Published online Oct 16, 2018. doi: 10.4253/wjge.v10.i10.283
Peer-review started: June 7, 2018
First decision: June 15, 2018
Revised: July 30, 2018
Accepted: August 12, 2018
Article in press: August 13, 2018
Published online: October 16, 2018
Processing time: 131 Days and 14.6 Hours
Liver resection surgery can be associated with significant perioperative mortality and morbidity. Extensive knowledge of the vascular anatomy is essential for successful, uncomplicated liver surgeries. Various imaging techniques like multidetector computed tomographic and magnetic resonance angiography are used to provide information about hepatic vasculature. Linear endoscopic ultrasound (EUS) can offer a detailed evaluation of hepatic veins, help in assessment of liver segments and can offer a possible route for EUS guided vascular endotherapy involving hepatic veins. A standard technique for visualization of hepatic veins by linear EUS has not been described. This review paper describes the normal EUS anatomy of hepatic veins and a standard technique for visualization of hepatic veins from four stations. With practice an imaging of all the hepatic veins is possible from four stations. The imaging from fundus of stomach is the easiest and most convenient method of imaging of hepatic veins. EUS of hepatic vein and the tributaries is an operator dependent technique and in expert hands may give a mapping comparable to computed tomographic and magnetic resonance imaging. EUS of hepatic veins can help in identification of individual sectors and segments of liver. EUS guided interventions involving hepatic veins may require approach from different stations.
Core tip: A standard technique for hepatic veins imaging by linear endoscopic ultrasound (EUS) has not been described. EUS of hepatic veins can help in identification of individual sectors and segments of liver. This review paper describes the normal EUS anatomy of hepatic veins and a standard technique for visualization of hepatic veins from four stations.
- Citation: Sharma M, Somani P, Rameshbabu CS. Linear endoscopic ultrasound evaluation of hepatic veins. World J Gastrointest Endosc 2018; 10(10): 283-293
- URL: https://www.wjgnet.com/1948-5190/full/v10/i10/283.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i10.283
