Published online May 16, 2018. doi: 10.4253/wjge.v10.i5.99
Peer-review started: January 10, 2018
First decision: January 23, 2018
Revised: February 10, 2018
Accepted: March 14, 2018
Article in press: March 15, 2018
Published online: May 16, 2018
Processing time: 126 Days and 16.5 Hours
To investigate the success rates of endosonography (EUS)-guided biliary drainage (EUS-BD) techniques after endoscopic retrograde cholangiopancreatography (ERCP) failure for management of biliary obstruction.
From Feb/2010 to Dec/2016, ERCP was performed in 3538 patients, 24 of whom (0.68%) suffered failure to cannulate the biliary tree. All of these patients were initially submitted to EUS-guided rendez-vous (EUS-RV) by means of a transhepatic approach. In case of failure, the next approach was an EUS-guided anterograde stent insertion (EUS-ASI) or an EUS-guided hepaticogastrostomy (EUS-HG). If a transhepatic approach was not possible or a guidewire could not be passed through the papilla, EUS-guided choledochoduodenostomy (EUS-CD) was performed.
Patients were submitted to EUS-RV (7), EUS-ASI (5), EUS-HG (6), and EUS-CD (6). Success rates did not differ among the various EUS-BD techniques. Overall, technical and clinical success rates were 83.3% and 75%, respectively. Technical success for each technique was, 71.4%, 100%, 83.3%, and 83.3%, respectively (P = 0.81). Complications occurred in 3 (12.5%) patients. All of these cases were managed conservatively, but one patient died after rescue percutaneous transhepatic biliary drainage (PTBD).
The choice of a particular EUS-BD technique should be based on patient’s anatomy and on whether the guidewire could be passed through the duodenal papilla.
Core tip: Endosonography-guided biliary drainage is an effective alternative in the failure of endoscopic retrograde cholangiopancreatography, with the potential to provide the least invasive and the lowest risk therapeutic modality for biliary drainage when compared to percutaneous transhepatic biliary drainage or surgery. For this procedure, access to the biliary tree can be obtained by transhepatic or transduodenal approaches. However, the transhepatic approach offers a good acoustic window for puncture of the biliary tree, a straight and easier to work with position of the echoendoscope, a better positioning of the guidewire, and a lower chance of bleeding or choleperitoneum.
- Citation: Ardengh JC, Lopes CV, Kemp R, dos Santos JS. Different options of endosonography-guided biliary drainage after endoscopic retrograde cholangio-pancreatography failure. World J Gastrointest Endosc 2018; 10(5): 99-108
- URL: https://www.wjgnet.com/1948-5190/full/v10/i5/99.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i5.99
Traditionally, endoscopic retrograde cholangiopancreatography (ERCP) is the standard approach to biliary drainage[1,2]. However, the procedure fails in up to 10% of patients, especially owing to anatomic variations, malignant duodenal obstructions and previous surgeries[3,4]. For these cases, percutaneous transhepatic biliary drainage (PTBD) or surgery has been used, despite the high morbidity and not negligible mortality caused by these procedures[5,6].
More recently, endosonography-guided biliary drainage (EUS-BD) has emerged as an effective alternative, with the potential to provide the least invasive and lowest risk therapeutic modality for biliary access and drainage[7,8]. A recent meta-analysis has reported technical and clinical success of 90% and 94%, respectively[9].
We aimed to evaluate the role of different EUS-BD techniques in case of ERCP failure, and to propose a systematic routine for EUS-BD according to the feasible access routes to the biliary tree.
This was a retrospective study with prospective data collection about the role of EUS-BD conducted at two tertiary-referral centers. Between February 2010 and December 2016, 3528 ERCPs were performed at these centers. Eligible cases included patients older than 18 years with unresectable biliopancreatic neoplasia, and patients with benign conditions referred to EUS-BD when access to the biliary tree and internal biliary drainage by ERCP were not possible. ERCP failure was considered when biliary cannulation could not be achieved even after advanced techniques (cannulation in addition to a pancreatic guidewire or stent, needle-knife access papillotomy over a pancreatic stent, cannulation through a duodenal stent, and back-loading of the duodenoscope over a duodenal guidewire to pass a luminal stricture). Exclusion criteria were an international normalized ratio (INR) > 1.5 or platelet count < 50000/μL, ascites around the puncture area, absence of an adequate acoustic window for hepatic or choledochal puncture, total gastrectomy, and patient refusal. After EUS-BD, four follow-up visits were scheduled for each patient during the first 90 d, or until their death. The study was approved by the Institutional Review Board (Approval No. 2.191.319), and all patients gave written informed consent for ERCP and EUS-BD before enrollment.
All EUS-BD procedures were performed by the same experienced endoscopist with Fujinon (FujiFilm Corporation, Nishiazabu 2-chome Minato, Ku, Tokyo) duodenoscopes (ED-530XT) and curvilinear array echoendoscopes (EG530UT2) coupled to SU-7000 or SU-8000 ultrasound units. The sequential EUS-BD procedures proposed for all patients were as follows: first, transhepatic puncture with a 19 gauge aspiration needle (EUSN-19 T, Cook, Winston Sallen, NC, United States) was tried. The EUS-RV technique was successful when the guidewire could be passed through the papilla and seized in the second portion of the duodenum. In case of papillary benign disease or absence of duodenal stenosis, retrograde treatment with a duodenoscope or echoendoscope was performed. An anterograde approach was attempted when tumoral duodenal infiltration or duodenal stenosis did not allow the capture of the guidewire in the duodenum. If the anterograde approach failed, Endosonography-guided hepatogastrostomy (EUS-HG) was the next alternative. In case of failure of the intrahepatic puncture due to unfavorable anatomy, cirrhosis or difficulty in maintaining the adequate position of the guidewire, patients were submitted to endosonography-guided choledocoduodenostomy (EUS-CD). If all approaches for EUS-BD were unsuccessful, patients were submitted to PTBD. Duodenal self-expandable metallic stents (SEMS) were used in all stenoses obstructing access to the papilla.
