©The Author(s) 2015.
World J Gastrointest Endosc. Apr 16, 2015; 7(4): 318-327
Published online Apr 16, 2015. doi: 10.4253/wjge.v7.i4.318
Published online Apr 16, 2015. doi: 10.4253/wjge.v7.i4.318
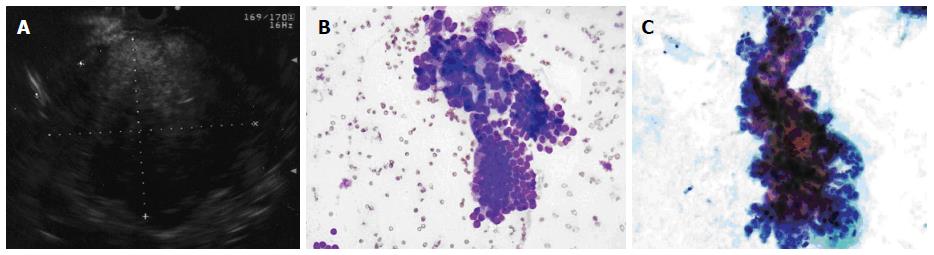
Figure 1 Pancreatic adenocarcinoma.
A: Endoscopic ultrasound image demonstrating a large pancreatic adenocarcinoma; B: Pancreatic adenocarcinoma. A crowded group of large, pleomorphic ductal cells with irregular hyperchromatic nuclei and prominent anisocytosis. These contrast well with an orderly sheet of benign ductal epithelial cells with round, uniform nuclei (bottom) (Diff-QuikTM stain, × 100); C: Similar in appearance malignant cells in a Papanicolaou-stained preparation (× 400).
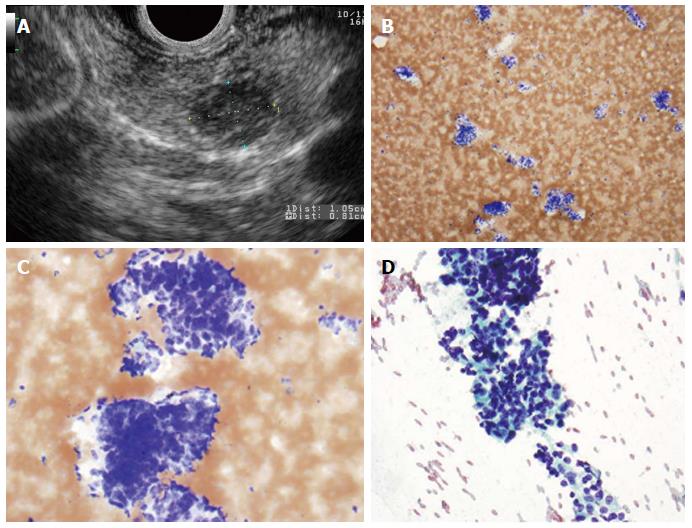
Figure 2 Pancreatic neuroendocrine neoplasm.
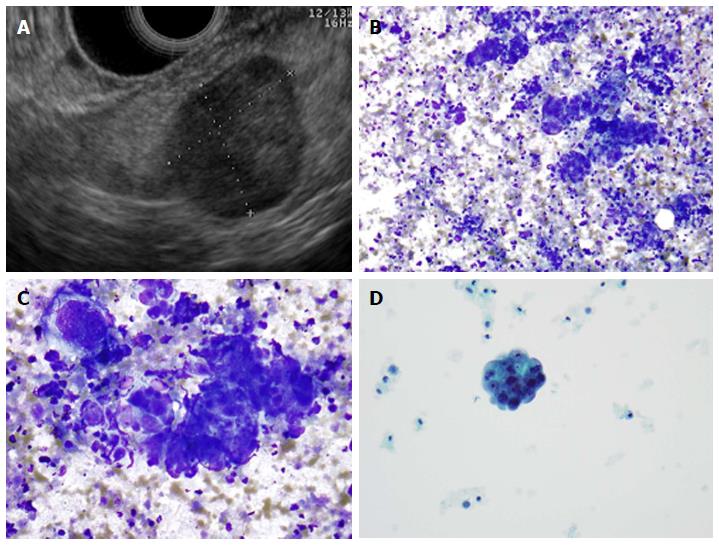
A: Endoscopic ultrasound image showing a 9 mm × 10 mm neuroendocrine tumor (insulinoma); B: Low-power view shows a cellular aspirate composed of clusters of uniform cells (Diff-QuikTM stain, × 100); C: High power view shows uniform cells with high N:C ratios and coarse chromatin (Diff-QuikTM stain, × 400); D: Papanicolaou stain highlights coarse, evenly distributed chromatin (× 400).