Liver resection surgery is associated with significant perioperative mortality and morbidity[1]. Despite refinements in hepatic surgical techniques, vascular complications still occur. A detailed knowledge of the vascular anatomy and pre-surgical planning of vascular anastomosis on a vessel-to-vessel basis is essential for successful, uncomplicated liver surgeries[2-5]. A wide variety of imaging strategies are used to provide comprehensive preprocedural information about hepatic angioarchitecture[6]. Currently multidetector computed tomographic (CT) and magnetic resonance angiography are complementary modalities of hepatic angioarchitecture evaluation[7]. Ultrasound offers the advantage of Doppler assessment[8,9]. Despite comprehensive evaluation many smaller vessels may not be picked up, however from a surgical point of view these smaller vessels are insignificant and are tied up during surgery. The identification of these smaller vessels and specifically accessory veins of liver is sometimes important as they may drain a complete segment of liver. Separate segmental venous anastomosis is required for such cases to maintain sufficient hepatic venous drainage and to prevent postoperative complications resulting from the venous obstruction. An adequate maintenance of segmental hepatic venous drainage is also important as there is no adequate venovenous shunt between hepatic venous systems[10,11]. Linear endoscopic ultrasound (EUS) can offer a detailed evaluation of hepatic veins, help in assessment of liver segments and can offer a possible route for EUS guided vascular endotherapy involving hepatic veins. A standard technique for visualization of hepatic veins by linear EUS has not been described. This article describes the normal EUS anatomy of hepatic veins.
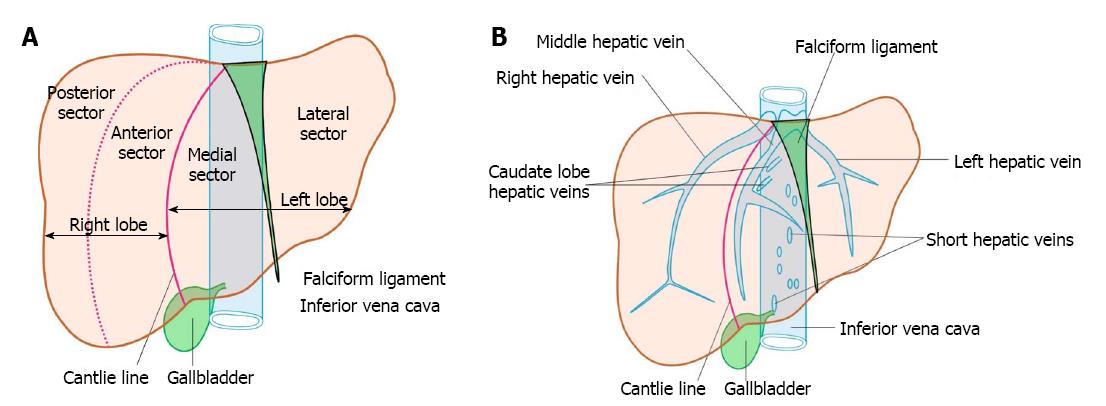
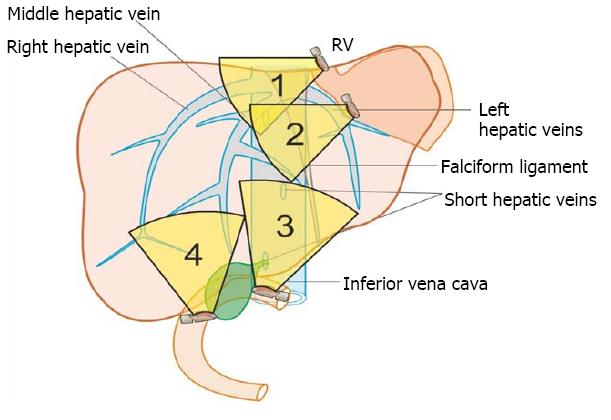
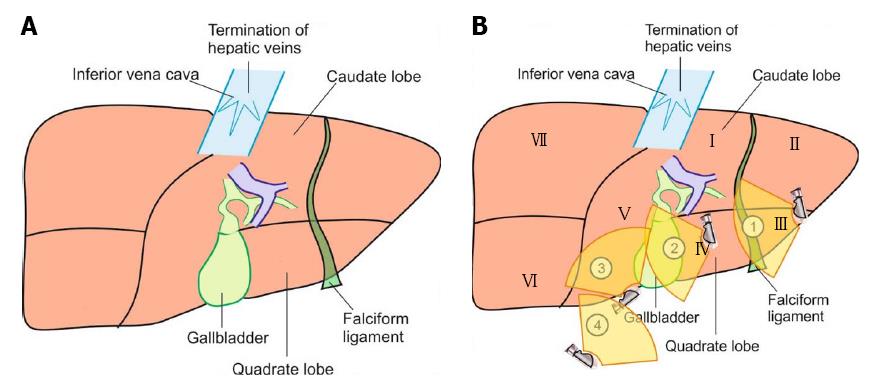
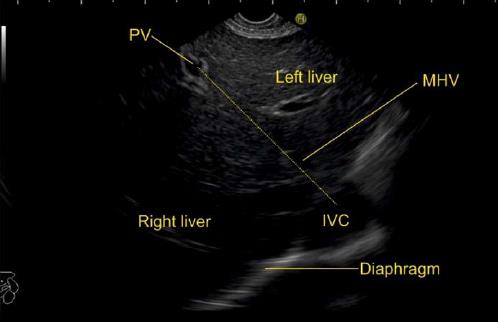