The procedures were always performed with the patient in the left lateral decubitus position, under deep sedation with the assistance of an anesthesiologist. After the procedure, patients were monitored for two hours, and intravenous antibiotics (ciprofloxacin and metronidazole) were given for 7 d.
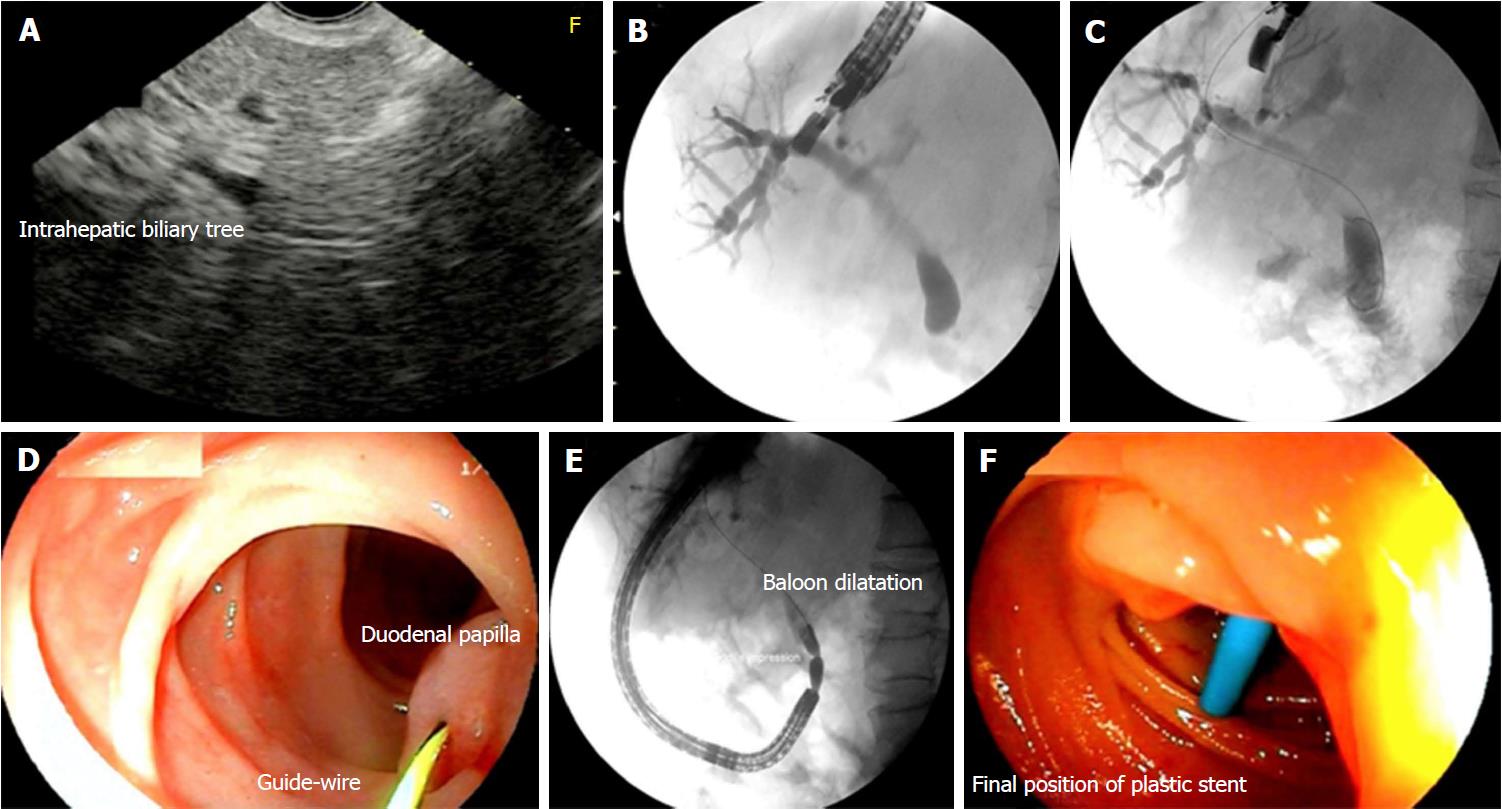
Endosonography-guided rendez-vous: When the duodenoscope could reach the major papilla, EUS-RV was tried and a curvilinear echoendoscope was used to obtain biliary access. The tip of the echoendoscope was positioned in the gastric fundus to access the intrahepatic bile duct. A 19 gauge EUS aspiration needle was used to puncture the bile duct close to the hepatic hilum, and to insert a large-caliber guidewire to deploy the stent. After fluoroscopic confirmation of the needle inside the bile duct, the guidewire was inserted through the obstruction and passed to the duodenum. Once the guidewire crossed the papilla, the guidewire was retrieved with a biopsy forceps or snare. Next, a metal stent was deployed by means of the over-the-wire technique[10].