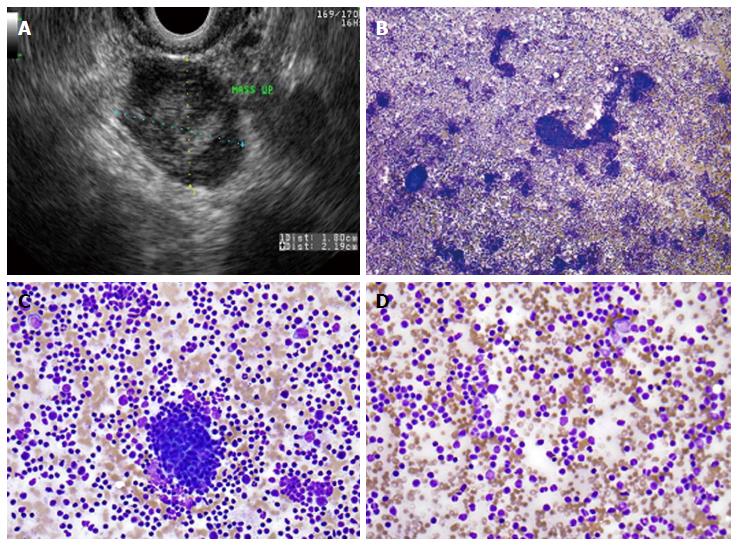
Figure 3 Primary pancreatic lymphoma.
A: Endoscopic ultrasound demonstrating a 1.8 cm × 2.2 cm lymphoma in the uncinate process of the pancreas; B: Low-power view showing a very cellular aspirate composed of discohesive lymphoid cells (Diff-QuikTM stain, × 100); C: High-power view showing an admixture of mature lymphocytes of various sizes with no more than a minimal atypia; lymphoid aggregates resembling a germinal center are also present (bottom); D: Small mature lymphocytes with cleaved and irregular nuclei raising suspicion for a mature B-cell lymhoma. (Diff-QuikTM stain, × 400).
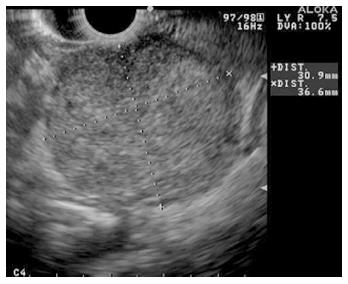
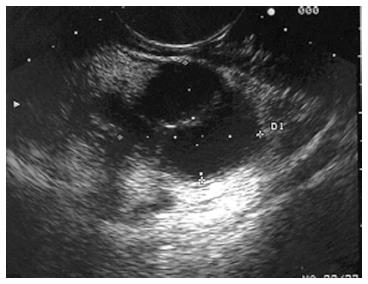
Figure 4 Endoscopic ultrasound image of large, 3.
5 cm × 4.4 cm, round, hypoechoic, heterogenous mass lesion arising from the tail of the pancreas.
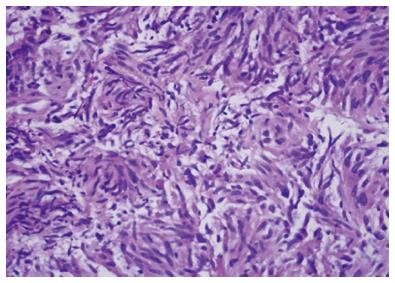
Figure 5 Cytology from a primary pancreatic gastrointestinal stromal tumor.