The anatomical classification of the liver, which divides the right and left lobe by the attachment of the falciform ligament is no longer accepted in routine terminology. The true physiological classification divides right and left hemi-liver by an imaginary line of Cantlie. Typically, the Cantlie’s line is 1 cm to the right of the middle hepatic vein (MHV), and corresponds to an important surgical plane in the sagittal axis that extends craniocaudally from the medial aspect of the gallbladder fossa to the left margin of inferior vena cava (IVC) (Figure 1A). Posteroinferiorly this line passes from gallbladder fossa to the main bifurcation of hepatic pedicle (portal triad) and then to retrohepatic IVC.
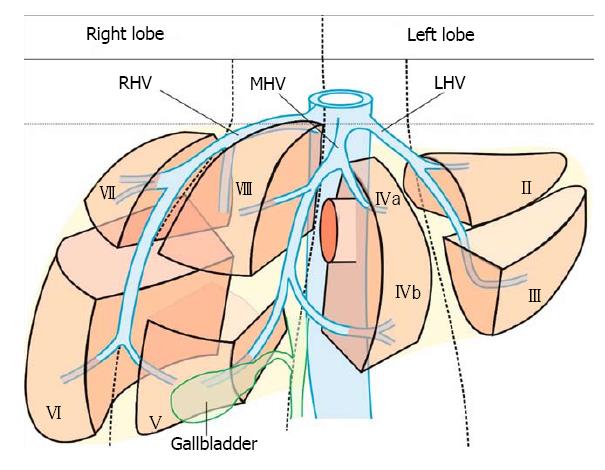
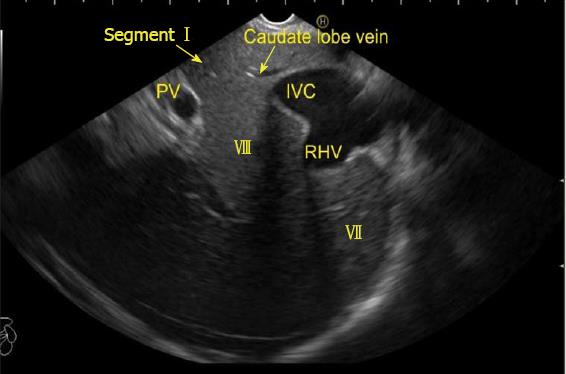
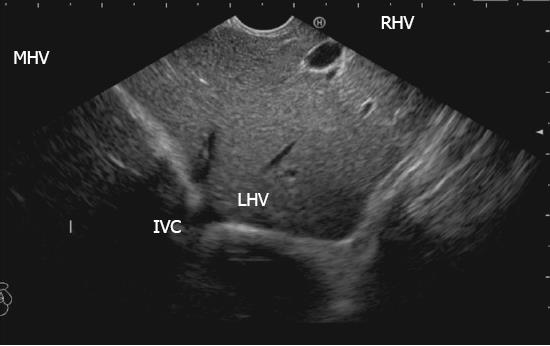
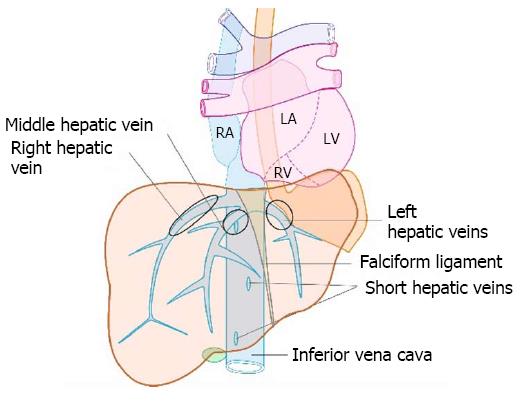
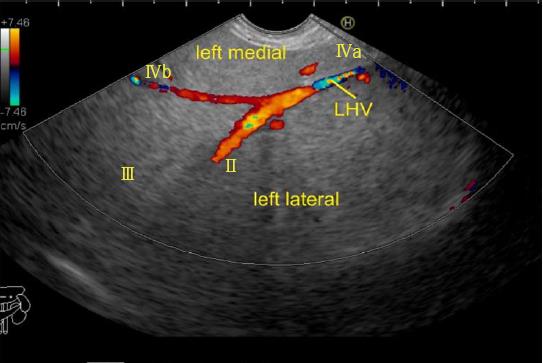
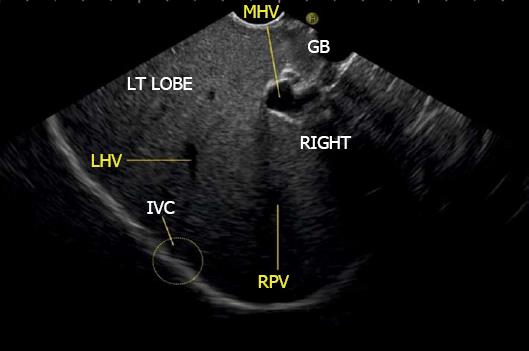
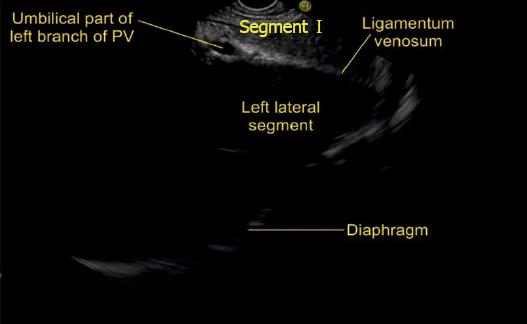
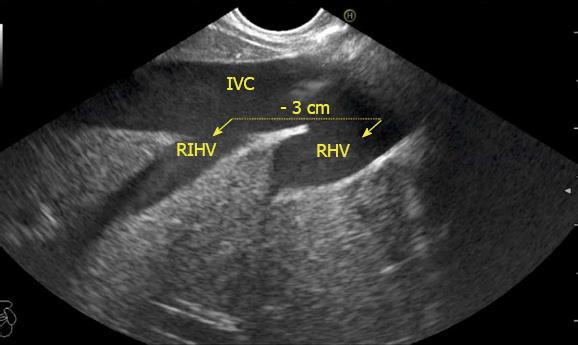
The hepatic veins are thin-walled anechoic vessels which do not have any valves, originate from the core (central) vein of the liver lobule and drain blood toward the IVC. The hepatic veins can be segregated into three major veins (right, middle and left) and many accessory veins or short hepatic veins. The three major hepatic veins are 6 to 15 mm in diameter, have no course outside liver and open directly into the supra hepatic part of IVC in the bare area of the liver (Figure 1B). The major veins are intersegmental in their course and divide the liver into four sectors; right anterior, right posterior, left medial and left lateral. The divisions separating the sectors are called portal fissures, which do not correspond to any superficially visible clefts but within each of which runs a hepatic vein. The right hepatic vein lies in the right portal fissure and separates the right hemi liver into anterior and posterior sectors. The right hepatic vein is the longest vein, passes through the segment I and lies parallel to the gallbladder fossa. The left hepatic vein (LHV) lies in the left portal fissure which is very close to the course of ligamentum venosum and separates the left hemi liver into medial and lateral sectors. The MHV lies in the middle portal fissure and separates the anterior division of right liver from medial division of left liver (Figure 1A). The accessory veins join the retro or intrahepatic part of IVC and are usually smaller in diameter (Figure 1B). The basic organisation of the segments and sectors of liver in relationship with hepatic vein tributaries is shown in Figure 2.