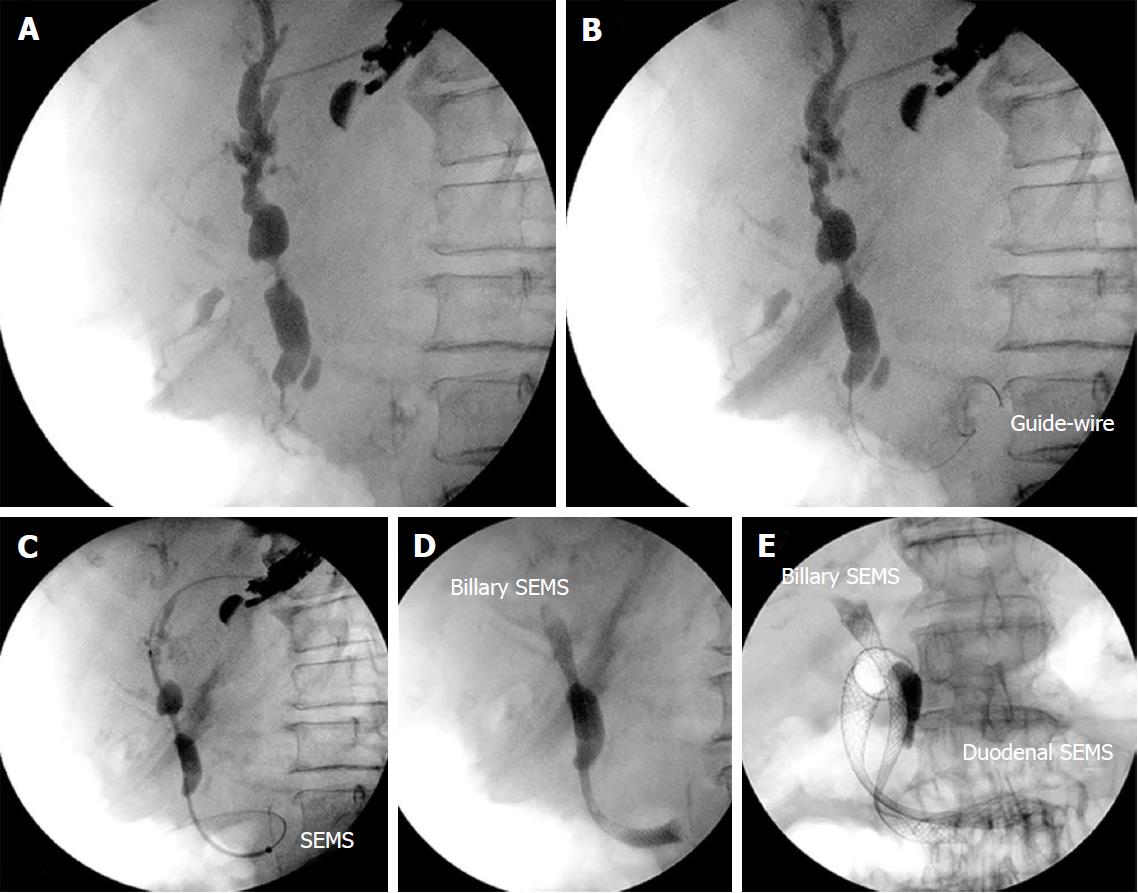
Endosonography-guided anterograde stent insertion: In the presence of neoplastic duodenal stenosis, when the guidewire could not be seized in the duodenum, the stent was placed in an anterograde way. Access to the intrahepatic bile duct was obtained using a 19 gauge aspiration needle. Once puncture of the bile duct was confirmed by fluoroscopy, the guidewire was inserted through the duodenal major papilla and positioned in the second portion of the duodenum. At this point, a SEMS was inserted through the gastric wall across the papilla.
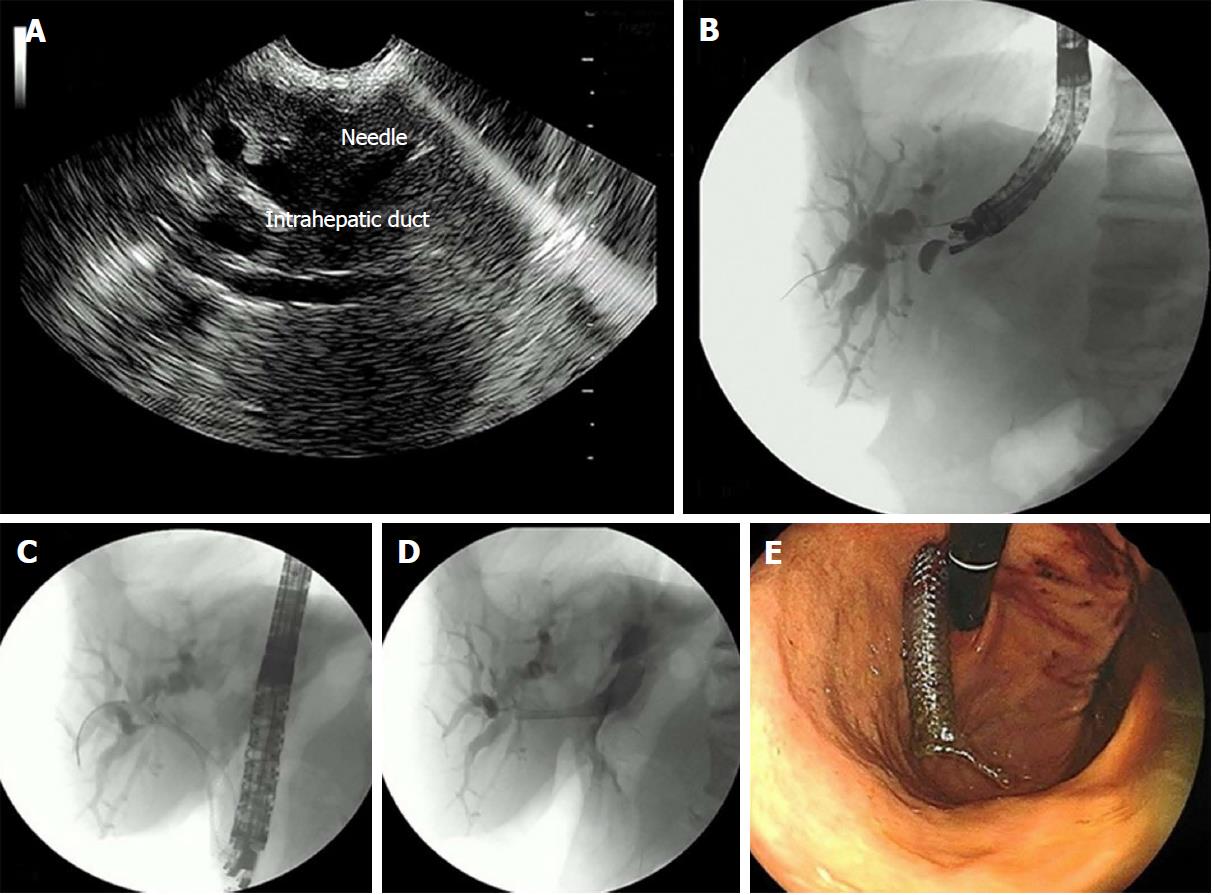
Endosonography-guided hepatogastrostomy: EUS-HG was tried after failure of the EUS-RV and EUS-anterograde stent insertion (EUS-ASI) techniques, in those cases whose hepatic puncture was