic intestinal T-cell lymphoma (MEITL), formally type 2, which is not associated with celiac disease[1]. MEITL is unique upon its immunophenotypic features compared to type 1 EATL[4] and has a higher incidence in Asian and Hispanic populations[5]. Ideal treatment of EATL consist of combination chemotherapy and hematopoietic cell transplantation (HCT)[6]. Here we present a unique case of EATL, in an African American patient without celiac disease.
A 60-yr-old African American female with a remote medical history of breast cancer, status post double mastectomy, presented with 1 wk of bilateral lower abdominal pain that was associated with diarrhea, early satiety, and a 30 lb. weight loss (over 3 mo).
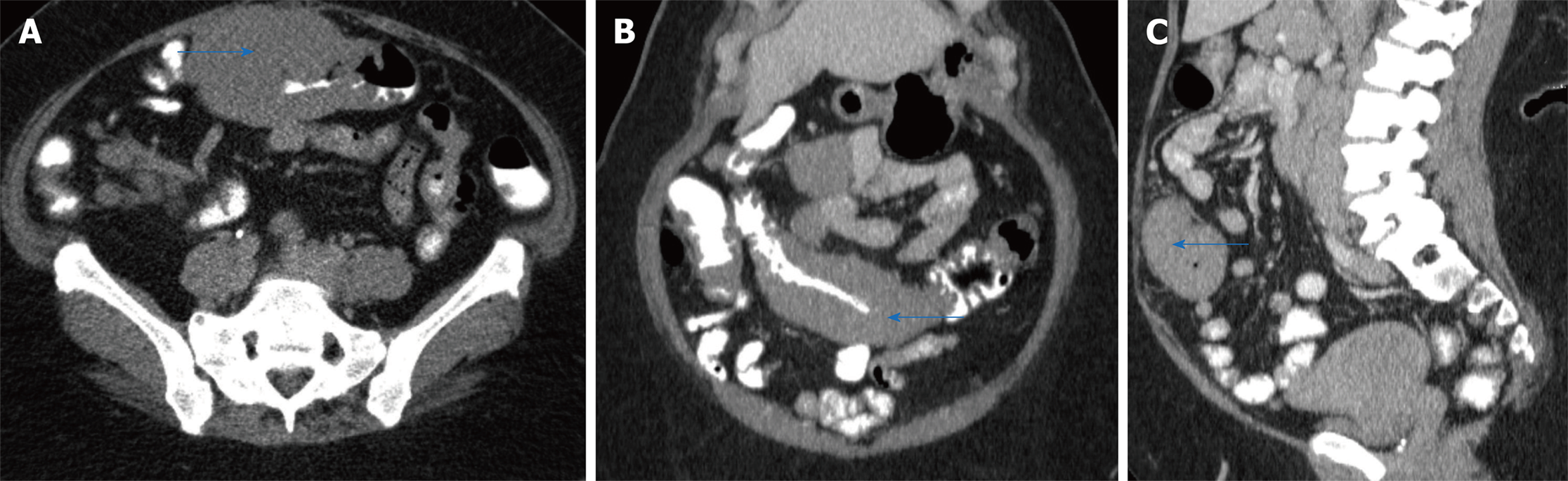
A computed tomography (CT) scan revealed a large circumferential mass involving the transverse colon which extended approximately 14 cm in length (Figure 1 A, B, and C). On physical exam, her vitals were stable. Her abdominal exam was soft and non-distended, with mild pain to palpation to her lower abdomen. Her laboratory work-up was notable for a mild leukocytosis (white blood count 13.4 bil/L, hemoglobin 15.7 g/dL, platelets 404 bil/L), hyponatremia and acute kidney injury ( sodium 123 mmol/L and creatinine 2.52 mg/dL (baseline creatinine was normal)). Her liver function tests (including albumin and protein level), lactic acid, lipase, infectious stool studies (i.e., Clostridium difficile infection, ova and parasites, Shiga toxin producing Escherichia coli, Salmonella, Shigella, Campylobacter, or Escherichia coli O157) were all normal.