The images given in this pictorial essay are taken by Pentax UTK 3870 UT from cases undergoing EUS examination. The imaging of hepatic veins is usually aided by proper identification of the IVC and the gallbladder both of which are discussed as important home bases for imaging of hepatic veins.
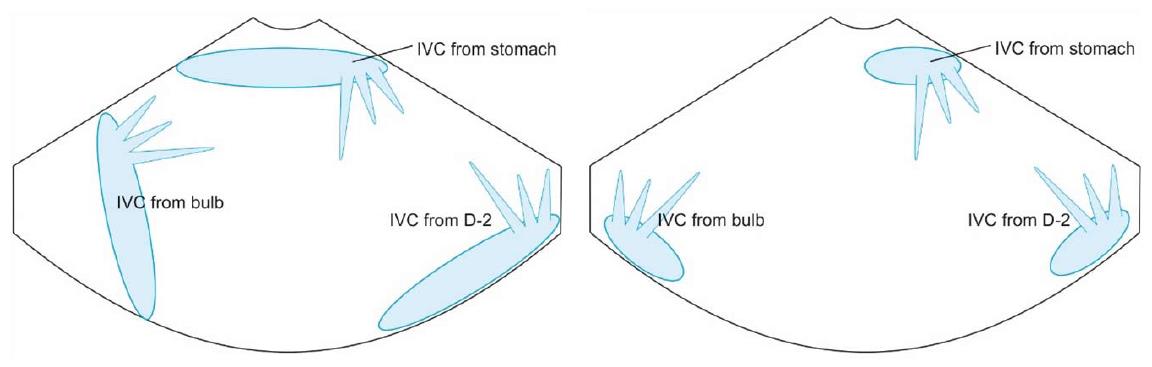
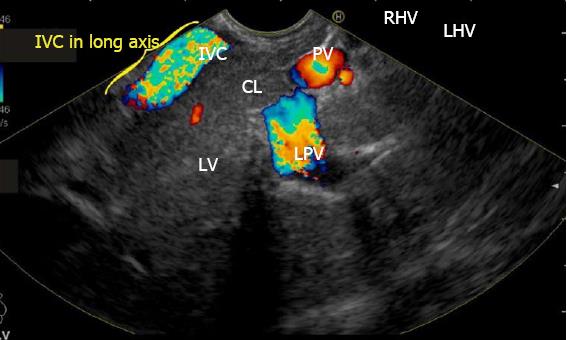
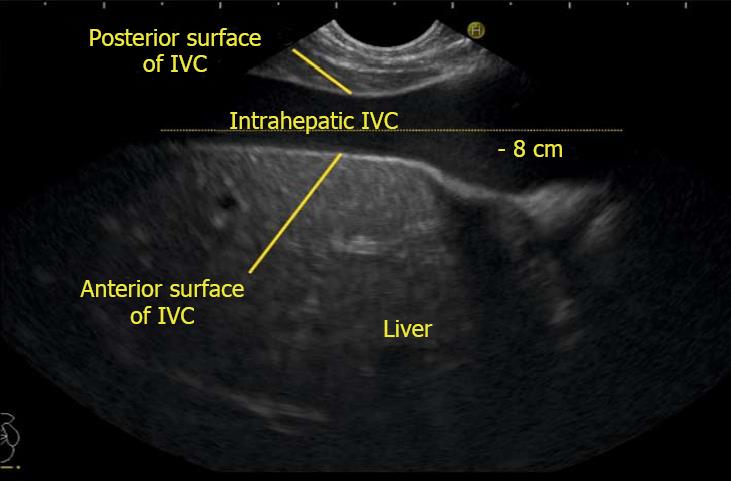
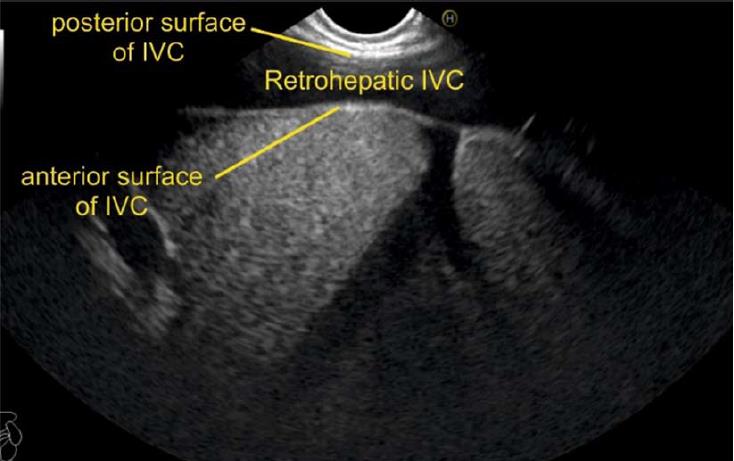
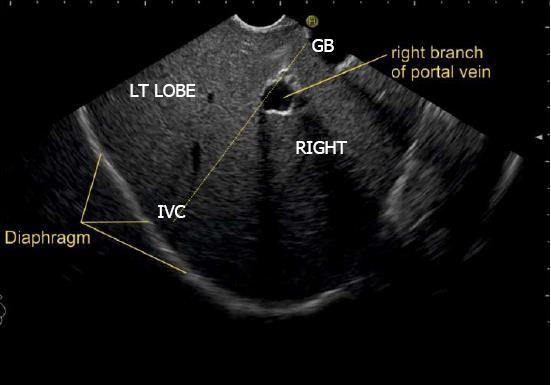
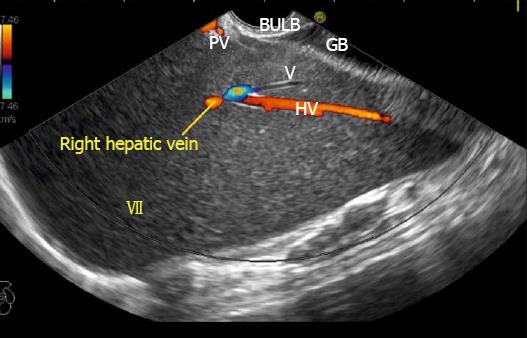
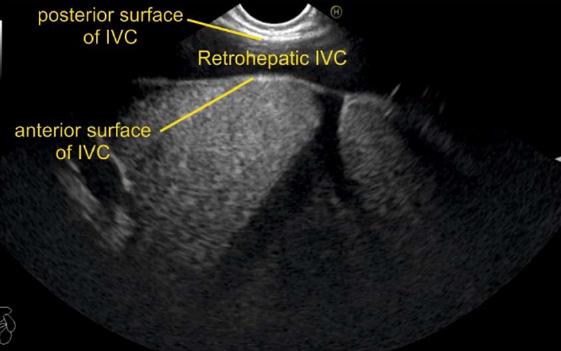
IVC can be visualized from different positions during EUS. The appearance of IVC may vary from rounded to an elongated axis depending on the axis of imaging and the angulation of the probe in these positions (Figures 3-7). It is usually possible to image the entire length (approximately 6 to 8 cm) of intrahepatic/retrohepatic part of IVC in a single frame at 1 to 3 cm distance from the probe in an axis parallel to the probe near the esophagogastric junction. In this position the surface closer to the probe corresponds to the posterior surface of IVC and the surface away from the probe corresponds to the anterior surface of IVC (Figure 8). The position and course of each of the hepatic vein is usually best assessed from the abdominal part of esophagus. Slight clockwise or anticlockwise rotation can trace the lateral surfaces of the IVC. The supra hepatic, or retro hepatic course of IVC can be followed for assessment of hepatic veins which join the anterior or lateral surface of IVC. No vein joins the posterior surface of IVC. During imaging from abdominal part of esophagus and stomach the spiral course of IVC in the liver is easily traced from above downwards from an anteriorly placed position of the IVC near the right atrium to a posteriorly placed position of the IVC in abdomen (Figures 4, 5, 8 and 9). The imaging of IVC and the hepatic veins is also possible from duodenal bulb and descending duodenum but the longer distance of hepatic veins and IVC from the bulb and descending duodenum may make it technically difficult to acquire similar amount of information (Figures 6 and 7).