successful but the guidewire could not be passed through the papilla. The dilated intrahepatic bile duct was punctured, and the guidewire was placed through the stenosis. The tract was dilated with a 6 Fr cystostome, and a fully covered metal stent was deployed, with care taken to leave more than 3 cm of the stent in the gastric lumen to avoid food obstruction.
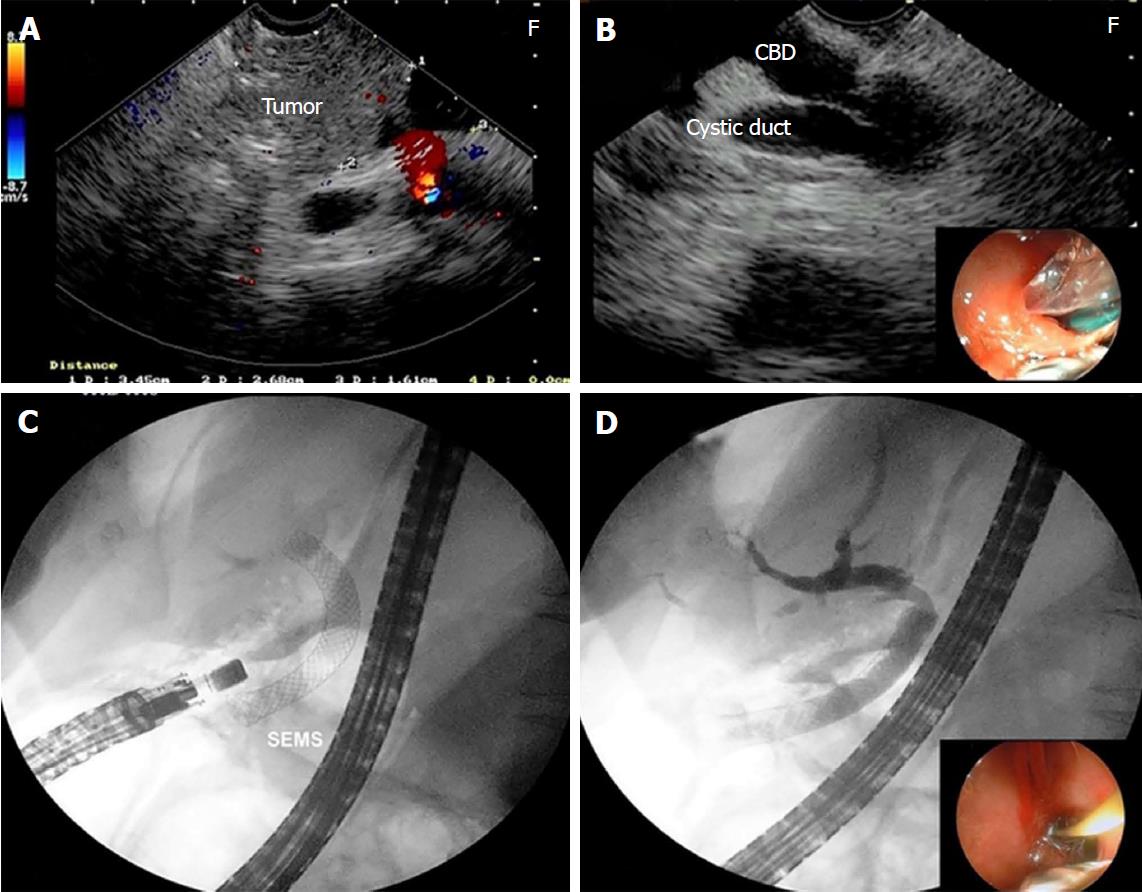
Endosonography-guided Choledocoduodenostomy: In patients for whom a transhepatic approach was not feasible, EUS-CD was performed with the identification of the extrahepatic bile duct from the duodenal bulb. Once the insertion of the guidewire into the bile duct was confirmed by cholangiography, the tract was dilated with a 6 Fr cystostome, and a fully covered self-expandable metal stent was inserted.