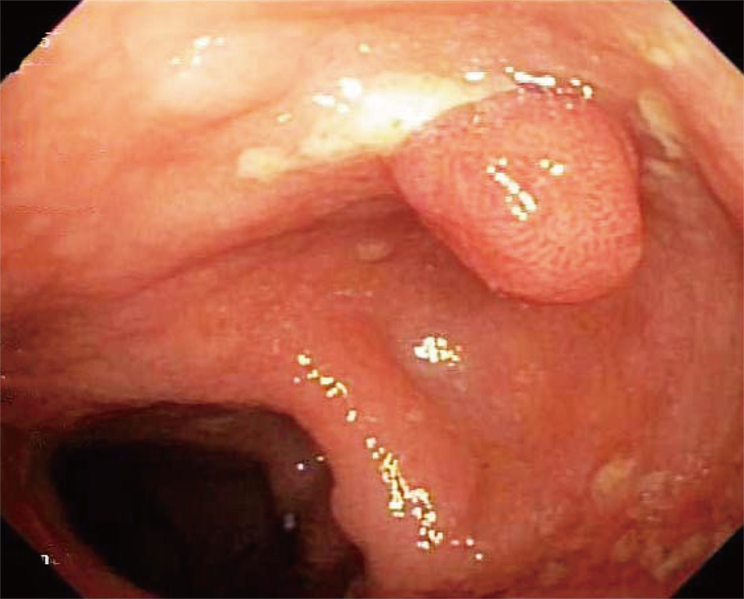
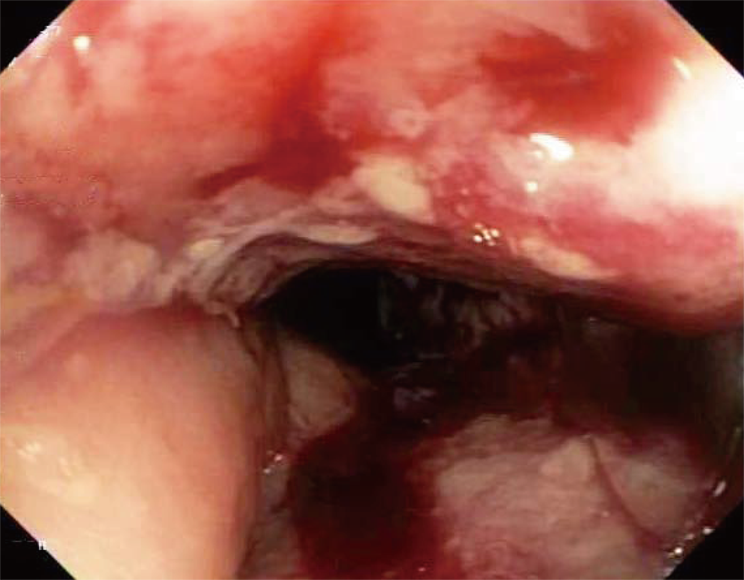
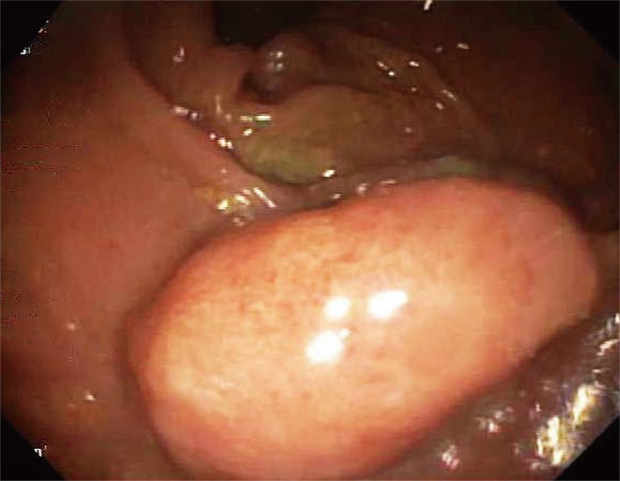
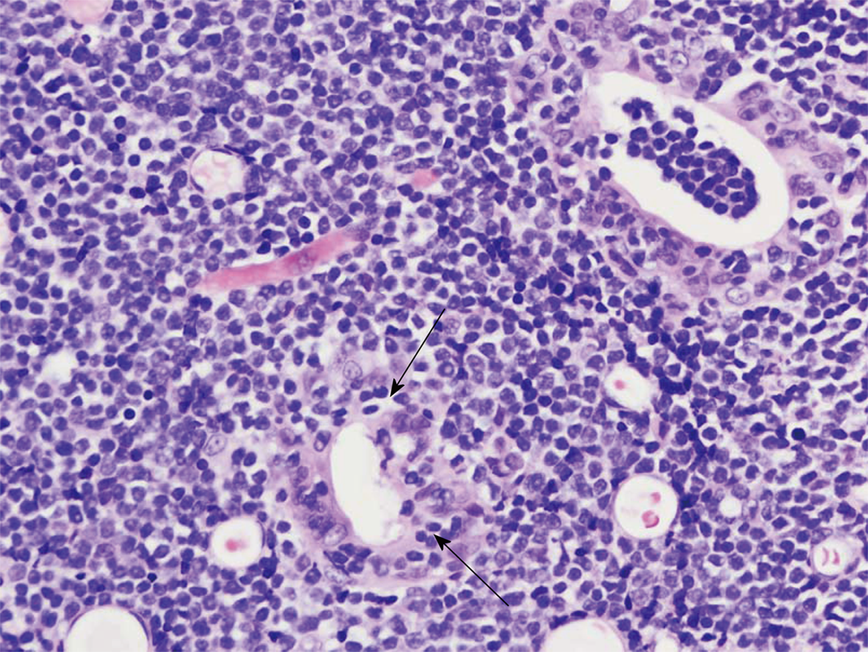
She underwent an esophagogastroduodenoscopy (EGD) and colonoscopy. EGD was notable for non-erosive gastropathy and normal appearing duodenum for which biopsies were obtained. Colonoscopy was notable for nodular ileal and colonic mucosa with multiple colonic polyps (Figure 2). In addition, she had a 15 cm malignant appearing stricture in the transverse colon (Figure 3). The largest colonic polyp was approximately 2.5 cm in her rectum (Figure 4). Pathology results of her stomach, duodenum, terminal ileum, colon, transverse stricture and all of her colonic polyps were notable for MEITL (Figure 5). There was no evidence of celiac disease on duodenal biopsy. There was no evidence of adenocarcinoma throughout her colon.
For the patient’s newly diagnosed high grade EATL, she underwent staging bone marrow biopsy and positron emission tomography (PET)/CT scan. Bone marrow biopsy was without overt morphologic or flow cytometry evidence of T-cell lymphoma or metastatic malignancy. PET/CT scan with abnormal F-18 fluorodeoxyglucose (FDG) activity associated with the transverse colonic mass, mesenteric lymphadenopathy, focal uptake within the rectum, intense uptake throughout the bone marrow, and portions of the spleen.
The patient was started on chemotherapy with EOCH (Etoposide, Oncovin, Cyclophosphamide, and Hydroxydaunorubicin). She was not a candidate for HCT given her functional status. Unfortunately, despite her aggressive chemotherapy regimen, her disease persisted. At her 6-month follow-up, her repeat PET/CT scan with abnormal FDG activity associated with the transverse colon and rectum. Repeat colonoscopy noted a large lymphoma polyploidy lesion.
The patient was diagnosed with EATL, immunochemical testing was not conclusive for MEITL however was suggestive of the disease.
The patient underwent chemotherapy with EOCH (Etoposide, Oncovin, Cyclophosphamide, and Hydroxydaunorubicin).
At her 6-mo follow-up, her repeat PET/CT scan with abnormal FDG activity associated with the transverse colon and rectum. Repeat colonoscopy noted a large lymphoma polyploidy lesion.
EATL is a rare form of PTCL that generally effects middle aged Caucasians. The majority of cases involve the small intestine (up to 90%), while the stomach and colon are less commonly involved (35% and 8% respectively)[3,4]. Additionally, many patients with EATL have celiac disease on histology[7]. Our patient is unique as she is an African American with involvement of entire GI tract, and no histological evidence of celiac disease.