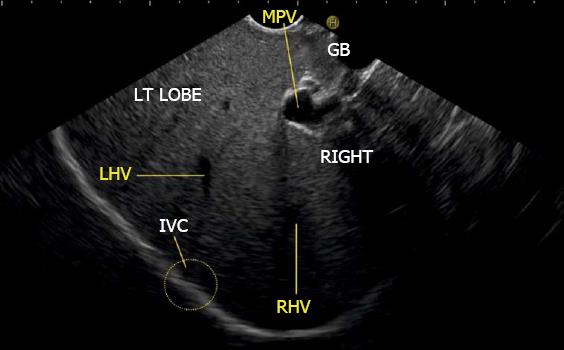
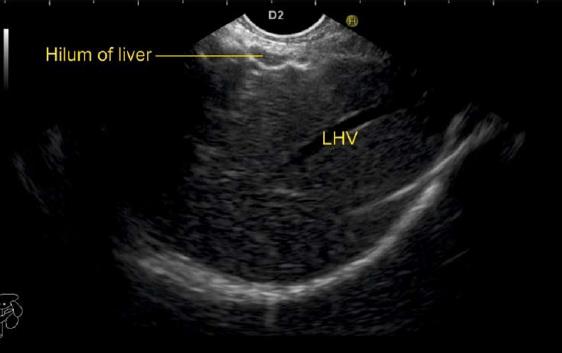
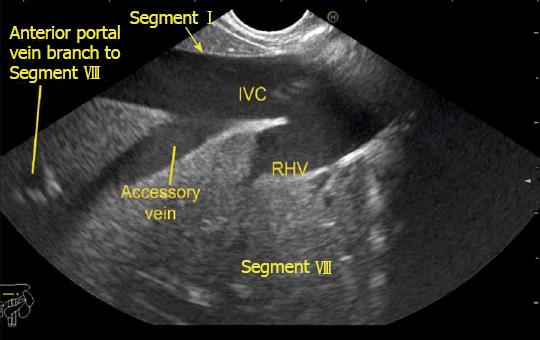
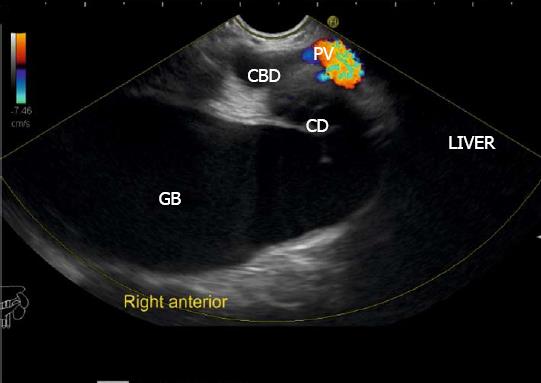
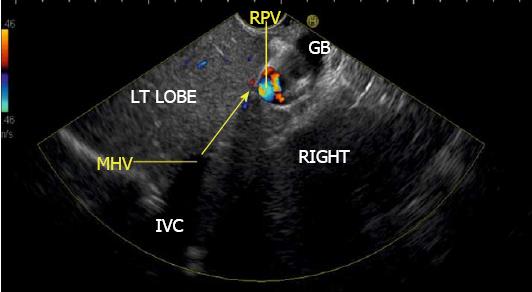
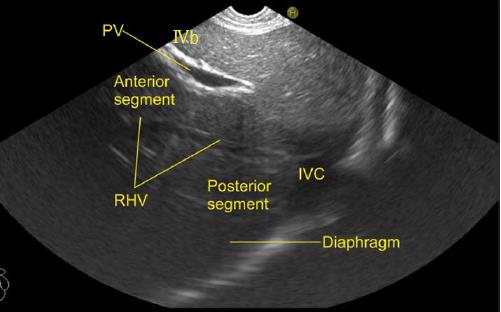
The gallbladder lies in a shallow fossa on the down sloping visceral surface of liver and can be visualized from the stomach, the duodenal bulb and from the descending part of duodenum. It is located near the right end of porta hepatis, its neck is highest, its fundus lowest. The location of gallbladder helps in following the course of hepatic vein; the right hepatic vein runs parallel to the upper surface of gallbladder (Figure 10), the MHV runs towards the neck of gallbladder (Figure 10) and the LHV runs away from the neck of the gallbladder (Figures 11 and 12).