Technical success was defined as adequate positioning of the stent as shown by endoscopic and fluoroscopic images. Clinical success was defined as a decrease of at least 50% in serum total bilirubin levels.
A linear model was adjusted for the calculation of the technical success prevalence ratios, generalized by Poisson distribution and by the linking logarithmic function using the Proc Genmod of SAS 9.3 software (SAS Institute Inc., Cary NC, United States) to determine whether the different approaches had any impact on efficacy, compared to the EUS-RV technique (P > 0.05).
During the study period, it was not possible to cannulate the biliary tree in 24 of 3528 (0.68%) patients submitted to ERCP. Thirteen men and 11 women with a mean age of 67.8 years old were included in the study. The most common symptom was jaundice in 96% of the patients, followed by abdominal pain and acute biliary pancreatitis in 21% and 8.3% of cases, respectively. The demographics, reasons for ERCP failure, indications for EUS-BD, as well as technical and clinical success are listed in Table 1.
| EUS-BD | EUS-RV | EUS-ASI | EUS-HG | EUS-CD | |
| n (%) | 24 (100) | 7 (29) | 5 (21) | 6 (25) | 6 (25) |
| Sex (M/F) | 13/11 | 5/2 | 1/4 | 4/2 | 3/3 |
| Age (range), yr | 67.8 (42-91) | 67.7 (42-84) | 60.8 (42-70) | 68.2 (50-81) | 73.5 (52-91) |
| Reasons for ERCP failure (n) | - | - | - | - | - |
| Malignant duodenal stenosis | 8 | 2 | 3 | 2 | 1 |
| Malignant papillary infiltration | 7 | 1 | 2 | 1 | 3 |
| Impossibility of access to the common bile duct or intrahepatic duct | 7 | 2 | 0 | 3 | 2 |
| Giant duodenal diverticulum | 1 | 1 | 0 | 0 | 0 |
| Billroth II gastrectomy without access to the duodenal papilla | 1 | 1 | 0 | 0 | 0 |
| Indications for EUS-BD | - | - | - | - | - |
| Malignant | 20 | 3 | 5 | 6 | 6 |
| Pancreatic cancer | 13 | 3 | 4 | 2 | 4 |
| Liver metastases of colon cancer | 4 | 0 | 0 | 3 | 1 |
| Cholangiocarcinoma | 1 | 0 | 0 | 1 | 0 |
| Duodenal lymphoma | 1 | 0 | 1 | 0 | 0 |
| Papillary cancer | 1 | 0 | 0 | 0 | 1 |
| Benign | 4 | 4 | 0 | 0 | 0 |
| Common bile duct stones | 2 | 2 | 0 | 0 | 0 |
| Biliary necrotizing acute pancreatitis | 1 | 1 | 0 | 0 | 0 |
| Recurrent acute pancreatitis due to sphincter of Oddi dysfunction | 1 | 1 | 0 | 0 | 0 |
| Technical success n (%) | 20 (83.3) | 5 (71.4) | 5 (100) | 5 (83.3) | 5 (83.3) |
| Clinical success (%) | 18 (75) | 4 (57.1) | 5 (100) | 4 (66.7) | 5 (83.3) |
| Complications (%) | 3 (12.5) | 2 (28.5) | 0 (0) | 1 (16.7) | 0 (0) |
The EUS-guided transhepatic approach was tried in all patients (Figure 1). In 18/24 (75%) cases, puncture of the bile duct was possible, but the passage of the guidewire through the papilla occurred only in 12 (50%) cases. The guidewire could be recovered in 5/7 cases, and the passage of the stent was performed by means of an EUS-RV technique (Figure 2). The complication rate for these cases was 28% (2/7), consisting of an intracavitary hemorrhage and a choleperitoneum, both managed conservatively. In 5 other cases the guidewire could not be recovered in the duodenum owing to duodenal stenosis (3) or papillary infiltration (2). For these cases, an EUS-ASI technique was the next option. In 6 other cases, the guidewire did not cross the papilla, and was positioned in the proximal common bile duct (4), and in the right lobe (1) and left lobe of the liver (1). For these cases, an EUS-HG was the next alternative. The remaining 6 patients for whom transhepatic approaches were not possible underwent EUS-CD.
Even after passage of the guidewire in the second duodenal portion, the recovery of the guidewire was not possible in 5 patients due to malignant duodenal stenosis (3) or papillary infiltration (2). For these cases, anterograde deployment of the biliary SEMS was performed (Figure 3). After passage of the biliary SEMS, a duodenal SEMS was delivered in 3 patients with neoplastic duodenal stenosis. The overall technical success was 100%.
EUS-HG through transhepatic puncture was tried in 6 patients in whom the guidewire was positioned in the common bile duct (4), right lobe (1) and left lobe of the liver (1) (Figure 4). In 5/6 (83.3%) cases, an uneventful passage of the biliary SEMS was possible. For a single patient with recurrent liver metastasis from colon cancer after hepatectomy, the introduction of the transhepatic guidewire was impossible. The technical success rate was 83.3%, with one patient developing a pneumoperitoneum after the procedure.
The insertion of the biliary stent through the duodenal puncture was tried in 6 patients as a rescue EUS-guided procedure for biliary drainage (Figure 5). All of these cases presented malignancies (Table 1). The correct positioning of the guidewire was achieved in 5/6 (83.3%), and one case was referred to PTBD. There was no complication.