Endoscopically, EATL often presents with nodularity, circumferential ulcers, and at times perforation. Identifiable masses or polyps are not generally appreciated[8]. In this case, the colonoscopy was notable for multiple large polyps (largest being 2.5 cm in the rectum) and a malignant appearing stricture.
Ideal treatment of EATL is consisted of combination chemotherapy and HCT. However, if patients are with poor functional status, HCT may not be an option. Sieniawski et al[6] demonstrated in a retrospective study that patients with EATL undergoing chemotherapy (ifosfamide, etoposide, epirubicin, and methotrexate) followed by HCT had progression free survival and overall survival (OS) at 5 yr of 52% and 60%, respectively. Chemotherapy alone has a much poorer prognosis with 5 yr OS of 10%-20%[8].
To review previous cases, we also performed a PubMed literature search. The search was conducted using the phrase “case report” and “monomorphic epitheliotropic intestinal T-cell lymphoma”. Six articles were identified between 2016 and 2018, involving nine patients. The majority of cases reported were in Southeast Asia. The patient’s ages ranged from 40 to 83 yr old and the majority of cases were male (66%). The most common presenting symptoms were diarrhea (44%) and weight loss (33%). Interestingly, one patient presented with severe dyspnea. The patient was noted to have a pleural effusion secondary to MEITL. Treatment regimens mainly consisted of various chemotherapy regimens with 33% of patients receiving stem cell transplants. Unfortunately, survival after diagnosis was poor and ranged from 5 d to 3 yr despite therapy (Table 1)[9-14].
| Author | Year and Location | Patient Age and Gender | Presenting symptom | History of celiac disease? | Treatment | Prognosis after diagnosis |
| Chen et al[9] | 2016, Singapore | 60 y/o, Male | Abdominal pain | No | CHOP + IVE/MTX + autologous stem cell transplant | Deceased at 2 wk |
| Ishibashi et al[10] | 2016, Japan | 60 y/o, Male | Persistent diarrhea and weight loss | No | CHASE and autologous stem cell transplant | Deceased at 3 yr |
| Ishibashi et al[10] | 2016, Japan | 40 y/o, Female | Diarrhea and weight loss | Not stated | THP-COP | Deceased at 2 mo |
| Ishibashi et al[10] | 2016, Japan | 50 y/o, Female | Abdominal distention | Not stated | CHOP + high dose MTX/cytarabine + allogeneic stem cell transplant | Deceased at 9 mo |
| Ishibashi et al[10] | 2016, Japan | 70 y/o, Male | Nausea | Not stated | SMILE | Deceased at 9 mo |
| Aiempanakit et al[11] | 2017, Thialand | 67 y/o, Male | Chronic diarrhea and weight loss | Not stated | Anthracycline based chemotherapy | Deceased at 2 mo |
| Antoniadou et al[12] | 2016, Greece | 76 y/o, Male | Severe dyspnea | Not stated | Unable to tolerate treatment and deceased | Deceased at Day 5 |
| Aoyama et al[13] | 2018, Japan | 83 y/o, Male | Fever and diarrhea | Not stated | CHOP followed by DeVIC | Deceased yet no timeframe stated |
| Pan et al[14] | 2018, Taiwan | 76 y/o, Female | Intermittent abdominal pain | No | CEOP | Deceased at 3.7 mo |
In conclusion, our patient continues to undergo therapy. Her subsequent PET scan and endoscopy have identified persistent disease. Our case is limited in that the patient has yet to complete chemotherapy. At her 6-mo follow-up, her repeat PET/CT scan with abnormal FDG activity associated with the transverse colon and rectum. Repeat colonoscopy noted a large lymphoma polyploidy lesion. There is no gold standard for monitoring EATL and repeat endoscopies can be decided on a case-by-case basis with guidance from oncology and further repeat imaging.