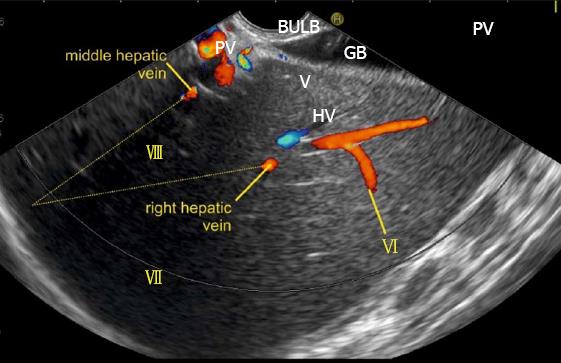
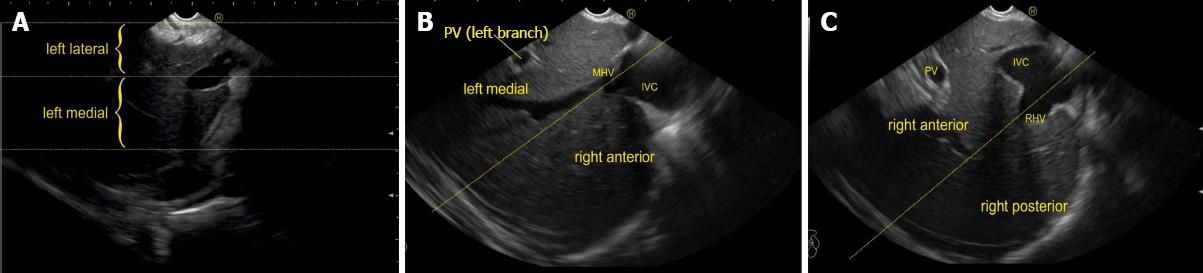
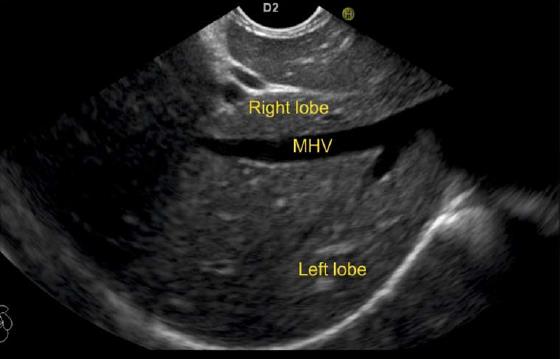
The course of hepatic veins and the hepatic vein branches is described from four stations: the abdominal part of esophagus, the fundus of stomach, the duodenal bulb and the descending duodenum (Figure 13). The imaging from each station may be done in following steps: (1) Demarcation of right and left lobe is done by following the course of MHV. The course of left and right hepatic vein helps in identification of the four sectors (Figure 14); (2) Further subdivision of the sectors into independent liver segments is possible by following the tributary free part of each hepatic vein and tracing the direction and path of travel of the tributaries (Figure 15); and (3) The location and side of appearance of 1st major tributary of each hepatic vein is helpful for segmental identification (Figures 15-18).
The abdominal part of esophagus lies very close to the entry point of left and MHV into the suprahepatic part of IVC. Initially the LHV is identified in an open position to the left (Figures 12 and 14A). The course of LHV divides the left lateral and left medial sector (Figure 14A). Slight clockwise rotation traces the joining of MHV at an angle of about 60° with the IVC (Figures 14B and 16). The presence of MHV divides the left medial (IVa) from right anterior sector (Figures 14B and 16). On further rotation, the right hepatic vein is seen, which divides the right anterior from right posterior sector (Figures 14C and 17). Usually in this position the merger of right hepatic vein is seen when the IVC is seen in an axis parallel to the probe (Figure 17). With a single movement of clockwise rotation from abdominal part of esophagus, the three hepatic veins can be identified within the portal fissures and the four sectors can be separated according to the order of appearance of hepatic veins (Figure 13).
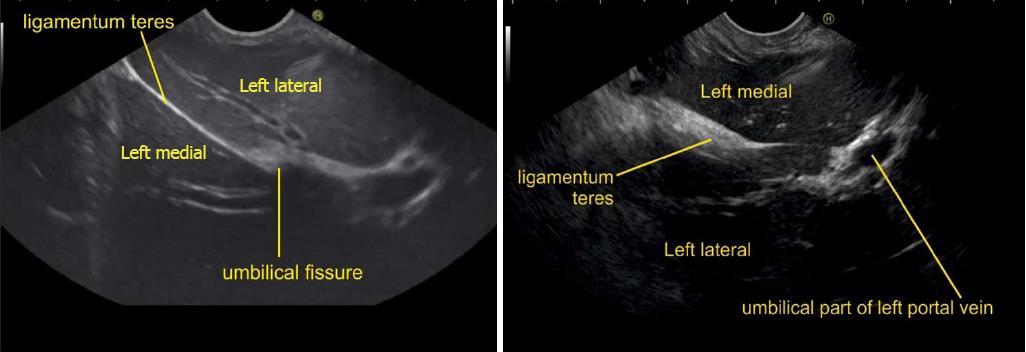
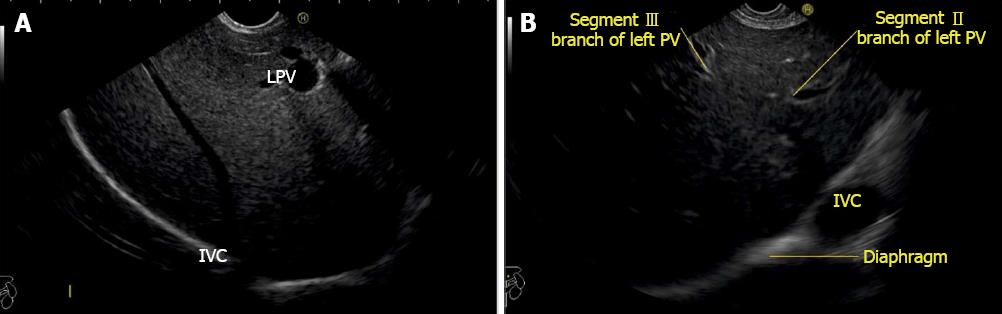
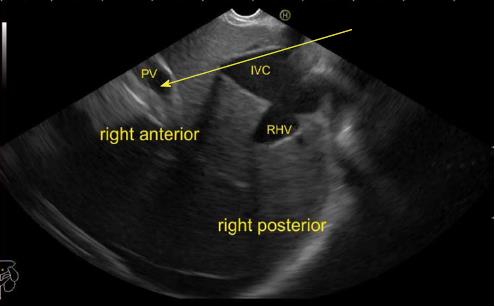
A EUS examination of most of the liver lobe, sectors and hepatic veins is possible from the visceral surface of liver which is in contact with stomach and forms the gastric impression on the under surface of liver (Figure 19). An open position to left places the tip of the transducer close to left lateral sector of liver in stomach. A clockwise rotation from an open position to the left brings into view the umbilical part of left branch of portal vein within the umbilical fissure which lies close to left edge of transverse fissure. Further clockwise rotation traces the transverse fissure from the left edge of the fissure to the right edge and moves the beam of probe from the left lateral sector to left medial sector (Figure 20). On continued rotation the beam moves towards the right anterior sector where the gallbladder is seen (Figure 21).