The overall technical success for EUS-BD was 83.3% (20/24). There was no significant difference among the various techniques (P = 0.81). Prior to EUS-BD, the mean levels of serum total and direct bilirubin were 13.3 mg/dL (5-29.9) and 9.1 (3-20.4) mg/dL, respectively. Ten days after EUS-BD, the mean levels were 2.3 (1.3-33) mg/dL, and 1.7 (0.6-22) mg/dL, respectively. The overall clinical success of EUS-BD was 75%.
Three (12.5%) complications occurred in patients submitted to EUS-BD: a pneumoperitoneum, a choleperitoneum, and an intracavitary liver hemorrhage. All of them were a consequence of the liver puncture in the hilum and were treated conservatively (Table 1). The patient with liver hemorrhage died three days after the PTBD due to acute respiratory and renal failure.
In our experience, an alternative to ERCP failure for biliary drainage was necessary in 0.68% of the cases, a finding similar to the rate of 0.62% in the experience of Holt et al[11]. Elderly people with malignant biliary obstruction are the most common candidates for the procedure[11], which was the case in our study, with patients at a median age of 68 years and with malignancies representing 83% of the cases. Endosonography-guided biliary drainage has been an alternative therapy to PTBD and surgery in ERCP failure[8,12]. PTBD, despite its satisfactory results, has a complication rate of about 30%, and surgery, although regarded as the definitive treatment for biliary drainage, is associated with high morbidity and mortality, especially for cases with terminal neoplastic disease[11,13,14].
Overall, the therapeutic success of EUS-BD ranges from 73% to 100%[15-19]. However, there is no consensus about the best EUS-BD technique[9]. Regarding particular EUS-BD techniques, there is a scarcity of comparative studies. Ogura et al[20] compared EUS-HG and EUS-CD for patients with jaundice and duodenal obstruction. Patients submitted to the transhepatic approach exhibited a longer patency of the biliary stent than those submitted to the transduodenal approach. In addition, the EUS-CD technique revealed a higher rate of complications, especially reflux cholangitis (OR = 10.285; 95%CI: 1.686-62.733; P = 0.012). Artifon et al[21] also evaluated the two techniques in a randomized clinical trial. There was no significant difference in effectiveness or safety between the two procedures. Technical and clinical success, as well as complications rates were 96%, 91%, and 20% for EUS-HG, respectively, and 91%, 77% and 12.5% for EUS-CD, respectively.
In an attempt to demonstrate the value of EUS-RV as the initial therapeutic option for biliary drainage in ERCP failure, Iwashita et al[22] performed the procedure using the transduodenal approach and using the transhepatic approach after failure of the transcholedochal approach. The authors concluded that EUS-RV is an effective and safe procedure, as also observed in our own experience. However, in contrast to the cited study, we began EUS-BD by the transhepatic approach, leaving the transduodenal approach only for the rescue option in the failure of the transhepatic approach.
In our experience, the transhepatic approach allows us to choose among three EUS-BD techniques according to the recovery or not of the guidewire, i.e., the EUS-RV, EUS-ASI and EUS-HG techniques. Our group has adopted a systematic EUS-BD routine starting with the transhepatic access to initially perform the EUS-RV or EUS-ASI technique. This approach offers a good acoustic window for puncture of the biliary tree, a straight and easier to work position of the echoendoscope, a better positioning of the guidewire, and a lower chance of bleeding or choleperitoneum, with both complications amenable to tamponade by the liver parenchyma[19,23]. In our study, beginning with the transhepatic approach, the overall technical success was 83%, and the clinical success (intention-to-treat) was 75%, similar to literature results[23]. On the other hand, the transduodenal approach permits an easier execution of only the EUS-CD or, although more laborious and time-consuming, the EUS-RV. In the failure of this approach, the transhepatic approach should be the rescue therapy.
Nevertheless, despite the good results of EUS-BD when using the transhepatic approach, the literature still mentions some concern about the risk of complications with the intrahepatic access[18,20,24]. The needle must traverse the peritoneal cavity, a procedure that might increase the risk of pneumo- and choleperitoneum. This complication occurred in one of our patients and was managed conservatively. Another issue is the movement of the stomach and liver during breathing and peristalsis, which might induce stent migration, trauma to the bilioenteric tract, and bile leakage. Finally, small-caliber intrahepatic ducts may not accommodate wider 8-mm to 10-mm metal stents, possibly predisposing to pneumoperitoneum and bile leakage due to incomplete sealing of the bilioenteric fistula[25,26]. For this reason, our goal during EUS-BD by means of the transhepatic approach is to obtain an intrahepatic duct of larger caliber as close as possible to the hepatic hilum.
In all of our cases in which the guidewire could not be reached in the duodenum due to stenosis or papillary infiltration, EUS-ASI succeeded without complications. The good performance and low complications rate of the EUS-ASI technique has been demonstrated in the literature[27].
On the other hand, if the patient has only a dilated biliary tree where the hepatic puncture is feasible but the guidewire could not reach the papilla, EUS-HG should be the next option. The greatest limitation in patients undergoing EUS-HG is the access to the right intrahepatic biliary tract and the progression of the guidewire to the common bile duct or its passage through the duodenal papilla. However, many authors justify selective drainage of the left intrahepatic biliary tract compared to the extrahepatic approach[7,28,29]. Both approaches have been shown to be effective and to involve low complications rates[21,26].