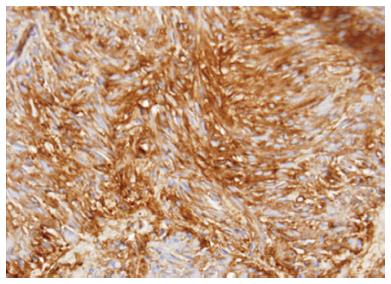
Figure 6 Pancreatic gastrointestinal stromal tumor, cytology demonstrates a spindle cell neoplasm with moderate nuclear pleomorphism which stains strongly positive for CD117 and negative for desmin, consistent with a gastrointestinal stromal tumor arising from the pancreas.
(Courtesy of Rashmi Agni, University of Wisconsin Department of Pathology and Laboratory Medicine).
Figure 7 Metastatic high-grade serous carcinoma of the ovary.
A: Endoscopic ultrasound image of a metastatic high-grade serous carcinoma of the ovary; B: Low-power view showing a cellular aspirate with a necrotic background (Diff-QuikTM stain, × 100); C: High-power view showing groups of malignant cells with large nuclei and prominent nucleoli. These cells are difficult to distinguish from a primary pancreatic ductal adenocarcinoma; however, necrotic background is not common in a primary tumor (Diff-QuikTM stain, × 400); D: Papanicolaou stain showing a cannon ball shaped group of malignant cells with large, round nuclei and prominent nucleoli, characteristic of serous ovarian carcinoma (× 400).
Figure 8 Endoscopic ultrasound image demonstrating a cystadenocarcinoma.
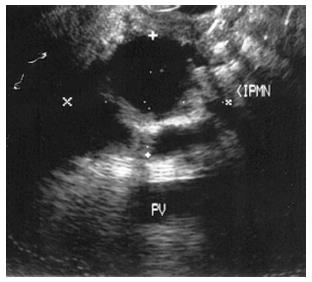
Figure 9 Endoscopic ultrasound image demonstrating an intraductal papillary-mucinous neoplasm.
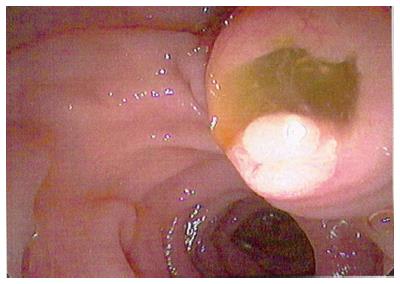
Figure 10 Endoscopic view of “fish mouth papilla” due to the presence of mucin within the main duct.
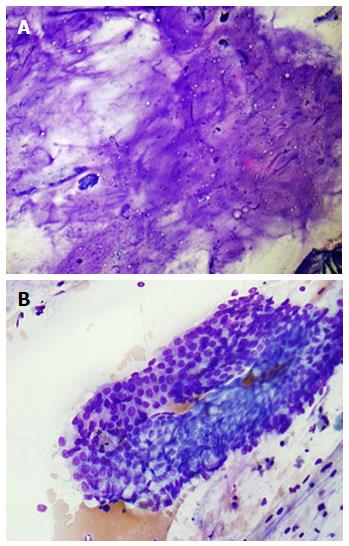
Figure 11 Pancreatic mucinous neoplasm.
A: Low-power view of pancreatic mucinous neoplasm showing copious thick, colloid-like mucin (Diff-QuikTM stain, × 100); B: High-power view of pancreatic mucinous neoplasm showing sheets of only mildly atypical columnar cells containing intracytoplasmic mucin; these cells are very difficult to distinguish from benign gastric or duodenal epithelium (Diff-QuikTM stain, × 400).
- Citation: Nelsen EM, Buehler D, Soni AV, Gopal DV. Endoscopic ultrasound in the evaluation of pancreatic neoplasms-solid and cystic: A review. World J Gastrointest Endosc 2015; 7(4): 318-327
- URL: https://www.wjgnet.com/1948-5190/full/v7/i4/318.htm
- DOI: https://dx.doi.org/10.4253/wjge.v7.i4.318