Imaging from duodenal bulb requires positioning of the scope in the duodenal bulb where clockwise and anticlockwise rotation results in appearance of left and right lobe (Figure 22). The presence of MHV is seen moving towards the neck of gall bladder and this divides the liver into right and left lobe (Figure 23). Further division into sectors is possible by clockwise rotation to visualize the left lobe (Figure 24) and anticlockwise rotation to visualize the right lobe (Figure 25). Imaging from the duodenal bulb usually visualizes the gallbladder neck near the liver hilum at 12 o’clock position, fundus at 3 o’clock position (Figures 23 and 25) and in this position the IVC is seen moving from 6 to 9 o’clock positions (Figure 24). A clockwise rotation moves the beam towards the duodenum and towards the retrorenal part of IVC whereas as an anticlockwise rotation traces the IVC towards the right lobe of liver.
The evaluation of the hepatic veins from descending duodenum is possible by extreme anti-clockwise rotation coupled with upwards angulation to prevent the slipping of the scope back into the stomach. During this rotation, the beam moves traces the IVC from behind the kidney towards the heart and sequentially rotates towards the axis of imaging across the right lobe of the liver, the gallbladder fossa and the left lobe of liver. In this position the IVC gradually moves from 9 o’clock position towards 4 o’clock position (Figure 26). During this rotation, the MHV (Figure 27), the RHV (Figure 28) and the LHV (Figure 29) appear one by one and help in identification of all the sectors of liver.
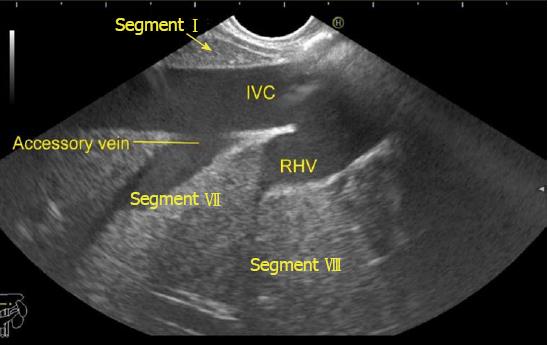
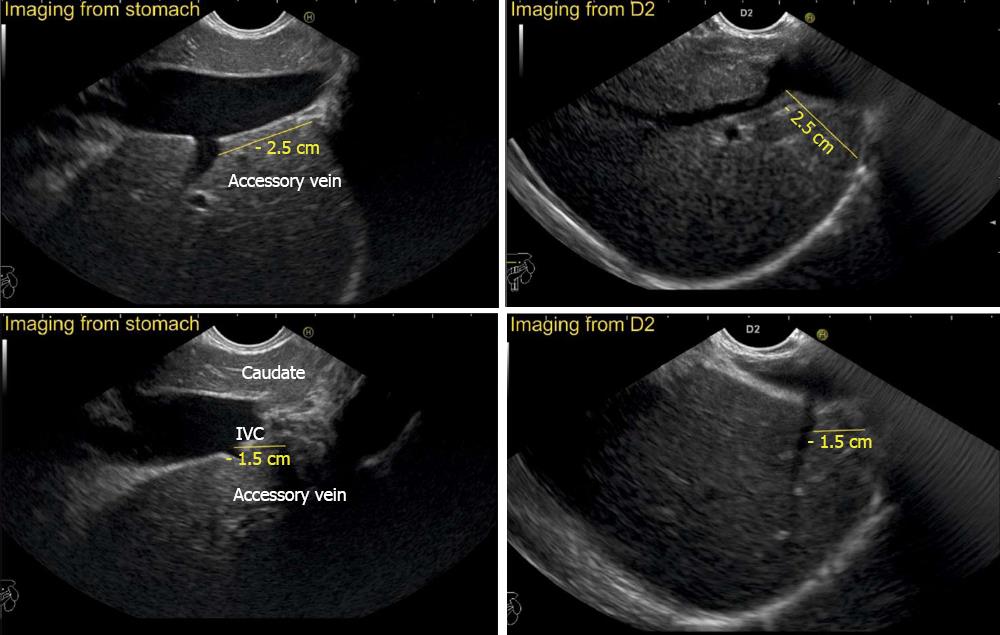
The accessory veins have significant variations in their number and size and the size may be larger, smaller or of the same size as the main hepatic veins. Larger size accessory veins usually provide independent and complete drainage of blood from a complete liver segment[11]. A universal classification of accessory veins is not given in literature and a simple description of accessory veins may mention all veins joining the IVC caudal to the main veins as right, middle or left inferior hepatic veins. Sometimes the accessory veins are classified into two groups according to the side that enter into IVC. The left side veins are called caudal hepatic veins, while the right sided veins are referred to as inferior right hepatic veins. On EUS the evaluation of the anterior and lateral wall of IVC below the joining of main hepatic vein is done in a craniocaudal axis (no vein joins the posterior aspect of IVC) for assessment of accessory veins (Figures 30-32). The number and diameter of hepatic veins joining IVC can be counted. The caudate lobe venous drainage is independent and occurs directly by two small fairly constant veins that enter the left side of IVC (Figure 5). In cases of liver donor, the caudate lobe usually remains in the donor because it directly drains into the IVC. The vena caval openings are considered as large openings with the diameter of 1.5-2 cm and medium when the diameter is 0.5-1.0 cm[11,12]. The distance of accessory vein from the main hepatic vein is important as it may be difficult to apply a single clamp if distance between accessory vein and the confluence of the hepatic vein 5 cm in the coronal plane.