Nonetheless, EUS-BD by transhepatic approach may not be possible in some cases, depending on the patient anatomy[19,30]. We observed EUS-RV failure due to the impossibility of puncturing the liver or the inability to maintain the stability of the guidewire, and the difficulty to seize the guidewire in the duodenal lumen. In such cases, an extrahepatic approach must be adopted. The transcholedochal approach has the benefit of being feasible in patients whose papilla cannot be reached and has the advantage of being close to the duodenum[7,31,32]. In the current study, the technical success rates were the same (83.3%) for EUS-HG and EUS-CD, in agreement with published series[20,21]. Except for a pneumoperitoneum in the intrahepatic group, no difference in major complications was found between EUS-HG and EUS-CD (16.6% vs 0%; P = 0.81).
As a whole, EUS-BD is a safer technique than PTBD and surgery, with complication rates ranging from 10% to 20%, although the severity of most cases is mild to moderate[10,13]. Our complication rate also agreed with that reported in other studies[10,13]. Three of our cases developed complications, representing an overall rate of 12.5%. All of these cases were managed conservatively, but a patient with intracavitary bleeding was submitted immediately to PTBD after EUS-BD failure, and died three days later.
Despite the small number of our patients, this study did not demonstrate any significant difference in technical success or complication rates among different techniques of EUS-BD, in agreement with other studies[19,23].
In summary, a rational algorithm for EUS-BD in case of obstructive biliary diseases and ERCP failure might begin with the transhepatic approach, followed by particular EUS-BD techniques based on the patient’s anatomy and feasibility to recover the guidewire.
Endoscopic retrograde cholangiopancreatography (ERCP) is the standard approach to biliary drainage, and, in the failure of the procedure, percutaneous transhepatic biliary drainage or surgery must be used. However, endosonography can guarantee the least invasive and lowest risk treatment for biliary drainage of these cases. This study presents the results of different techniques for endosonography-guided biliary drainage in case of ERCP failure.
In case of ERCP failure, patients must be submitted to surgery or percutaneous transhepatic biliary drainage at different places in the hospital and with a long delay in treatment, conditions which can increase the morbidity and risks for the patient. Endosonography-guided biliary drainage can be performed immediately after ERCP failure, decreasing the time and risk of definitive treatment of the patient.
The main objectives of the study were to evaluate the success rates of endosonography (EUS)-guided biliary drainage techniques after ERCP failure for the management of biliary obstruction, and to propose a rational approach based on the access to the biliary tree and feasibility to recover the guidewire.
In our experience, an alternative to ERCP failure for biliary drainage was necessary in 24 of 3538 (0.68%) cases. Elderly people with malignant biliary obstruction were the most common candidates for the procedure. The sequential endosonography-guided biliary drainage (EUS-BD) procedures proposed for all patients were transhepatic puncture in order to perform the EUS-guided rendez-vous technique. An anterograde approach was attempted when the capture of the guidewire in the duodenum was not possible. If the anterograde approach failed, EUS-guided Hepatogastrostomy was the next alternative. In case of failure of the intrahepatic puncture, patients were submitted to EUS-guided choledochoduodenostomy (EUS-CD).
Patients were submitted to EUS-guided rendez-vous (7), EUS-guided anterograde stent insertion (5), EUS-guided hepaticogastrostomy (6), and EUS-CD (6). Success rates did not differ among the various EUS-BD technique. Overall, technical and clinical success rates were 83.3% and 75%, respectively. The technical success for each technique was 71.4%, 100%, 83.3%, and 83.3%, respectively (P = 0.81). Complications occurred in 3 (12.5%) patients. All of these cases were managed conservatively, but one patient died after a rescue percutaneous transhepatic biliary drainage. Regarding particular EUS-BD techniques, there is a scarcity of comparative studies, and a consensus about the best technique has not been established.
A rational approach to EUS-guided biliary drainage in case of obstructive biliary disease and ERCP failure should begin with the transhepatic approach, followed by particular EUS-guided biliary drainage techniques based on the patient’s anatomy and feasibility to recover the guidewire in the duodenum.
EUS-guided biliary drainage should be included in the therapeutic arsenal for the management of malignant biliary obstruction in case of ERCP failure, and should be the choice rather than surgery or percutaneous transhepatic biliary drainage.