| 1. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5642] [Article Influence: 564.2] [Reference Citation Analysis (1)] |
| 2. | Zettl A, deLeeuw R, Haralambieva E, Mueller-Hermelink HK. Enteropathy-type T-cell lymphoma. Am J Clin Pathol. 2007;127:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, Macintyre E, Cerf-Bensussan N, Brousse N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. 2000;356:203-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 464] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 4. | Delabie J, Holte H, Vose JM, Ullrich F, Jaffe ES, Savage KJ, Connors JM, Rimsza L, Harris NL, Müller-Hermelink K, Rüdiger T, Coiffier B, Gascoyne RD, Berger F, Tobinai K, Au WY, Liang R, Montserrat E, Hochberg EP, Pileri S, Federico M, Nathwani B, Armitage JO, Weisenburger DD. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the international peripheral T-cell lymphoma project. Blood. 2011;118:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 242] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 5. | Deleeuw RJ, Zettl A, Klinker E, Haralambieva E, Trottier M, Chari R, Ge Y, Gascoyne RD, Chott A, Müller-Hermelink HK, Lam WL. Whole-genome analysis and HLA genotyping of enteropathy-type T-cell lymphoma reveals 2 distinct lymphoma subtypes. Gastroenterology. 2007;132:1902-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 160] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Sieniawski M, Angamuthu N, Boyd K, Chasty R, Davies J, Forsyth P, Jack F, Lyons S, Mounter P, Revell P, Proctor SJ, Lennard AL. Evaluation of enteropathy-associated T-cell lymphoma comparing standard therapies with a novel regimen including autologous stem cell transplantation. Blood. 2010;115:3664-3670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 7. | Isaacson PG, O'Connor NT, Spencer J, Bevan DH, Connolly CE, Kirkham N, Pollock DJ, Wainscoat JS, Stein H, Mason DY. Malignant histiocytosis of the intestine: a T-cell lymphoma. Lancet. 1985;2:688-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Gale J, Simmonds PD, Mead GM, Sweetenham JW, Wright DH. Enteropathy-type intestinal T-cell lymphoma: clinical features and treatment of 31 patients in a single center. J Clin Oncol. 2000;18:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 219] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Chen Y, Tan SY, Petersson BF, Khor YM, Gopalakrishnan SK, Tan D. Occult recurrence of monomorphic epitheliotropic intestinal T-cell lymphoma and the role of MATK gene expression in diagnosis. Hematol Oncol. 2017;35:852-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Ishibashi H, Nimura S, Kayashima Y, Takamatsu Y, Aoyagi K, Harada N, Kadowaki M, Kamio T, Sakisaka S, Takeshita M. Multiple lesions of gastrointestinal tract invasion by monomorphic epitheliotropic intestinal T-cell lymphoma, accompanied by duodenal and intestinal enteropathy-like lesions and microscopic lymphocytic proctocolitis: a case series. Diagn Pathol. 2016;11:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Aiempanakit K, Amatawet C, Chiratikarnwong K, Auepemkiate S, Kayasut K, Suwiwat S, Apinantriyo B. Erythema multiforme-like cutaneous lesions in monomorphic epitheliotropic intestinal T-cell lymphoma: a rare case report. J Cutan Pathol. 2017;44:183-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Antoniadou F, Dimitrakopoulou A, Voutsinas PM, Vrettou K, Vlahadami I, Voulgarelis M, Korkolopoulou P, Kafasi N, Mikou P. Monomorphic epitheliotropic intestinal T-cell lymphoma in pleural effusion: A case report. Diagn Cytopathol. 2017;45:1050-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Aoyama Y, Tsunemine H, Zushi Y, Maruoka H, Goto Y, Kodaka T, Itoh T, Takahashi T. Colonal monomorphic epitheliotropic intestinal T-cell lymphoma with novel phenotype of cytoplasmic CD3 expression. J Clin Exp Hematop. 2018;58:102-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Pan ST, Ko YH, Tan SY, Chuang SS. Primary cutaneous peripheral T-cell lymphoma with a late relapse solely in the ileum mimicking monomorphic epitheliotropic intestinal T-cell lymphoma. Pathol Res Pract. 2018;214:2106-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected byan in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Elzanan MHE, Jha AK S- Editor: Yan JP L- Editor: A E- Editor: Song H