EUS of hepatic vein and the tributaries is an operator dependent technique and in expert hands may give a mapping comparable to CT and magnetic resonance imaging. EUS of hepatic veins can help in identification of individual sectors and segments of liver. EUS offers additional superiority in assessing the flow dynamics of individual hepatic veins and can provide an opportunity for assessment of the anatomical features of hepatic vein length, diameter, pattern of joining, and evaluation of segmental venous drainage. Knowledge of the presence of supernumerary right hepatic veins or an inferior hepatic vein may facilitate extrahepatic or intrahepatic venous ligation during resection of the right hemi liver[13-16]. Studies done in animal models have shown a possible route for EUS guided intrahepatic portosystemic shunt from IVC and hepatic vein to portal vein[17]. The EUS anatomy of portal venous system has been well defined[18-20]. The assessment of hepatic veins can be also useful for assessing the path and possible techniques of specific hepatic vein puncture in planning a EUS guided procedures involving hepatic veins and portal vein (Figure 33).
This article describes a standard technique for visualization of hepatic veins. With practice an imaging of all the hepatic veins is possible from four stations. The imaging from fundus of stomach is the easiest and most convenient method of imaging of hepatic veins. EUS guided interventions may require approach from different stations. Knowledge of the hepatic venous territories and “venous drainage map” may provide useful information for complex liver surgeries and therapeutic procedure involving hepatic veins.
Pran Prakash (Graphic designer) for his contribution.
| 1. | Koea J. Getting started as a hepatobiliary surgeon: lessons learned from the first 100 hepatectomies as a consultant. N Z Med J. 2005;118:U1322. [PubMed] |
| 2. | Van Thiel DH, Wright HI, Fagiuoli S, Caraceni P, Rodriguez-Rilo H. Preoperative evaluation of a patient for hepatic surgery. J Surg Oncol Suppl. 1993;3:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Ozeki Y, Uchiyama T, Katayama M, Sugiyama A, Kokubo M, Matsubara N. Extended left hepatic trisegmentectomy with resection of main right hepatic vein and preservation of middle and inferior right hepatic veins. Surgery. 1995;117:715-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Fan ST, Lo CM, Liu CL, Yong BH, Chan JK, Ng IO. Safety of donors in live donor liver transplantation using right lobe grafts. Arch Surg. 2000;135:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 340] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 5. | Chen WT, Liang JL, Chen MH, Liao CC, Huang TL, Chen TY, Tsang LL, Ou HY, Hsu HW, Lazo MZ. Noncontrast Magnetic Resonance Angiography Using Inflow Sensitive Inversion Recovery Technique for Vascular Evaluation in Pre-liver Transplantation Recipients. Transplant Proc. 2016;48:1032-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Soyer P, Bluemke DA, Choti MA, Fishman EK. Variations in the intrahepatic portions of the hepatic and portal veins: findings on helical CT scans during arterial portography. AJR Am J Roentgenol. 1995;164:103-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 7. | Sahani D, Mehta A, Blake M, Prasad S, Harris G, Saini S. Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics. 2004;24:1367-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Appleton CP, Hatle LK, Popp RL. Superior vena cava and hepatic vein Doppler echocardiography in healthy adults. J Am Coll Cardiol. 1987;10:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Honda H, Yanaga K, Onitsuka H, Kaneko K, Murakami J, Masuda K. Ultrasonographic anatomy of veins draining the left lobe of the liver. Feasibility of live related transplantation. Acta Radiol. 1991;32:479-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Nayak SB, Surendran S, Nelluri VM, Kumar N, Aithal AP. A South Indian Cadaveric Study About the Relationship of Hepatic Segment of Inferior Vena Cava with the Liver. J Clin Diagn Res. 2016;10:AC04-AC07. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Nayak SB, Deepthinath R, Kumar N, Shetty P, Kumar V, Aithal A, Shetty SD. Evaluation of Numerical and Positional Variations of the Hepatic Veins: A Cadaveric Study. J Cardiovasc Echogr. 2016;26:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Camargo AM, Teixeira GG, Ortale JR. Anatomy of the ostia venae hepaticae and the retrohepatic segment of the inferior vena cava. J Anat. 1996;188:59-64. [PubMed] |
| 13. | Makuuchi M, Hasegawa H, Yamazaki S, Bandai Y, Watanabe G, Ito T. The inferior right hepatic vein: ultrasonic demonstration. Radiology. 1983;148:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Makuuchi M, Sugawara Y. Technical progress in living donor liver transplantation for adults. HPB (Oxford). 2004;6:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kamel IR, Lawler LP, Fishman EK. Variations in anatomy of the middle hepatic vein and their impact on formal right hepatectomy. Abdom Imaging. 2003;28:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Erbay N, Raptopoulos V, Pomfret EA, Kamel IR, Kruskal JB. Living donor liver transplantation in adults: vascular variants important in surgical planning for donors and recipients. AJR Am J Roentgenol. 2003;181:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Schulman AR, Ryou M, Aihara H, Abidi W, Chiang A, Jirapinyo P, Sakr A, Ajeje E, Ryan MB, Thompson CC. EUS-guided intrahepatic portosystemic shunt with direct portal pressure measurements: a novel alternative to transjugular intrahepatic portosystemic shunting. Gastrointest Endosc. 2017;85:243-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Rameshbabu CS, Wani ZA, Rai P, Abdulqader A, Garg S, Sharma M. Standard imaging techniques for assessment of portal venous system and its tributaries by linear endoscopic ultrasound: a pictorial essay. Endosc Ultrasound. 2013;2:16-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 19. | Sharma M, Babu CS, Garg S, Rai P. Portal venous system and its tributaries: a radial endosonographic assessment. Endosc Ultrasound. 2012;1:96-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Sharma M, Rameshbabu CS. Collateral pathways in portal hypertension. J Clin Exp Hepatol. 2012;2:338-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Goral V, Hosoe N, Skok P S- Editor: Gong ZM L- Editor: A E- Editor: Wu YXJ