| 1. | Carr-Locke DL. Overview of the role of ERCP in the management of diseases of the biliary tract and the pancreas. Gastrointest Endosc. 2002;56:S157-S160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Fogel EL, Sherman S, Devereaux BM, Lehman GA. Therapeutic biliary endoscopy. Endoscopy. 2001;33:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Peng C, Nietert PJ, Cotton PB, Lackland DT, Romagnuolo J. Predicting native papilla biliary cannulation success using a multinational Endoscopic Retrograde Cholangiopancreatography (ERCP) Quality Network. BMC Gastroenterol. 2013;13:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Williams EJ, Ogollah R, Thomas P, Logan RF, Martin D, Wilkinson ML, Lombard M. What predicts failed cannulation and therapy at ERCP? Results of a large-scale multicenter analysis. Endoscopy. 2012;44:674-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Ferrucci JT Jr, Mueller PR, Harbin WP. Percutaneous transhepatic biliary drainage: technique, results, and applications. Radiology. 1980;135:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 157] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Smith AC, Dowsett JF, Russell RC, Hatfield AR, Cotton PB. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstruction. Lancet. 1994;344:1655-1660. [PubMed] |
| 7. | Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004;59:100-107. [PubMed] |
| 8. | Dhir V, Itoi T, Khashab MA, Park DH, Yuen Bun Teoh A, Attam R, Messallam A, Varadarajulu S, Maydeo A. Multicenter comparative evaluation of endoscopic placement of expandable metal stents for malignant distal common bile duct obstruction by ERCP or EUS-guided approach. Gastrointest Endosc. 2015;81:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 9. | Khan MA, Akbar A, Baron TH, Khan S, Kocak M, Alastal Y, Hammad T, Lee WM, Sofi A, Artifon EL. Endoscopic Ultrasound-Guided Biliary Drainage: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:684-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 10. | Khashab MA, Dewitt J. EUS-guided biliary drainage: is it ready for prime time? Yes! Gastrointest Endosc. 2013;78:102-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Holt BA, Hawes R, Hasan M, Canipe A, Tharian B, Navaneethan U, Varadarajulu S. Biliary drainage: role of EUS guidance. Gastrointest Endosc. 2016;83:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Sharaiha RZ, Khan MA, Kamal F, Tyberg A, Tombazzi CR, Ali B, Tombazzi C, Kahaleh M. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: a systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 300] [Article Influence: 33.3] [Reference Citation Analysis (2)] |
| 13. | Cotton PB, Eisen GM, Aabakken L, Baron TH, Hutter MM, Jacobson BC, Mergener K, Nemcek A Jr, Petersen BT, Petrini JL, Pike IM, Rabeneck L, Romagnuolo J, Vargo JJ. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 2016] [Article Influence: 126.0] [Reference Citation Analysis (1)] |
| 14. | Gupta K, Perez-Miranda M, Kahaleh M, Artifon EL, Itoi T, Freeman ML, de-Serna C, Sauer B, Giovannini M; InEBD STUDY GROUP. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Khashab MA, Levy MJ, Itoi T, Artifon EL. EUS-guided biliary drainage. Gastrointest Endosc. 2015;82:993-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Moole H, Bechtold ML, Forcione D, Puli SR. A meta-analysis and systematic review: Success of endoscopic ultrasound guided biliary stenting in patients with inoperable malignant biliary strictures and a failed ERCP. Medicine (Baltimore). 2017;96:e5154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 18. | Park DH, Song TJ, Eum J, Moon SH, Lee SS, Seo DW, Lee SK, Kim MH. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversion technique for an occluded biliary metal stent after a failed ERCP (with videos). Gastrointest Endosc. 2010;71:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Tyberg A, Desai AP, Kumta NA, Brown E, Gaidhane M, Sharaiha RZ, Kahaleh M. EUS-guided biliary drainage after failed ERCP: a novel algorithm individualized based on patient anatomy. Gastrointest Endosc. 2016;84:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Ogura T, Chiba Y, Masuda D, Kitano M, Sano T, Saori O, Yamamoto K, Imaoka H, Imoto A, Takeuchi T. Comparison of the clinical impact of endoscopic ultrasound-guided choledochoduodenostomy and hepaticogastrostomy for bile duct obstruction with duodenal obstruction. Endoscopy. 2016;48:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Artifon EL, Marson FP, Gaidhane M, Kahaleh M, Otoch JP. Hepaticogastrostomy or choledochoduodenostomy for distal malignant biliary obstruction after failed ERCP: is there any difference? Gastrointest Endosc. 2015;81:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Iwashita T, Yasuda I, Mukai T, Iwata K, Ando N, Doi S, Nakashima M, Uemura S, Mabuchi M, Shimizu M. EUS-guided rendezvous for difficult biliary cannulation using a standardized algorithm: a multicenter prospective pilot study (with videos). Gastrointest Endosc. 2016;83:394-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Poincloux L, Rouquette O, Buc E, Privat J, Pezet D, Dapoigny M, Bommelaer G, Abergel A. Endoscopic ultrasound-guided biliary drainage after failed ERCP: cumulative experience of 101 procedures at a single center. Endoscopy. 2015;47:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 24. | Park DH. Endoscopic ultrasonography-guided hepaticogastrostomy. Gastrointest Endosc Clin N Am. 2012;22:271-280, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Chan SM, Teoh AY. Endoscopic ultrasound-guided biliary drainage: a review. Curr Treat Options Gastroenterol. 2015;13:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Khashab MA, Messallam AA, Penas I, Nakai Y, Modayil RJ, De la Serna C, Hara K, El Zein M, Stavropoulos SN, Perez-Miranda M. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs choledochoduodenostomy approaches. Endosc Int Open. 2016;4:E175-E181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 27. | Weilert F. Prospective evaluation of simplified algorithm for EUS-guided intra-hepatic biliary access and anterograde interventions for failed ERCP. Surg Endosc. 2014;28:3193-3199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Harbin WP, Mueller PR, Ferrucci JT Jr. Transhepatic cholangiography: complicatons and use patterns of the fine-needle technique: a multi-institutional survey. Radiology. 1980;135:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 111] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Kahaleh M, Hernandez AJ, Tokar J, Adams RB, Shami VM, Yeaton P. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Giovannini M, Dotti M, Bories E, Moutardier V, Pesenti C, Danisi C, Delpero JR. Hepaticogastrostomy by echo-endoscopy as a palliative treatment in a patient with metastatic biliary obstruction. Endoscopy. 2003;35:1076-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 31. | Isayama H, Nakai Y, Kawakubo K, Kawakami H, Itoi T, Yamamoto N, Kogure H, Koike K. The endoscopic ultrasonography-guided rendezvous technique for biliary cannulation: a technical review. J Hepatobiliary Pancreat Sci. 2013;20:413-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Kim YS, Gupta K, Mallery S, Li R, Kinney T, Freeman ML. Endoscopic ultrasound rendezvous for bile duct access using a transduodenal approach: cumulative experience at a single center. A case series. Endoscopy. 2010;42:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 102] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Andrianello S, Garg P, Govindarajan GK S- Editor: Cui LJ L- Editor: A E- Editor: Li D